Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application
Abstract
:1. Introduction
2. 3D Printing Technologies for HA-Based Nanocomposites
2.1. Inkjet-Based 3D Printing
2.2. Stereolithography (SLA)-Based 3D Printing
2.3. Extrusion-Based 3D Printing
2.4. Laser-Assisted 3D Printing
3. Hydroxyapatite (HA) and HA-Based Nanocomposites via 3D Printing
3.1. Hydroxyapatite
3.2. Hydroxyapatite (HA)/Polymer-Based Nanocomposites
3.2.1. HA/Collagen Nanocomposites
3.2.2. Hydroxyapatite (HA)/Gelatin Nanocomposites
3.2.3. Hydroxyapatite (HA)/Silk Nanocomposites
3.2.4. Hydroxyapatite (HA)/Alginate Nanocomposites
3.2.5. Hydroxyapatite (HA)/Cellulose Nanocomposites
3.2.6. Hydroxyapatite (HA)/Chitosan Nanocomposites
3.2.7. Other Hydroxyapatite (HA)/Natural Polymer-Based Nanocomposites
3.2.8. Hydroxyapatite (HA)/Poly (Lactic Acid) Based Nanocomposites
3.2.9. Hydroxyapatite (HA)/Poly-ε-Caprolactone Nanocomposites
3.2.10. Hydroxyapatite (HA)/Polymethyl Methacrylate Nanocomposites
3.2.11. Hydroxyapatite (HA)/Polyvinyl Alcohol Nanocomposites
3.2.12. Hydroxyapatite (HA)/Poly(Propylene Fumarate) Nanocomposites
3.2.13. Other Hydroxyapatite (HA)/Synthetic Polymer-Based Nanocomposites
3.2.14. Hydroxyapatite (HA)/Natural Polymer/Synthetic Polymer-Based Nanocomposites
3.3. Hydroxyapatite (HA)-Based Ceramics
3.3.1. Hydroxyapatite (HA)/β-Tricalcium Phosphate (BCP) Based Ceramics
3.3.2. Hydroxyapatite (HA)/Bioglass Based Ceramics
3.3.3. HA-Based Composites of Titanium Ceramics
3.3.4. Other HA-Based Composites Containing Metals
4. Desired Properties
4.1. Porosity
4.2. Mechanical Properties
4.3. Biocompatibility
4.4. Biodegradability
4.5. Other Properties
5. Applications of HA-Based Nanocomposites
5.1. HA-Based Nanocomposites Constructs in Bone TE
5.2. HA-Based Nanocomposites Constructs in Cartilage TE
5.3. HA-Based Nanocomposites Constructs in Dental Applications
5.4. HA-Based Nanocomposites Constructs in Drug Delivery Applications
6. Next Generation of Hydroxyapatite (HA)-Based Nanocomposite Application in TE
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopi, D.; Kavitha, L.; Ramya, S.; Rajeswari, D. Chemical and green routes for the synthesis of multifunctional pure and substituted nanohydroxyapatite for biomedical applications. In Engineering of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; Chapter 15; pp. 485–521. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J. 1—Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–19. [Google Scholar] [CrossRef]
- Fernando, S.; McEnery, M.; Guelcher, S.A. 16—Polyurethanes for bone TE. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 481–501. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels—A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Okoro, O.V.; Shavandi, A. An assessment of the utilization of waste apple slurry in bio-succinic acid and bioenergy production. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, M.A.; Sun, Z. Bio-mimetic composite scaffold from mussel shells, squid pen and crab chitosan for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from N ew Z ealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.-D.; Ling, J. Biomimetic Collagen–Hydroxyapatite Composite Fabricated via a Novel Perfusion-Flow Mineralization Technique. Tissue Eng. Part C Methods 2013, 19, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Lin, C.J.; Feng, Z.D.; Tian, Z.W. Hydroxyapatite/metal composite coatings prepared by multi-step electrodeposition method. J. Mater. Sci. Lett. 1998, 17, 1077–1079. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite and gelatin composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J. Biomed. Mater. Res. Part A 2005, 72, 136–145. [Google Scholar] [CrossRef]
- Ito, Y.; Hasuda, H.; Kamitakahara, M.; Ohtsuki, C.; Tanihara, M.; Kang, I.-K.; Kwon, O.H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J. Biosci. Bioeng. 2005, 100, 43–49. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Voicu, G.; Ghitulica, C.; Vasile, B.S.; Ficai, D.; Trandafir, V. Self-assembled collagen/hydroxyapatite composite materials. Chem. Eng. J. 2010, 160, 794–800. [Google Scholar] [CrossRef]
- Li, H.; Zhao, N.; Liu, Y.; Liang, C.; Shi, C.; Du, X.; Li, J. Fabrication and properties of carbon nanotubes reinforced Fe/hydroxyapatite composites by in situ chemical vapor deposition. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1128–1132. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Redepenning, J.; Venkataraman, G.; Chen, J.; Stafford, N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. Biomed. Mater. Res. Part A 2003, 66, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Hench, L.L.; Du, J.; Choy, K.-L.; Guo, J. Preparation of Hydroxyapatite Fibers by Electrospinning Technique. J. Am. Ceram. Soc. 2004, 87, 1988–1991. [Google Scholar] [CrossRef]
- Nosrati, H.; Mamoory, R.S.; Le, D.Q.S.; Bünger, C.E.; Emameh, R.Z.; Dabir, F. Gas injection approach for synthesis of hydroxyapatite nanorods via hydrothermal method. Mater. Charact. 2020, 159, 110071. [Google Scholar] [CrossRef]
- Nosrati, H.; Sarraf Mamoory, R.; Svend Le, D.Q.; Bünger, C.E. Fabrication of gelatin/hydroxyapatite/3D-graphene scaffolds by a hydrogel 3D-printing method. Mater. Chem. Phys. 2020, 239, 122305. [Google Scholar] [CrossRef]
- Nosrati, H.; Sarraf-Mamoory, R.; Le, D.Q.S.; Perez, M.C.; Bünger, C.E. Evaluation of Argon-Gas-Injected Solvothermal Synthesis of Hydroxyapatite Crystals Followed by High-Frequency Induction Heat Sintering. Cryst. Growth Des. 2020, 20, 3182–3189. [Google Scholar] [CrossRef]
- Ishengoma, F.R.; Mtaho, A.B. 3D printing: Developing countries perspectives. arXiv 2014, arXiv:1410.5349. Available online: https://arxiv.org/abs/1410.5349 (accessed on 27 March 2021).
- Hull, C. Co-Founder and Chief Technology Officer. Ann H.J. Smead Aerosp. Eng. Sci. 2021. Available online: https://www.colorado.edu/aerospace/charles-hull (accessed on 2 February 2021).
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C 2021, 118. [Google Scholar] [CrossRef]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in TE and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, D.; Rauf, S.; Hauser, C. 3D bioprinting technology for regenerative medicine applications. Int. J. Bioprint. 2016, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.D.; Hoath, S.D.; Hutchings, I.M. Inkjet printing—The physics of manipulating liquid jets and drops. J. Phys. Conf. 2008, 105, 012001. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet printing for biosensor fabrication: Combining chemistry and technology for advanced manufacturing. Lab Chip 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Li, X.P.; Li, C.C.; Yang, M.M.; Wei, Q.H. Binder droplet impact mechanism on a hydroxyapatite microsphere surface in 3D printing of bone scaffolds. J. Mater. Sci. 2015, 50, 5014–5023. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds-Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.X.; Buchanan, F.; Mitchell, C.; Dunne, N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Strobel, L.A.; Rath, S.N.; Maier, A.K.; Beier, J.P.; Arkudas, A.; Greil, P.; Horch, R.E.; Kneser, U. Induction of bone formation in biphasic calcium phosphate scaffolds by bone morphogenetic protein-2 and primary osteoblasts. J. Tissue Eng. Regen. Med. 2014, 8, 176–185. [Google Scholar] [CrossRef]
- Warnke, P.H.; Seitz, H.; Warnke, F.; Becker, S.T.; Sivananthan, S.; Sherry, E.; Liu, Q.; Wiltfang, J.; Douglas, T. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: Characterization and biocompatibility investigations. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 212–217. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by SLA. U.S. Patent US4575330A, 11 March 1986. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.J.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.C.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, D.N.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Gantumur, E.; Nakahata, M.; Kojima, M.; Sakai, S. Extrusion-Based Bioprinting through Glucose-Mediated Enzymatic Hydrogelation. Int. J. Bioprint. 2020, 6, 250. [Google Scholar] [CrossRef]
- Sakai, S.; Mochizuki, K.; Qu, Y.; Mail, M.; Nakahata, M.; Taya, M. Peroxidase-catalyzed microextrusion bioprinting of cell-laden hydrogel constructs in vaporized ppm-level hydrogen peroxide. Biofabrication 2018, 10, 045007. [Google Scholar] [CrossRef]
- Barry, J.J.A.; Evseev, A.V.; Markov, M.A.; Upton, C.E.; Scotchford, C.A.; Popov, V.K.; Howdle, S.M. In vitro study of hydroxyapatite-based photocurable polymer composites prepared by laser stereolithography and supercritical fluid extraction. Acta Biomater. 2008, 4, 1603–1610. [Google Scholar] [CrossRef]
- Woesz, A.; Rumpler, M.; Stampfl, J.; Varga, F.; Fratzl-Zelman, N.; Roschger, P.; Klaushofer, K.; Fratzl, P. Towards bone replacement materials from calcium phosphates via rapid prototyping and ceramic gelcasting. Mater. Sci. Eng. C 2005, 25, 181–186. [Google Scholar] [CrossRef]
- Chen, Q.; Zou, B.; Lai, Q.; Wang, Y.; Xue, R.; Xing, H.; Fu, X.; Huang, C.; Yao, P. A study on biosafety of HAP ceramic prepared by SLA-3D printing technology directly. J. Mech. Behav. Biomed. Mater. 2019, 98, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Van Hede, D.; Plougonven, E.; Nolens, G.; Verlée, B.; De Pauw, M.C.; Lambert, F. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J. Biomed. Mater. Res. Part A 2020, 108, 412–425. [Google Scholar] [CrossRef]
- Wang, Z.J.; Kumar, H.; Tian, Z.L.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Lee, J.-W. Extrusion Bioprinting. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–152. [Google Scholar] [CrossRef]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef] [Green Version]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Khalyfa, A.; Vogt, S.; Weisser, J.; Grimm, G.; Rechtenbach, A.; Meyer, W.; Schnabelrauch, M. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J. Mater. Sci. Mater. Med. 2007, 18, 909–916. [Google Scholar] [CrossRef]
- Michna, S.; Wu, W.; Lewis, J.A. Concentrated hydroxyapatite inks for direct-write assembly of 3-D periodic scaffolds. Biomaterials 2005, 26, 5632–5639. [Google Scholar] [CrossRef]
- Sun, L.; Parker, S.T.; Syoji, D.; Wang, X.; Lewis, J.A.; Kaplan, D.L. Direct-write assembly of 3D silk/hydroxyapatite scaffolds for bone co-cultures. Adv. Healthc. Mater. 2012, 1, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Khodaei, M.; Amini, K.; Valanezhad, A. Fabrication and Characterization of Poly Lactic Acid Scaffolds by Fused Deposition Modeling for Bone Tissue Engineering. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 248–251. [Google Scholar] [CrossRef]
- You, F.; Eames, B.F.; Chen, X. Application of Extrusion-Based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Juengst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Rodriguez-Salvador, M.; Ruiz-Cantu, L. Revealing emerging science and technology research for dentistry applications of 3D bioprinting. Int. J. Bioprint. 2018, 5. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Remy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amedee, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Keriquel, V.; Guillemot, F.; Arnault, I.; Guillotin, B.; Miraux, S.; Amédée, J.L.; Fricain, J.C.; Catros, S. In vivo bioprinting for computer- and robotic-assisted medical intervention: Preliminary study in mice. Biofabrication 2010, 2, 014101. [Google Scholar] [CrossRef]
- Shirazi, S.F.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.; Adib Kadri, N.; Osman, N.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone TE applications. Int. J. Nanomed. 2013, 8, 4197. [Google Scholar]
- Gao, C.; Yang, B.; Hu, H.; Liu, J.; Shuai, C.; Peng, S. Enhanced sintering ability of biphasic calcium phosphate by polymers used for bone scaffold fabrication. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3802–3810. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Li, X.; Wei, Q.; Chai, W.; Wang, S.; Che, Y.; Lu, T.; Zhang, B. 3D fabrication and characterization of phosphoric acid scaffold with a HA/β-TCP weight ratio of 60:40 for bone TE applications. PLoS ONE 2017, 12, e0174870. [Google Scholar] [CrossRef]
- Lin, K.F.; He, S.; Song, Y.; Wang, C.M.; Gao, Y.; Li, J.Q.; Tang, P.; Wang, Z.; Bi, L.; Pei, G.X. Low-Temperature Additive Manufacturing of Biomimic Three-Dimensional Hydroxyapatite/Collagen Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 6905–6916. [Google Scholar] [CrossRef]
- Ardelean, I.L.; Gudovan, D.; Ficai, D.; Ficai, A.; Andronescu, E.; Albu-Kaya, M.G.; Neacsu, P.; Ion, R.N.; Cimpean, A.; Mitran, V. Collagen/hydroxyapatite bone grafts manufactured by homogeneous/heterogeneous 3D printing. Mater. Lett. 2018, 231, 179–182. [Google Scholar] [CrossRef]
- Huang, T.; Fan, C.; Zhu, M.; Zhu, Y.; Zhang, W.; Li, L. 3D-printed scaffolds of biomineralized hydroxyapatite nanocomposite on silk fibroin for improving bone regeneration. Appl. Surf. Sci. 2019, 467–468, 345–353. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and Biocompatible Macroporous Scaffolds with Tunable Performances Prepared Based on 3D Printing of the Pre-Crosslinked Sodium Alginate/Hydroxyapatite Hydrogel Ink. Macromol. Mater. Eng. 2019, 304, 1800698. [Google Scholar] [CrossRef]
- Myung, S.-W.; Kim, B.-H. Oxygen and nitrogen plasma etching of three-dimensional hydroxyapatite/chitosan scaffolds fabricated by additive manufacturing. Jpn. J. Appl. Phys. 2015, 55, 01AB07. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone TE application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Moncal, K.K.; Heo, D.N.; Godzik, K.P.; Sosnoski, D.M.; Mrowczynski, O.D.; Rizk, E.; Ozbolat, V.; Tucker, S.M.; Gerhard, E.M.; Dey, M.; et al. 3D printing of poly(ε-caprolactone)/poly(d,l-lactide-co-glycolide)/hydroxyapatite composite constructs for bone tissue engineering. J. Mater. Res. 2018, 33, 1972–1986. [Google Scholar] [CrossRef]
- Esmi, A.; Jahani, Y.; Yousefi, A.A.; Zandi, M. PMMA-CNT-HAp nanocomposites optimized for 3D-printing applications. Mater. Res. Express 2019, 6, 085405. [Google Scholar] [CrossRef]
- Ergul, N.M.; Unal, S.; Kartal, I.; Kalkandelen, C.; Ekren, N.; Kilic, O.; Chi-Chang, L.; Gunduz, O. 3D printing of chitosan/poly(vinyl alcohol) hydrogel containing synthesized hydroxyapatite scaffolds for hard-TE. Polym. Test. 2019, 79, 106006. [Google Scholar] [CrossRef]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D Printed Polycaprolactone/Gelatin/Bacterial Cellulose/Hydroxyapatite Composite Scaffold for Bone Tissue Engineering. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Yeon, Y.K.; Park, H.S.; Lee, J.M.; Lee, J.S.; Lee, Y.J.; Sultan, M.T.; Seo, Y.B.; Lee, O.J.; Kim, S.H.; Park, C.H. New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J. Biomater. Sci. Polym. Ed. 2018, 29, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Gwak, S.J.; Seo, K.D.; Lee, S.; Yun, J.H.; Cho, Y.S.; Lee, S.J. Fabrication of Three-Dimensional Composite Scaffold for Simultaneous Alveolar Bone Regeneration in Dental Implant Installation. Int. J. Mol. Sci. 2020, 21, 1863. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Kim, Y.-J. Fabrication of strontium-substituted hydroxyapatite scaffolds using 3D printing for enhanced bone regeneration. J. Mater. Sci. 2021, 56, 1673–1684. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Godec, D.; Cano, S.; Holzer, C.; Gonzalez-Gutierrez, J.J.M. Optimization of the 3D Printing Parameters for Tensile Properties of Specimens Produced by Fused Filament Fabrication of 17-4PH Stainless Steel. Materials 2020, 13, 774. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ouyang, J.; Liang, W.; Yan, Z.C.; Stadler, F.; Lao, C. Development and characterizations of novel aqueous-based LSCF suspensions for inkjet printing. Ceram. Int. 2018, 44, 13381–13388. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Chockalingam, K.; Jawahar, N.; Ramanathan, K.N.; Banerjee, P.S. Optimization of stereolithography process parameters for part strength using design of experiments. Int. J. Adv. Manuf. Technol. 2006, 29, 79–88. [Google Scholar] [CrossRef]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D Printing of Personalized Artificial Bone Scaffolds. 3D Print. Addit. Manuf. 2015, 2, 56–64. [Google Scholar] [CrossRef]
- Gentry, S.P.; Halloran, J.W. Depth and width of cured lines in photopolymerizable ceramic suspensions. J. Eur. Ceram. Soc. 2013, 33, 1981–1988. [Google Scholar] [CrossRef]
- de Hazan, Y.; Penner, D. SiC and SiOC ceramic articles produced by stereolithography of acrylate modified polycarbosilane systems. J. Eur. Ceram. Soc. 2017, 37, 5205–5212. [Google Scholar] [CrossRef]
- Santoliquido, O.; Colombo, P.; Ortona, A. Additive Manufacturing of ceramic components by Digital Light Processing: A comparison between the “bottom-up” and the “top-down” approaches. J. Eur. Ceram. Soc. 2019, 39, 2140–2148. [Google Scholar] [CrossRef]
- El Magri, A.; El Mabrouk, K.; Vaudreuil, S.; Ebn Touhami, M. Experimental investigation and optimization of printing parameters of 3D printed polyphenylene sulfide through response surface methodology. J. Appl. Polym. Sci. 2021, 138, 49625. [Google Scholar] [CrossRef]
- Moradi, M.; Moghadam, M.K.; Shamsborhan, M.; Bodaghi, M. The Synergic Effects of FDM 3D Printing Parameters on Mechanical Behaviors of Bronze Poly Lactic Acid Composites. J. Compos. Sci. 2020, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Damien, E.; Hing, K.; Saeed, S.; Revell, P.A. A preliminary study on the enhancement of the osteointegration of a novel synthetic hydroxyapatite scaffold in vivo. J. Biomed. Mater. Res. Part A 2003, 66A, 241–246. [Google Scholar] [CrossRef]
- Liu, X. Cell responses to two kinds of nanohydroxyapatite with different sizes and crystallinities. Int. J. Nanomed. 2012, 7, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Lennon, A.; Buchanan, F.; McCarthy, H.O.; Dunne, N. Binder jetting additive manufacturing of hydroxyapatite powders: Effects of adhesives on geometrical accuracy and green compressive strength. Addit. Manuf. 2020, 101645. [Google Scholar] [CrossRef]
- Liu, Z.B.; Liang, H.X.; Shi, T.S.; Xie, D.Q.; Chen, R.Y.; Han, X.; Shen, L.D.; Wang, C.J.; Tian, Z.J. Additive manufacturing of hydroxyapatite bone scaffolds via digital light processing and in vitro compatibility. Ceram. Int. 2019, 45, 11079–11086. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive Manufacturing Methods for Producing Hydroxyapatite and Hydroxyapatite-Based Composite Scaffolds: A Review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Herranz-Blanco, B.; Almeida, P.V.; Hirvonen, J.; Santos, H.A. Multifaceted polymersome platforms: Spanning from self-assembly to drug delivery and protocells. Prog. Polym. Sci. 2016, 60, 51–85. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Averous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Xu, W.; Wu, X.; Sun, W. Review on polymer/layered silicates nanocomposites. J. Chin. Ceram. Soc. 2016. [Google Scholar] [CrossRef]
- Bedell, M.L.; Navara, A.M.; Du, Y.; Zhang, S.; Mikos, A.G. Polymeric Systems for Bioprinting. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Desimone, E.; Schacht, K.; Jungst, T.; Groll, J.; Scheibel, T. Biofabrication of 3D constructs: Fabrication technologies and spider silk proteins as bioinks. Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zheng, S.; Hu, X.; Li, L.; Li, W.; Parungao, R.; Wang, Y.; Nie, Y.; Liu, T.; Song, K. Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting. Polymers 2020, 12, 1237. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Echave, M.C.; SBurgo, L.; LPedraz, J.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical insights into bioinks for 3D printing. Chem. Soc. Rev. 2019, 48, 4049–4086. [Google Scholar] [CrossRef] [Green Version]
- Samadikuchaksaraei, A.; Gholipourmalekabadi, M.; Erfani Ezadyar, E.; Azami, M.; Mozafari, M.; Johari, B.; Kargozar, S.; Jameie, S.B.; Korourian, A.; Seifalian, A.M. Fabrication and In vivo evaluation of an osteoblast-conditioned nano-hydroxyapatite/gelatin composite scaffold for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2016, 104, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.K.; Ferreira, J.; Luo, T.J.M.; Geng, H.X.; Lin, F.C.; Ko, C.C. Direct scaffolding of biomimetic hydroxyapatite-gelatin nanocomposites using aminosilane cross-linker for bone regeneration. J. Mater. Sci. Mater. Med. 2012, 23, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comeau, P.; Willett, T. Printability of Methacrylated Gelatin upon Inclusion of a Chloride Salt and Hydroxyapatite Nano-Particles. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Özsağıroğlu, T.B.; Nasün-Saygılı, G. The Impact of Gelatin Weight Ratio on Hydroxyapatite-gelatin Composites and Their SBF Behaviour. Macromol. Symp. 2015, 352, 8–15. [Google Scholar] [CrossRef]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Rui, L.R.; Kaplan, D.L. Silk-Based Biomaterials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Wang, L.; Fang, M.; Xia, Y.J.; Hou, J.X.; Nan, X.R.; Zhao, Z.; Wang, X.Y. Preparation and biological properties of silk fibroin/nano-hydroxyapatite/graphene oxide scaffolds with an oriented channel-like structure. RSC Adv. 2020, 10, 10118–10128. [Google Scholar] [CrossRef]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef] [Green Version]
- Mottaghitalab, F.; Hosseinkhani, H.; Shokrgozar, M.A.; Mao, C.; Yang, M.; Farokhi, M. Silk as a potential candidate for bone TE. J. Control. Release 2015, 215, 112–128. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Ohgo, K.; Asakura, T. Preparation and characterization of regenerated Bombyx mori silk fibroin fiber with high strength. Express Polym. Lett. 2008, 2, 885–889. [Google Scholar] [CrossRef]
- Wang, Q.S.; Han, G.C.; Yan, S.Q.; Zhang, Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Li, X.; Zhou, G.; Fan, H.; Fan, Y. Electrospun sulfated silk fibroin nanofibrous scaffolds for vascular tissue engineering. Biomaterials 2011, 32, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, G.W.; Wang, Y.F.; Zhao, H.S.; Xiong, S.; Wu, Y.; Heng, B.C.; An, C.R.; Zhu, G.H.; Xie, D.H. Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop. Biomaterials 2015, 49, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, E.; Eckardt, J.; Hermansson, A.M.; Larsson, A.; Loren, N.; Altskar, A.; Strom, A. Microstructural, mechanical and mass transport properties of isotropic and capillary alginate gels. Soft Matter 2014, 10, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, S.; Pan, J.; Shi, R.; Liu, H.; Lyu, Y.; Han, X.; Li, Y.; Yang, Y.; Xu, Z.; et al. Time-responsive osteogenic niche of stem cells: A sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials 2018, 163, 25–42. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, Y.; Deng, Y.; Sun, Y.; Yang, H.; Wei, S. Peptide-incorporated 3D porous alginate scaffolds with enhanced osteogenesis for bone tissue engineering. Colloids Surf. B Biointerfaces 2016, 143, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/Hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575. [Google Scholar] [CrossRef]
- Colovic, B.; Jokanovic, V.; Petrovic, M. Self assembly of biomimetic hydroxyapatite on the surface of different polymer thin films. J. Ceram. Process. Res. 2012, 13, 398–404. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Lin, H.R.; Yeh, Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71b, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Turlybekuly, A.; Sagidugumar, A.; Otarov, Y.; Magazov, N.; Pogrebnjak, A.; Savitskaya, I.; Akatan, K.; Kistaubayeva, A.; Talipova, A. Bacterial Cellulose/Hydroxyapatite Printed Scaffolds for Bone Engineering. In Nanomaterials in Biomedical Application and Biosensors, Proceedings of the 9th IEEE International Conference on Nanomaterials: Applications & Properties, Oddesa, Ukraine, 10–15 September 2019; Springer: Singapore, 2020; pp. 1–7. [Google Scholar]
- Favi, P.M.; Ospina, S.P.; Kachole, M.; Gao, M.; Atehortua, L.; Webster, T.J. Preparation and characterization of biodegradable nano hydroxyapatite–bacterial cellulose composites with well-defined honeycomb pore arrays for bone tissue engineering applications. Cellulose 2016, 23, 1263–1282. [Google Scholar] [CrossRef]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. 2—Natural polymer biomaterials: Advanced applications. In New Functional Biomaterials for Medicine and Healthcare; Ivanova, E.P., Bazaka, K., Crawford, R.J., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 32–70. [Google Scholar]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Mescher, A. Junqueira’s Basic Histology: Text and Atlas; McGraw-Hill Medical: New York, NY, USA, 2013. [Google Scholar]
- Tigli, R.S.; Gumusderelioglu, M. Chondrogenesis on BMP-6 Loaded Chitosan Scaffolds in Stationary and Dynamic Cultures. Biotechnol. Bioeng. 2009, 104, 601–610. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Domenech, M.; Polocorrales, L.; Ramirezvick, J.E.; Freytes, D.O. TE Strategies for Myocardial Regeneration: Acellular Versus Cellular Scaffolds? Tissue Eng. Part B Rev. 2016, 22, 438–458. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.F.; Liang, J.N.; Cui, Y.H.; Xu, S.; Zhao, N.R. Fabrication of novel bioactive hydroxyapatite-chitosan-silica hybrid scaffolds: Combined the sol-gel method with 3D plotting technique. Carbohydr. Polym. 2018, 197, 183–193. [Google Scholar] [CrossRef]
- Ang, T.H.; Sultana, F.S.A.; Hutmacher, D.W.; Wong, Y.S.; Fuh, J.Y.H.; Mo, X.M.; Loh, H.T.; Burdet, E.; Teoh, S.H. Fabrication of 3D chitosan-hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C 2002, 20, 35–42. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Yang, A.; Peng, X.; Wang, X.; Zhang, X. Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J. Mater. Mater. Med. 2005, 16, 213–219. [Google Scholar]
- Shavandi, A.; Hosseini, S.; Okoro, O.V.; Nie, L.; Babadi, F.E.; Melchels, F. 3D Bioprinting of Lignocellulosic Biomaterials. Adv. Healthc. Mater. 2020. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.H.; Liu, C.; Ao, Q.; Tian, X.H.; Fan, J.; Tong, H.; Wang, X.H. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef] [Green Version]
- Wenz, A.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials 2004, 25, 3829–3835. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.-H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomater. Guildf. 2005. [Google Scholar] [CrossRef]
- Sailaja, G.S.; Velayudhan, S.; Sunny, M.C.; Sreenivasan, K.; Varma, H.K.; Ramesh, P. Hydroxyapatite filled chitosan-polyacrylic acid polyelectrolyte complexes. J. Mater. Sci. 2003, 38, 3653–3662. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Tokuchi, K.; Fukuzaki, H.; Koyama, Y.; Takakuda, K.; Monma, H.; Tanaka, J. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J. Biomed. Mater. Res. 2001. [Google Scholar] [CrossRef]
- Freddi, G.; Monti, P.; Nagura, M.; And, Y.G.; Tsukada, M. Structure and molecular conformation of tussah silk fibroin films: Effect of heat treatment. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 841–847. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.Y.; Ha, W.S.; Park, S.Y. Structural changes and their effect on mechanical properties of silk fibroin/chitosan blends. J. Appl. Polym. Sci. 1999, 74, 2571–2575. [Google Scholar] [CrossRef]
- Wang, L.; Nemoto, R.; Senna, M. Microstructure and Chemical States of Hydroxyapatite/silk Fibroin Nanocomposites Synthesized via A Wet-mechanochemical Route. J. Nanoparticle Res. 2002, 4, 535–540. [Google Scholar] [CrossRef]
- Wang, L.; Nemoto, R.; Senna, M. Changes in microstructure and physico-chemical properties of hydroxyapatite–silk fibroin nanocomposite with varying silk fibroin content. J. Eur. Ceram. Soc. 2004, 24, 2707–2715. [Google Scholar] [CrossRef]
- Wang, L.; Li, C. Preparation and physicochemical properties of a novel hydroxyapatite/chitosan–silk fibroin composite. Carbohydr. Polym. 2007, 68, 740–745. [Google Scholar] [CrossRef]
- Peter, M.; Ganesh, N.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation and characterization of chitosan–gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2010, 80, 687–694. [Google Scholar] [CrossRef]
- Verma, D.; Katti, K.S.; Katti, D.R.; Mohanty, B. Mechanical response and multilevel structure of biomimetic hydroxyapatite/polygalacturonic/chitosan nanocomposites. Mater. Sci. Eng. C 2008, 28, 399–405. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Beneventi, D.; Dufresne, A. Biomimetic Mineralization of Three-Dimensional Printed Alginate/TEMPO-Oxidized Cellulose Nanofibril Scaffolds for Bone Tissue Engineering. Biomacromolecules 2018, 19, 4442–4452. [Google Scholar] [CrossRef]
- Van den Eynde, M.; Van Puyvelde, P. 3D Printing of Poly(lactic acid). In Industrial Applications of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 139–158. [Google Scholar]
- Fini, M.; Giannini, S.; Giardino, R.; Giavaresi, G.; Rocca, M. Resorbable device for fracture fixation: In vivo degradation and mechanical behaviour. Int. J. Artif. Organs 1995, 18, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.; Monti, P.; Simoni, R. Vibrational and thermal study on the in vitro and in vivo degradation of a poly(lactic acid)-based bioabsorbable periodontal membrane. J. Mater. Sci. Mater. Med. 2002, 13, 469–475. [Google Scholar] [CrossRef]
- Takayama, T.; Uchiumi, K.; Ito, H.; Kawai, T.; Todo, M. Particle size distribution effects on physical properties of injection molded HA/PLA composites. Adv. Compos. Mater. 2013, 22, 327–337. [Google Scholar] [CrossRef]
- Nejati, E.; Mirzadeh, H.; Zandi, M. Synthesis and characterization of nano-hydroxyapatite rods/poly(l-lactide acid) composite scaffolds for bone tissue engineering. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1589–1596. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, S.; Li, X.; Weng, J. Shape memory properties of poly(d,l-lactide)/hydroxyapatite composites. Biomaterials 2006, 27, 4288–4295. [Google Scholar] [CrossRef] [PubMed]
- Leenslag, J.W.; Pennings, A.J.; Bos, R.R.M.; Rozema, F.R.; Boering, G. Resorbable materials of poly(l-lactide). VI. Plates and screws for internal fracture fixation. Biomaterials 1987, 8, 70–73. [Google Scholar] [CrossRef]
- Böstman, O.M. Absorbable implants for the fixation of fractures. J. Bone Jt. Surg. Am. 1991, 73, 148–153. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.L.; Zheng, Y.F. Effect of surface modified hydroxyapatite on the tensile property improvement of HA/PLA composite. Appl. Surf. Sci. 2008, 255, 494–497. [Google Scholar] [CrossRef]
- Deng, X.; Hao, J.; Wang, C. Preparation and mechanical properties of nanocomposites of poly(d,l-lactide) with Ca-deficient hydroxyapatite nanocrystals. Biomaterials 2001, 22, 2867–2873. [Google Scholar] [CrossRef]
- Shikinami, Y.; Matsusue, Y.; Nakamura, T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly l-lactide (F-u-HA/PLLA). Biomaterials 2005, 26, 5542–5551. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Suetsugu, Y.; Tanaka, J.; Akao, M. Preparation and mechanical properties of calcium phosphate/copoly-l-lactide composites. J. Mater. Sci. Mater. Med. 1997, 8, 361–364. [Google Scholar] [CrossRef]
- Yamaji, S.; Kobayashi, S. Effect of in vitro hydrolysis on the compressive behavior and strain rates dependence of tricalcium phosphate/poly(l-lactic acid) composites. Adv. Compos. Mater. 2013, 22, 1–11. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sakamoto, K. Bending and Compressive Properties of Crystallized TCP/PLLA Composites. Adv. Compos. Mater. 2009, 18, 287–295. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, P.; He, C.; Qiu, X.; Liu, A.; Chen, L.; Chen, X.; Jing, X. Nano-composite of poly(l-lactide) and surface grafted hydroxyapatite: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296–6304. [Google Scholar] [CrossRef]
- Takayama, T.; Todo, M.; Takano, A. The effect of bimodal distribution on the mechanical properties of hydroxyapatite particle filled poly(l-lactide) composites. J. Mech. Behav. Biomed. Mater. 2009, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Qiu, X.; Sun, J.; Deng, M.; Chen, X.; Jing, X. Grafting polymerization of l-lactide on the surface of hydroxyapatite nano-crystals. Polymer 2004, 45, 6699–6706. [Google Scholar] [CrossRef]
- Naik, A.; Best, S.M.; Cameron, R.E. The influence of silanisation on the mechanical and degradation behaviour of PLGA/HA composites. Mater. Sci. Eng. C 2015, 48, 642–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Bostman, O.M. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: A three- to nine-year follow-up study. J. Bone Jt. Surg. Br. 1998, 80, 333–338. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-l-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Nie, L.; Suo, J.P.; Zou, P.; Feng, S.B. Preparation and Properties of Biphasic Calcium Phosphate Scaffolds Multiply Coated with HA/PLLA Nanocomposites for Bone Tissue Engineering Applications. J. Nanomater. 2012, 2012, 213549. [Google Scholar] [CrossRef]
- Verheyen, C.C.P.M.; Wijn, J.R.D.; Blitterswijk, C.A.V.; Groot, K.D. Evaluation of hydroxylapatite/poly(l-lactide) composites: Mechanical behavior. J. Biomed. Mater. Res. 1992, 26, 1277–1296. [Google Scholar] [CrossRef]
- Furukawa, T.; Matsusue, Y.; Yasunaga, T.; Shikinami, Y.; Okuno, M.; Nakamura, T. Biodegradation behavior of ultra-high-strength hydroxyapatite/poly(l-lactide) composite rods for internal fixation of bone fractures. Biomaterials 2000, 21, 889–898. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kwon, J.S.; Park, S.H.; Park, J.H.; Jang, S.H.; Yin, X.Y.; Yun, J.H.; Kim, J.H.; Min, B.H.; Lee, J.H.; et al. A computer-designed scaffold for bone regeneration within cranial defect using human dental pulp stem cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile preparation of bioactive nanoparticle/poly(ε-caprolactone) hierarchical porous scaffolds via 3D printing of high internal phase Pickering emulsions. J. Colloid Interface Sci. 2019, 545, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.J.; Miller, S.T.; Zhu, G.; Yasko, A.W.; Mikos, A.G. In vivo degradation of a poly(propylene fumarate)/β-tricalcium phosphate injectable composite scaffold. J. Biomed. Mater. Res. 1998, 41, 1–7. [Google Scholar] [CrossRef]
- Petersmann, S.; Spoerk, M.; Huber, P.; Lang, M.; Pinter, G.; Arbeiter, F. Impact Optimization of 3D-Printed Poly(methyl methacrylate) for Cranial Implants. Macromol. Mater. Eng. 2019, 304, 1900263. [Google Scholar] [CrossRef]
- Tontowi, A.E.; Kuswanto, D.; Sihaloho, R.I.; Sosiati, H. Composite of [HA/PMMA] for 3D-printer material application. AIP Conf. Proc. 2016, 1755, 150020. [Google Scholar]
- Mahammod, B.P.; Barua, E.; Deb, P.; Deoghare, A.B.; Pandey, K.M. Investigation of Physico-mechanical Behavior, Permeability and Wall Shear Stress of Porous HA/PMMA Composite Bone Scaffold. Arab. J. Sci. Eng. 2020, 45, 5505–5515. [Google Scholar] [CrossRef]
- Lal, B.; Ghosh, M.; Agarwal, B.; Gupta, D.; Roychoudhury, A. A novel economically viable solution for 3D printing-assisted cranioplast fabrication. Br. J. Neurosurg. 2020, 34, 280–283. [Google Scholar] [CrossRef]
- Duran, C.; Subbian, V.; Giovanetti, M.T.; Simkins, J.R.; Beyette, F.R. Experimental desktop 3D printing using dual extrusion and water-soluble polyvinyl alcohol. Rapid Prototyp. J. 2015, 21, 528–534. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, G.H.; Hou, R.X.; Xu, H.P.; Li, Y.P.; Fu, J. Controllable promotion of chondrocyte adhesion and growth on PVA hydrogels by controlled release of TGF-beta 1 from porous PLGA microspheres. Colloid Surf. B 2015, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Du, G.L.; Nie, L.; Gao, G.R.; Sun, Y.N.; Hou, R.X.; Zhang, H.; Chen, T.; Fu, J. Tough and Biocompatible Hydrogels Based on In Situ Interpenetrating Networks of Dithiol-Connected Graphene Oxide and Poly(vinyl alcohol). ACS Appl. Mater. Interfaces 2015, 7, 3003–3008. [Google Scholar] [CrossRef]
- Hou, R.X.; Nie, L.; Du, G.L.; Xiong, X.P.; Fu, J. Natural polysaccharides promote chondrocyte adhesion and proliferation on magnetic nanoparticle/PVA composite hydrogels. Colloid Surf. B 2015, 132, 146–154. [Google Scholar] [CrossRef]
- Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials 2019, 13, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, C.K.; Leong, K.F.; Tan, K.H.; Wiria, F.E.; Cheah, C.M. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci. Mater. Med. 2004, 15, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Deng, Y.L.; Li, P.; Hou, R.X.; Shavandi, A.; Yang, S.F. Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration. ACS Omega 2020, 5, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, D.; Suo, J.P.; Zou, P.; Feng, S.B.; Yang, Q.; Yang, S.H.; Ye, S.N. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze-drying method for bone tissue engineering applications. Colloid Surf. B 2012, 100, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Li, X.C.; Wang, Z.; Hu, K.H.; Cai, R.H.; Li, P.; Han, Y.T.; Sun, M.; Yuan, H.Y.; Suo, J.P.; et al. In vitro biomineralization on poly(vinyl alcohol)/biphasic calcium phosphate hydrogels. Bioinspir. Biomim. Nanobiomater. 2020, 9, 122–128. [Google Scholar] [CrossRef]
- Chai, W.H.; Wei, Q.H.; Yang, M.M.; Ji, K.; Guo, Y.H.; Wei, S.M.; Wang, Y.N. The printability of three water based polymeric binders and their effects on the properties of 3D printed hydroxyapatite bone scaffold. Ceram. Int. 2020, 46, 6663–6671. [Google Scholar] [CrossRef]
- Domb, A.J.; Manor, N.; Elmalak, O. Biodegradable bone cement compositions based on acrylate and epoxide terminated poly(propylene fumarate) oligomers and calcium salt compositions. Biomaterials 1996, 17, 411–417. [Google Scholar] [CrossRef]
- He, S.; Timmer, M.D.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)-diacylate macromers as crosslinking agents and characterization of their degradation products. Polymer 2001, 42, 1251–1260. [Google Scholar] [CrossRef]
- Wilson, J.A.; Luong, D.; Kleinfehn, A.P.; Sallam, S.; Wesdemiotis, C.; Becker, M.L. Magnesium Catalyzed Polymerization of End Functionalized Poly(propylene maleate) and Poly(propylene fumarate) for 3D Printing of Bioactive Scaffolds. J. Am. Chem. Soc. 2018, 140, 277–284. [Google Scholar] [CrossRef]
- Kim, K.; Dean, D.; Wallace, J.; Breithaupt, R.; Mikos, A.G.; Fisher, J.P. The influence of stereolithographic scaffold architecture and composition on osteogenic signal expression with rat bone marrow stromal cells. Biomaterials 2011, 32, 3750–3763. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.Y.; Le Fer, G.; Dean, D.; Becker, M.L. 3D Printing of Poly(propylene fumarate) Oligomers: Evaluation of Resin Viscosity, Printing Characteristics and Mechanical Properties. Biomacromolecules 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- Le Fer, G.; Luo, Y.Y.; Becker, M.L. Poly(propylene fumarate) stars, using architecture to reduce the viscosity of 3D printable resins. Polym. Chem. 2019, 10, 4655–4664. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.; Lu, L.; Jabbari, E.; Currier, B.L.; Yaszemski, M.J. Fabrication and Characterization of Poly(Propylene Fumarate) Scaffolds with Controlled Pore Structures Using 3-Dimensional Printing and Injection Molding. Tissue Eng. 2006, 12, 2801. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.; Yaszemski, M.J.; Lu, L. Physical properties and cellular responses to crosslinkable poly(propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials 2008, 29, 2839–2848. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Wang, S.; Dadsetan, M.; Yaszemski, M.J.; Lu, L. Enhanced cell ingrowth and proliferation through three-dimensional nanocomposite scaffolds with controlled pore structures. Biomacromolecules 2010, 11, 682. [Google Scholar] [CrossRef] [Green Version]
- Trachtenberg, J.E.; Placone, J.K.; Smith, B.T.; Fisher, J.P.; Mikos, A.G. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J. Biomater. Sci. Polym. E 2017, 28, 532–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Ahn, G.; Kim, D.S.; Cho, D.W. Development of nano- and microscale composite 3D scaffolds using PPF/DEF-HA and micro-stereolithography. Microelectron. Eng. 2009, 86, 1465–1467. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Charles, L.F.; Shaw, M.T.; Wei, J.R.O. Fabrication and mechanical properties of PLLA/PCL/HA composites via a biomimetic, dip coating, and hot compression procedure. J. Mater. Sci. Mater. Med. 2010, 21, 1845–1854. [Google Scholar] [CrossRef]
- Park, S.D.; Todo, M.; Arakawa, K.; Tsuji, H.; Takenoshita, Y. Fracture properties of bioabsorbable HA/PLLA/PCL composite material. Proc. SPIE Int. Soc. Opt. Eng. 2005, 5852. [Google Scholar] [CrossRef]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Todo, M.; Kagawa, T. Improvement of fracture energy of HA/PLLA biocomposite material due to press processing. J. Mater. Sci. 2008. [Google Scholar] [CrossRef]
- Todo, M.; Park, S.; Arakawa, K.; Takenoshita, Y. Effect of Particle Shape on the Fracture Behavior of HA/PLLA Composite Material. J. Jpn. Soc. Compos. Mater. 2005, 31, 177–183. [Google Scholar] [CrossRef]
- Todo, M.; Sang, D.P.; Arakawa, K.; Takenoshita, Y. Relationship between microstructure and fracture behavior of bioabsorbable HA/PLLA composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2221–2225. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Poly(A-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 2015, 44, 446–455. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; Bray, L.J.; Barnett, N.L.; Harkin, D.G. Evaluation of silk sericin as a biomaterial: In vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2013, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.; Trivedy, K.; Nirmal, K.S. The silk proteins, sericin and fibroin in silkworm, Bombyx mori Linn.—A review. Caspian J. Environ. Sci. 2007, 5, 63–76. [Google Scholar]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite nanoparticles: A review of preparation methodologies. J. Appl. Biomater. Biomech. 2004, 2, 74–80. [Google Scholar]
- Helmus, M.; Scott, M.J. Enhanced Biocompatibility Coatings for Medical Implants. U.S. Patent No. EP1051210A2, 15 November 2000. [Google Scholar]
- Zebarjad, S.M.; Sajjadi, S.A.; Sdrabadi, T.E.; Yaghmaei, A.; Naderi, B. A Study on Mechanical Properties of PMMA/Hydroxyapatite Nanocomposite. Engineering 2011, 3, 795–801. [Google Scholar]
- Tontowi, A.E.; Raharjo, K.P.; Sihaloho, R.I.; Baroroh, D.K. Comparison of Design Method for Making Composite of (PMMA/HA/Sericin). In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2017; pp. 85–90. [Google Scholar]
- Tontowi, A.E.; Anggraeni, D.; Saragih, H.T.; Raharjo, K.P.N.; Utami, P. Experimental study of 3D-printable biocomposite of [HA/PMMA/Sericin] materials. Adv. Mater. Lett. 2017, 8, 857–861. [Google Scholar]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan–polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, N.; Ravichandran, P.; Reddy, P.N.; Ramamurty, N.; Rao, K.P. Collagen–chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials 2001, 22, 1943–1951. [Google Scholar] [CrossRef]
- Mi, F.L.; Tan, Y.C.; Liang, H.F.; Sung, H.W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Wang, L.; Khor, E.; Wee, A.; Lim, L.Y. Chitosan-alginate PEC membrane as a wound dressing: Assessment of incisional wound healing. J. Biomed. Mater. Res. 2010, 63, 610–618. [Google Scholar] [CrossRef]
- Cai, X.; Tong, H.; Shen, X.; Chen, W.; Yan, J.; Hu, J. Preparation and characterization of homogeneous chitosan-polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009, 5, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Fan, Y.; Liu, X.; Li, X.; Li, P.; Wang, J.; Sha, Z.; Feng, Q. Repair of bone defect in femoral condyle using microencapsulated chitosan, nanohydroxyapatite/collagen and poly(l-lactide)-based microsphere-scaffold delivery system. Artif. Organs 2011, 35, E119–E128. [Google Scholar] [CrossRef]
- Li, X.; Ye, F.; Li, G.L.; Cui, J.; Liu, Y.X.; Yang, L.Q.; Cong, L.; Li, B. 3D Printed Hydroxyapatite/Silk Fibroin/Polycaprolactone Artificial Bone Scaffold and Bone Tissue Engineering Materials Constructed with Double-Transfected Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor Mesenchymal Stem Cells to Repair Rabbit Radial Bone Defects. Nanosci. Nanotechnol. Lett. 2020, 12, 368–375. [Google Scholar] [CrossRef]
- Kim, H.; Hwangbo, H.; Koo, Y.; Kim, G. Fabrication of Mechanically Reinforced Gelatin/Hydroxyapatite Bio-Composite Scaffolds by Core/Shell Nozzle Printing for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 3401. [Google Scholar] [CrossRef]
- Antonetti, C.; Ciorba, S.; Licursi, D.; Coccia, V.; Cotana, F.; Galletti, A.M.R. Production of levulinic acid and n-butyl levulinate from the waste biomasses grape pomace and Cynara cardunculus L. In Proceedings of the 1st International Electronic Conference on Catalysts Science, Online, 10–30 November 2020; p. 30. [Google Scholar]
- Bouler, J.-M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Li, X.Y.; Wang, X.H.; Li, D.N.; Chung, S.; Chen, C.; Lee, I.S. Osteogenesis of 3D printed macro-pore size biphasic calcium phosphate scaffold in rabbit calvaria. J. Biomater. Appl. 2019, 33, 1168–1177. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Wu, Q.; Wu, B. Fabrication of HA/β-TCP scaffolds based on micro-syringe extrusion system. Rapid Prototyp. J. 2013, 19, 319–326. [Google Scholar] [CrossRef]
- Franchin, G.; Wahl, L.; Colombo, P. Direct ink writing of ceramic matrix composite structures. J. Am. Ceram. Soc. 2017, 100, 4397–4401. [Google Scholar] [CrossRef]
- Siemens, A.G. Manufacturing of SiO2-Coated β-TCP Structures by 3D Printing using a Preceramic Polymer as Printing Binder and Silica Source. J. Ceram. Sci. Technol. 2017, 9, 37–42. [Google Scholar]
- Sachs, E.; Cima, M.; Williams, P.; Brancazio, D.; Cornie, J. Three dimensional printing: Rapid tooling and prototypes directly from a CAD model. J. Eng. Ind. 1992, 114, 481–488. [Google Scholar] [CrossRef]
- Zocca, A.; Elsayed, H.; Bernardo, E.; Gomes, C.; Lopez-Heredia, M.; Knabe, C.; Colombo, P.; Günster, J.J.B. 3D-printed silicate porous bioceramics using a non-sacrificial preceramic polymer binder. Biofabrication 2015, 7, 025008. [Google Scholar] [CrossRef]
- Musskaya, O.N.; Krut’ko, V.K.; Kulak, A.I.; Filatov, S.A.; Batyrev, E.V.; Safronova, T.V. Calcium Phosphate Compositions with Polyvinyl Alcohol for 3D Printing. Inorg. Mater. Appl. Res. 2020, 11, 192–197. [Google Scholar] [CrossRef]
- Tang, H.-H.; Chiu, M.-L.; Yen, H.-C. Slurry-based selective laser sintering of polymer-coated ceramic powders to fabricate high strength alumina parts. J. Eur. Ceram. Soc. 2011, 31, 1383–1388. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, D.S.; Park, M.S.; Yun, J.S.J.N. Sintering Process Optimization for 3YSZ Ceramic 3D-Printed Objects Manufactured by Stereolithography. Nanomaterials 2021, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.Q.; Sun, H.; Wu, L.N.; Ma, L.; Xing, F.; Kong, Q.Q.; Fan, Y.J.; Zhou, C.C.; Zhang, X.D. 3D printing of calcium phosphate bioceramic with tailored biodegradation rate for skull bone tissue reconstruction. Bio-Des. Manuf. 2019, 2, 161–171. [Google Scholar] [CrossRef]
- Touri, M.; Moztarzadeh, F.; Osman, N.A.A.; Dehghan, M.M.; Mozafari, M. 3D–printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Mater. Sci. Eng. C 2018, 84, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gomez, L.; Elizondo, M.E.; Kontoyiannis, P.D.; Koons, G.L.; Dacunha-Marinho, B.; Zhang, X.; Ajayan, P.; Jansen, J.A.; Melchiorri, A.J.; Mikos, A.G. Three-Dimensional Extrusion Printing of Porous Scaffolds Using Storable Ceramic Inks. Tissue Eng. Part C Methods 2020, 26, 292–305. [Google Scholar] [CrossRef]
- Hench, L.L. Opening paper 2015-some comments on bioglass: Four eras of discovery and development. Biomed. Glasses 2015, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and Bioactive Glasses and Their Impact on Healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Pleural, N.; New, N. Pleural effusion. Postgrad. Med. J. 2005, 81, 702–710. [Google Scholar]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.; Wu, Q.; Yan, W. Early peri-implant osteogenesis with functionally graded nanophase hydroxyapatite/bioglass coating on Ti alloys. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2007; Volume 19. [Google Scholar]
- Pengbo, W.; Hongyan, S.; Ming, Y.; Wantao, C. The osteointergration and osteoinduction of titanium implant with nHA/BG gradient coating in rabbits. J. Pract. Stomatol. 2016, 32, 749–751. [Google Scholar]
- Bellucci, D.; Salvatori, R.; Cannio, M.; Luginina, M.; Orrù, R.; Montinaro, S.; Anesi, A.; Chiarini, L.; Cao, G.; Cannillo, V. Bioglass and bioceramic composites processed by Spark Plasma Sintering (SPS): Biological evaluation Versus SBF test. Biomed. Glasses 2018, 4, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Chanchareonsook, N.; Tideman, H.; Lee, S.; Hollister, S.J.; Flanagan, C.; Jansen, J.A. Mandibular reconstruction with a bioactive-coated cementless Ti6Al4V modular endoprosthesis in Macaca fascicularis. Int. J. Oral Maxillofac. Surg. 2014, 43, 758–768. [Google Scholar] [CrossRef]
- Xu, C.; Bai, Y.; Yang, S.; Yang, H.; Stout, D.A.; Tran, P.A.; Yang, L. A versatile three-dimensional foam fabrication strategy for soft and hard tissue engineering. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.G.; Chen, C.Z.; Jin, Q.P.; Li, H.C.; Pan, Y.K. HA/Bioglass composite films deposited by pulsed laser with different substrate temperature. Appl. Phys. A 2013, 114, 897–902. [Google Scholar] [CrossRef]
- Qi, X.; Pei, P.; Zhu, M.; Du, X.Y.; Xin, C.; Zhao, S.C.; Li, X.L.; Zhu, Y.F. Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedmajidi, S.; Seyedmajidi, S.; Alaghehmand, H.; Hajian-Tilaki, K.; Haghanifar, S.; Zabihi, E.; Rajabnia, R.; Seyedmajidi, M. Synthesis and Characterization of Hydroxyapatite/Bioactive Glass Nanocomposite Foam and Fluorapatite/Bioactive Glass Nanocomposite Foam by Gel Casting Method as Cell Scaffold for Bone Tissue. Eurasian J. Anal. Chem. 2018, 13. [Google Scholar] [CrossRef]
- Seyedmajidi, M.; Haghanifar, S.; Hajian-Tilaki, K.; Seyedmajidi, S. Histopathological, histomorphometrical, and radiological evaluations of hydroxyapatite/bioactive glass and fluorapatite/bioactive glass nanocomposite foams as cell scaffolds in rat tibia: An in vivo study. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Salih, V.; Knowles, J.C. Hydroxyapatite and titania sol-gel composite coatings on titanium for hard tissue implants; mechanical and in vitro biological performance. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 1–8. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Shipley, H.; McDonnell, D.; Culleton, M.; Coull, R.; Lupoi, R.; O’Donnell, G.; Trimble, D. Optimisation of process parameters to address fundamental challenges during selective laser melting of Ti-6Al-4V: A review. Int. J. Mach. Tools Manuf. 2018, 128, 1–20. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R. Metals for Biomedical Applications; InTech: London, UK, 2011. [Google Scholar]
- Xia, L.; Xie, Y.; Fang, B.; Wang, X.; Lin, K. In situ modulation of crystallinity and nano-structures to enhance the stability and osseointegration of hydroxyapatite coatings on Ti-6Al-4V implants. Chem. Eng. J. 2018, 347, 711–720. [Google Scholar] [CrossRef]
- Habibovic, P.; Barrere, F.; Van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Robertson, S.F.; Bandyopadhyay, A.; Bose, S. Titania nanotube interface to increase adhesion strength of hydroxyapatite sol-gel coatings on Ti-6Al-4V for orthopedic applications. Surf. Coat. Technol. 2019, 372, 140–147. [Google Scholar] [CrossRef]
- Qi, J.; Yang, Y.; Zhou, M.; Chen, Z.; Chen, K. Effect of transition layer on the performance of hydroxyapatite/titanium nitride coating developed on Ti-6Al-4V alloy by magnetron sputtering. Ceram. Int. 2019, 45, 4863–4869. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Kang, C.; Zhang, J.; Xu, Z.; Jiang, C.; Luo, P.; Fu, Z.; Ding, M.; Lv, Y. Synthesis of magnetic Fe-doped hydroxyapatite nanocages with highly efficient and selective adsorption for Cd2+. Mater. Lett. 2019, 253, 144–147. [Google Scholar] [CrossRef]
- Unabia, R.B.; Bonebeau, S.; Candidato, R.T., Jr.; Jouin, J.; Noguera, O.; Pawłowski, L. Investigation on the structural and microstructural properties of copper-doped hydroxyapatite coatings deposited using solution precursor plasma spraying. J. Eur. Ceram. Soc. 2019, 39, 4255–4263. [Google Scholar] [CrossRef]
- Dittler, M.L.; Unalan, I.; Grünewald, A.; Beltrán, A.M.; Grillo, C.A.; Destch, R.; Gonzalez, M.C.; Boccaccini, A.R. Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc-loaded hydroxyapatite nanoparticles for tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 182, 110346. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, characterization, and antimicrobial activity of magnesium-doped hydroxyapatite suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Veerla, S.C.; Kim, D.R.; Kim, J.; Sohn, H.; Yang, S.Y. Controlled nanoparticle synthesis of Ag/Fe co-doped hydroxyapatite system for cancer cell treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 311–323. [Google Scholar] [CrossRef]
- Kumar, V.B.; Khajuria, D.K.; Karasik, D.; Gedanken, A. Silver and gold doped hydroxyapatite nanocomposites for enhanced bone regeneration. Biomed. Mater. 2019, 14, 055002. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Hou, M.J.; Wang, T.W.; Sun, M.; Hou, R.X. Nanostructured selenium-doped biphasic calcium phosphate with in situ incorporation of silver for antibacterial applications. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Park, S.; Miller, A.L.; Terzic, A.; Lu, L. Strontium-substituted hydroxyapatite stimulates osteogenesis on poly(propylene fumarate) nanocomposite scaffolds. J. Biomed. Mater. Res. Part A 2019, 107, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.B.; Li, Z.Y.; Lam, W.M.; Wong, J.C.; Darvell, B.W.; Luk, K.D.K.; Lu, W.W. Solubility of strontium-substituted apatite by solid titration. Acta Biomater. 2009, 5, 1678–1685. [Google Scholar] [CrossRef] [Green Version]
- Jianqiang, X.; Yaoqi, Y.; Rong, W.; Yuhui, S.; Weibin, Z. Hydrothermal Preparation and Characterization of Ultralong Strontium-Substituted Hydroxyapatite Whiskers Using Acetamide as Homogeneous Precipitation Reagent. Theentificworldjournal 2014, 2014, 863137. [Google Scholar]
- Yan, J.; Sun, J.F.; Chu, P.K.; Han, Y.; Zhang, Y.M. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. Part A 2013, 101A, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomedicine 2017, 13, 231–239. [Google Scholar] [CrossRef]
- Gonzalez, P.; Schwarzer, E.; Scheithauer, U.; Kooijmans, N.; Moritz, T. Additive Manufacturing of Functionally Graded Ceramic Materials by Stereolithography. JoVE 2019, e57943. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Vleugels, J.; Kruth, J.P. Additive manufacturing of ceramics: A review. J. Ceram. Sci. Technol. 2014, 5, 245–260. [Google Scholar]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- He, L.; Fei, F.; Wang, W.; Song, X. Support-Free Ceramic Stereolithography of Complex Overhanging Structures Based on an Elasto-viscoplastic Suspension Feedstock. ACS Appl. Mater. Interfaces 2019, 11, 18849–18857. [Google Scholar] [CrossRef]
- Hu, K.; Wei, Y.; Lu, Z.; Wan, L.; Li, P. Design of a Shaping System for Stereolithography with High Solid Loading Ceramic Suspensions. 3D Print. Addit. Manuf. 2018, 5, 311–318. [Google Scholar] [CrossRef]
- Safonov, A.; Maltsev, E.; Chugunov, S.; Tikhonov, A.; Konev, S.; Evlashin, S.; Popov, D.; Pasko, A.; Akhatov, I.J.A.S. Design and fabrication of complex-shaped ceramic bone implants via 3d printing based on laser stereolithography. Appl. Sci. 2020, 10, 7138. [Google Scholar] [CrossRef]
- Bae, C.-J.; Ramachandran, A.; Chung, K.; Park, S. Ceramic Stereolithography: Additive Manufacturing for 3D Complex Ceramic Structures. J. Korean Ceram. Soc. 2017, 54, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Chaput, C.; Chartier, T. Fabrication of ceramics by stereolithography. Rapid Technol. E J. 2007, 4. Available online: https://www.rtejournal.de/ausgabe4/1163 (accessed on 23 March 2021).
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Kuboki, Y.; Takita, H.; Kobayashi, D.; Tsuruga, E.; Inoue, M.; Murata, M.; Nagai, N.; Dohi, Y.; Ohgushi, H. BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: Topology of osteogenesis. J. Biomed. Mater. Res. 2015, 39, 190–199. [Google Scholar] [CrossRef]
- Svehla, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. Part A 2000, 51, 15–22. [Google Scholar] [CrossRef]
- Lee, C.M.; Yang, S.W.; Jung, S.C.; Kim, B.H. Oxygen Plasma Treatment on 3D-Printed Chitosan/Gelatin/Hydroxyapatite Scaffolds for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2017, 17, 2747–2750. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D printed scaffolds with gradient porosity based on a cellulose nanocrystal hydrogel. Nanoscale 2018, 10, 4421–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Kang, J.F.; Sun, C.N.; Li, D.C.; Cao, Y.; Jin, Z.M. Mapping porous microstructures to yield desired mechanical properties for application in 3D printed bone scaffolds and orthopaedic implants. Mater. Des. 2017, 133, 62–68. [Google Scholar] [CrossRef]
- Zhang, S.; Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. A review on the use of computational methods to characterize, design, and optimize tissue engineering scaffolds, with a potential in 3D printing fabrication. J. Biomed. Mater. Res. B 2019, 107, 1329–1351. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, R.; Zandsalimi, K.; Rasoulianboroujeni, M.; Tayebi, L. Challenges in Three-Dimensional Printing of Bone Substitutes. Tissue Eng. Part B Rev. 2019, 25, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.R.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Qin, Z.; Huang, W.; Cao, S.; Kaplan, D.L.; Buehler, M.J. Design and function of biomimetic multilayer water purification membranes. Sci. Adv. 2017, 3, e1601939. [Google Scholar] [CrossRef] [Green Version]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Lee, J.B.; Maeng, W.Y.; Koh, Y.H.; Kim, H.E. Porous Calcium Phosphate Ceramic Scaffolds with Tailored Pore Orientations and Mechanical Properties Using Lithography-Based Ceramic 3D Printing Technique. Materials 2018, 11, 1711. [Google Scholar] [CrossRef] [Green Version]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Chen, S.S.; Shi, Y.F.; Zhang, X.; Ma, J. 3D printed hydroxyapatite composite scaffolds with enhanced mechanical properties. Ceram. Int. 2019, 45, 10991–10996. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cell Nanomed. B 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Saroia, J.; Wang, Y.E.; Wei, Q.H.; Zhang, K.; Lu, T.L.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Hung, K.-C.; Chen, C.-W. Biodegradable polymer scaffolds. J. Mater. Chem. B 2016, 4, 7493–7505. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Jaszcz, K.; Jurczyk, S.; Chladek, G. The novel semi-biodegradable interpenetrating polymer networks based on urethane-dimethacrylate and epoxy-polyester components as alternative biomaterials. Acta Bioeng. Biomech. 2015, 17, 13–22. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Shuai, C.; Wu, P.; Zhong, Y.; Feng, P.; Gao, C.; Huang, W.; Zhou, Z.; Chen, L.; Shuai, C. Polyetheretherketone/poly (glycolic acid) blend scaffolds with biodegradable properties. J. Biomater. Sci. Polym. Ed. 2016, 27, 1434–1446. [Google Scholar] [CrossRef]
- Feng, P.; Wu, P.; Gao, C.D.; Yang, Y.W.; Guo, W.; Yang, W.J.; Shuai, C.J. A Multimaterial Scaffold with Tunable Properties: Toward Bone Tissue Repair. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Le, T.Y.L.; Daly, S.; Wang, Y.W.; Maitz, P.K.; Schindeler, A.; Dehghani, F. Formation of porous biodegradable scaffolds based on poly(propylene carbonate) using gas foaming technology. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Hu, N.; Liu, J.; Zhai, X.Y.; Wu, M.M.; Hu, C.S.; Li, L.; Lai, Y.X.; Pan, H.B.; Lu, W.W.; et al. Three-Dimensional Printing of Biodegradable Piperazine-Based Polyurethane-Urea Scaffolds with Enhanced Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 9415–9424. [Google Scholar] [CrossRef]
- Odelius, K.; Hoglund, A.; Kumar, S.; Hakkarainen, M.; Ghosh, A.K.; Bhatnagar, N.; Albertsson, A.C. Porosity and Pore Size Regulate the Degradation Product Profile of Polylactide. Biomacromolecules 2011, 12, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Dilisio, M.F.; Dietz, N.E.; Agrawal, D.K. Recent strategies in cartilage repair: A systemic review of the scaffold development and tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 2343–2354. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.Y.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, S.J.; Lee, H.; Park, S.A.; Lee, J.Y. Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr. Polym. 2018, 196, 217–224. [Google Scholar] [CrossRef]
- Shao, H.; He, J.; Lin, T.; Zhang, Z.; Zhang, Y.; Liu, S. 3D gel-printing of hydroxyapatite scaffold for bone tissue engineering. Ceram. Int. 2019, 45, 1163–1170. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Golubevas, R.; Stankeviciute, Z.; Zarkov, A.; Golubevas, R.; Hansson, L.; Raudonis, R.; Kareiva, A.; Garskaite, E. Acrylate–gelatin–carbonated hydroxyapatite (cHAP) composites for dental bone-tissue applications. Mater. Adv. 2020, 1, 1675–1684. [Google Scholar] [CrossRef]
- Szczes, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interfaces 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Yuan, H.F.; Zheng, X.Y.; Liu, W.; Zhang, H.; Shao, J.J.; Yao, J.X.; Mao, C.Y.; Hui, J.F.; Fan, D.D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloid Surf. B 2020, 192. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pal, U. 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J. Drug Deliv. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Jose, R.; Archana, P.S.; Nair, A.S.; Balamurugan, R.; Venugopal, J.; Teo, W.E. Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine. J. Mater. Sci. 2010, 45, 6283–6312. [Google Scholar] [CrossRef]
- Pei, X.; Ma, L.; Zhang, B.Q.; Sun, J.X.; Sun, Y.; Fan, Y.J.; Gou, Z.R.; Zhou, C.C.; Zhang, X.D. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9. [Google Scholar] [CrossRef]
- Song, X.L.; Tetik, H.; Jirakittsonthon, T.; Parandoush, P.; Yang, G.; Lee, D.; Ryu, S.; Lei, S.T.; Weiss, M.L.; Lin, D. Biomimetic 3D Printing of Hierarchical and Interconnected Porous Hydroxyapatite Structures with High Mechanical Strength for Bone Cell Culture. Adv. Eng. Mater. 2019, 21. [Google Scholar] [CrossRef] [Green Version]
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Zhang, F.J.; Tsang, W.P.; Wan, C.; Wu, C. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 2017, 120, 11–21. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Jablonski, A.; Jatteau, S.; Sliwka, M.; Ziabka, M.; Menaszek, E. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing—For nasal cartilages and subchondral bone reconstruction. Mater. Des. 2018, 155, 297–306. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Shen, B.-Y.; Wang, Y.-H.; Lin, B.; Lee, H.-M.; Hsieh, M.-F. Healing of osteochondral defects implanted with biomimetic scaffolds of poly (ε-caprolactone)/hydroxyapatite and glycidyl-methacrylate-modified hyaluronic acid in a minipig. Int. J. Mol. Sci. 2018, 19, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—encompassing the facets of dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucar, Y.; Meric, I.A.; Ekren, O. Layered Manufacturing of Dental Ceramics: Fracture Mechanics, Microstructure, and Elemental Composition of Lithography-Sintered Ceramic. J. Prosthodont. 2019, 28, E310–E318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Chong, B.S. 3D imaging, 3D printing and 3D virtual planning in endodontics. Clin. Oral Investig. 2018, 22, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.W.; Fang, Y.F.; Liao, Y.X.; Chen, G.; Gao, C.X.; Zhu, P.Z. 3D Printing and Digital Processing Techniques in Dentistry: A Review of Literature. Adv. Eng. Mater. 2019, 21. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Hydroxyapatite-alginate nanocomposite as drug delivery matrix for sustained release of ciprofloxacin. J. Biomed. Nanotechnol. 2011, 7, 759–767. [Google Scholar] [CrossRef]
- Bi, Y.-G.; Lin, Z.-T.; Deng, S.-T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Wang, T.W.; Wang, C.; Wang, Z.; Yang, Y.A.; Li, P.; Cai, R.H.; Sun, M.; Yuan, H.Y.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloid Surf. A 2020, 585. [Google Scholar] [CrossRef]
- Nie, L.; Deng, Y.; Zhang, Y.; Zhou, Q.; Shi, Q.; Zhong, S.; Sun, Y.; Yang, Z.; Sun, M.; Politis, C.; et al. Silver-doped biphasic calcium phosphate/alginate microclusters with antibacterial property and controlled doxorubicin delivery. J. Appl. Polym. Sci. 2021, 138, 50433. [Google Scholar] [CrossRef]
- Rodzeń, K.; Sharma, P.K.; McIlhagger, A.; Mokhtari, M.; Dave, F.; Tormey, D.; Sherlock, R.; Meenan, B.J.; Boyd, A.J.P. The Direct 3D Printing of Functional PEEK/Hydroxyapatite Composites via a Fused Filament Fabrication Approach. Polymers 2021, 13, 545. [Google Scholar] [CrossRef]
- Robles-Aguila, M.J.; Reyes-Avendano, J.A.; Mendoza, M.E. Structural analysis of metal-doped (Mn, Fe, Co, Ni, Cu, Zn) calcium hydroxyapatite synthetized by a sol-gel microwave-assisted method. Ceram. Int. 2017, 43, 12705–12709. [Google Scholar] [CrossRef]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Yazici, M.; Gulec, A.E.; Gurbuz, M.; Gencer, Y.; Tarakci, M. Biodegradability and antibacterial properties of MAO coatings formed on Mg-Sr-Ca alloys in an electrolyte containing Ag doped hydroxyapatite. Thin Solid Films 2017, 644, 92–98. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]


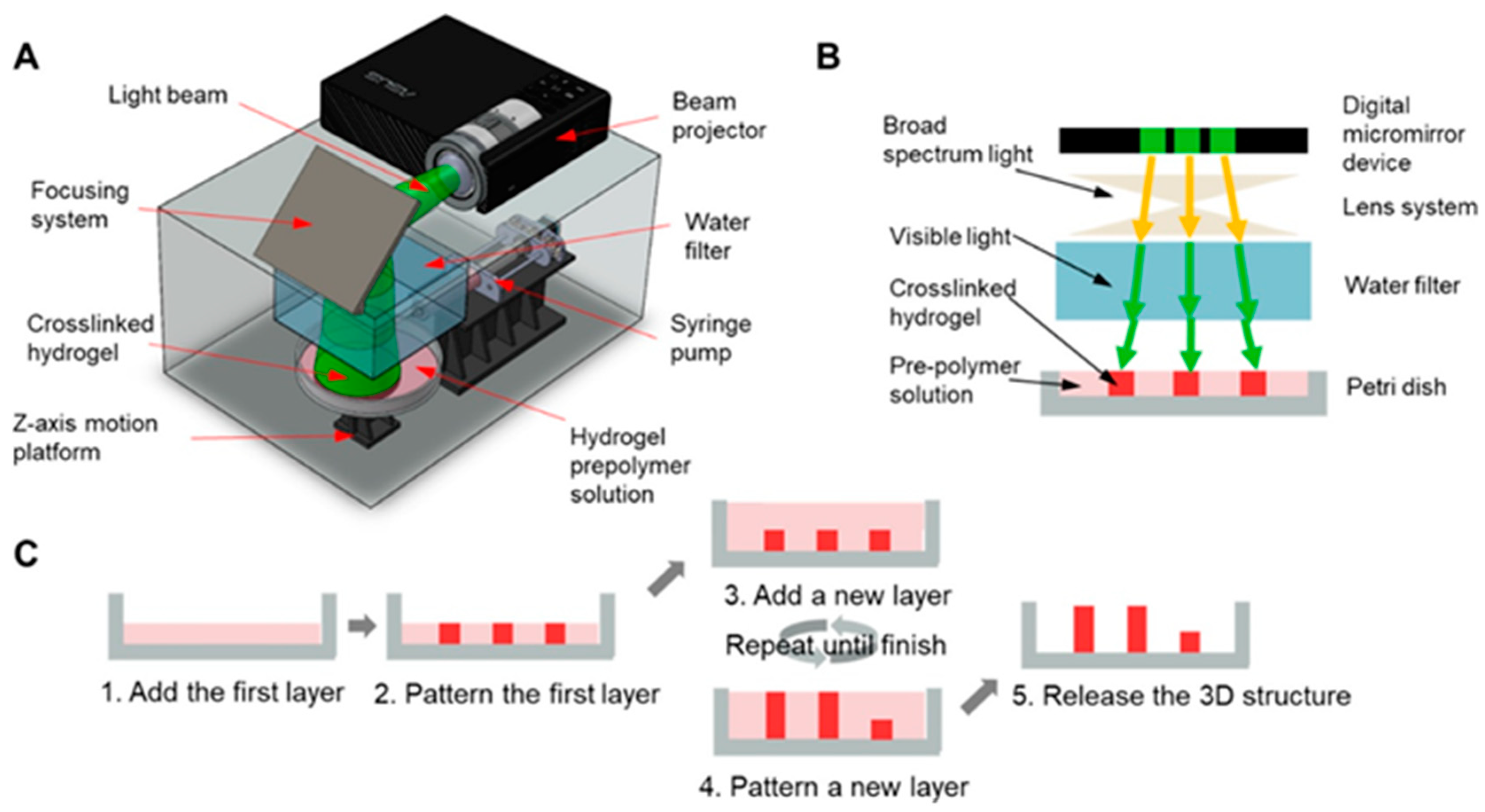






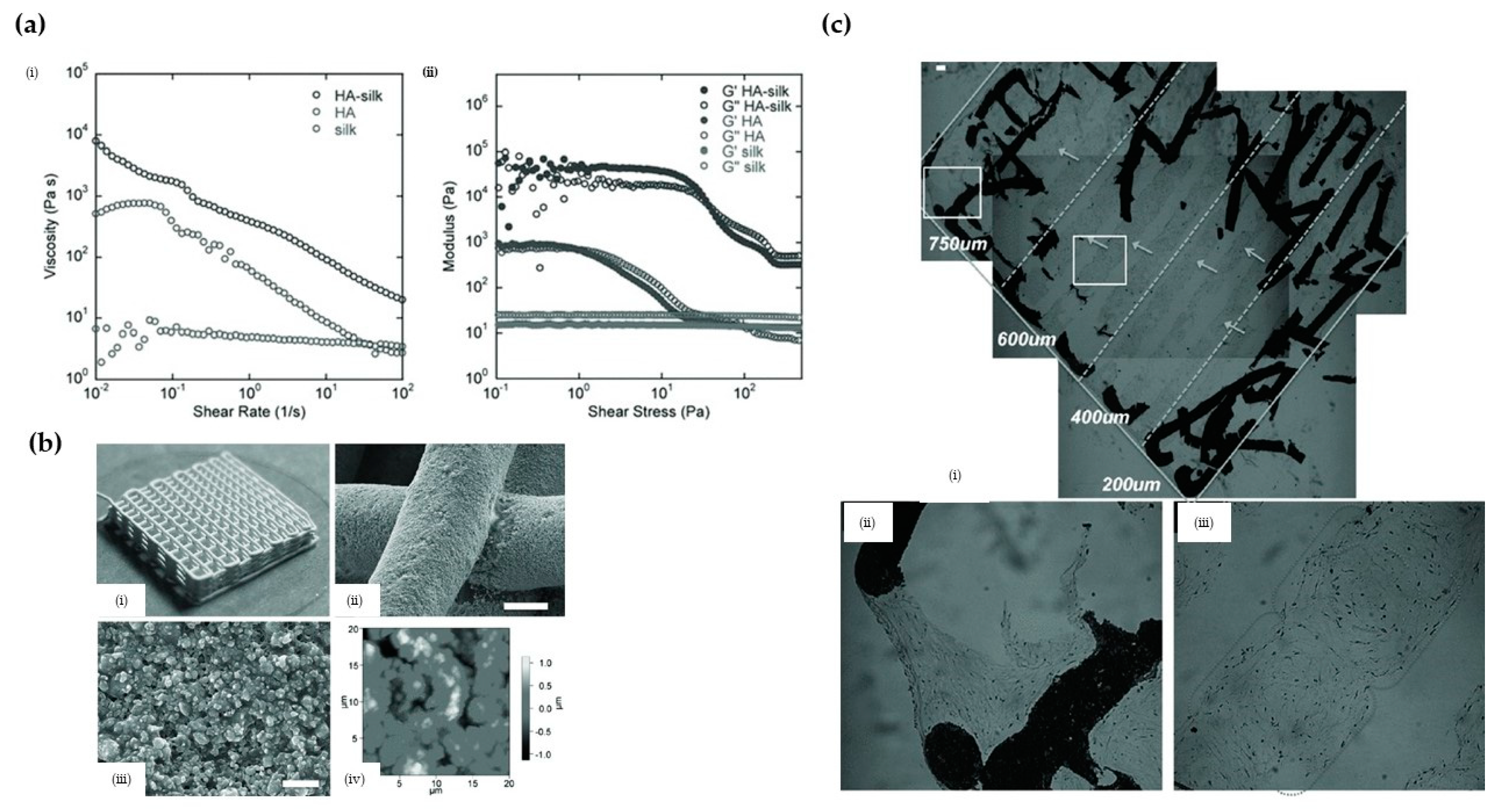
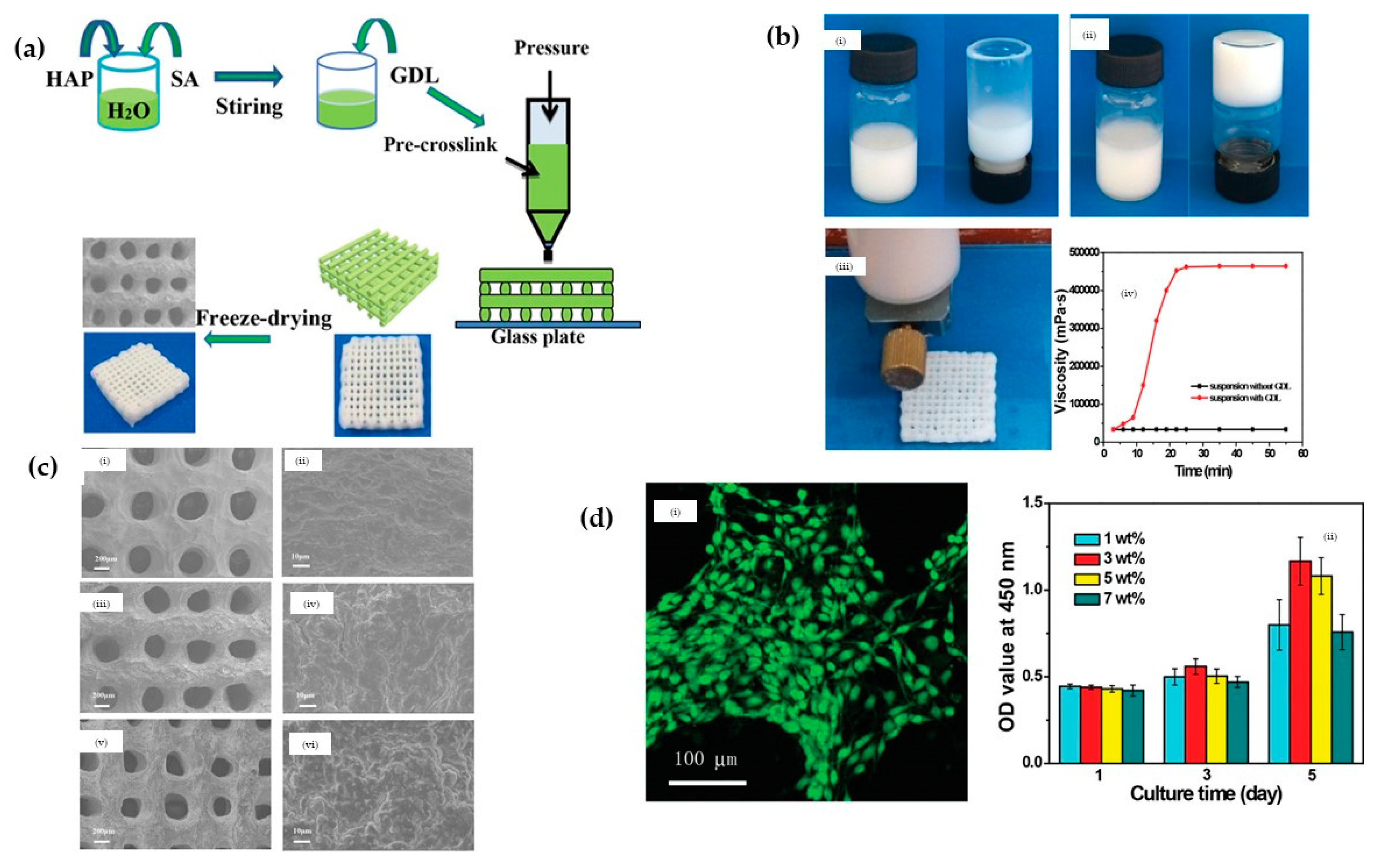







| HA-Composites Fabrication Methods | Brief Description | Sources |
|---|---|---|
| Biomimetic mineralization | In this approach, the composite material is decorated in a solution of bioactive substances or simulated electrolyte body fluid solution (SBF). In such a solution, the increased concentration of calcium ions induces the nucleation of hydroxyapatite crystals on the selected composite material. | [14] |
| Electrochemical deposition | In this method, the hydroxyapatite composite is deposited onto the surface of a conductor using an electrolysis processes such that the solution contains the calcium ions and phosphate ions for (HA) and the relevant composite candidate (i.e., dissolved chitosan). | [15] |
| Lyphilisation | The composite materials (i.e., graphene and HA) are dispersed in an organic solvent after which the mixture is frozen. Sublimation of the frozen solution is subsequently achieved by reducing the pressure. | [16] |
| Electrospinning | This approach is employed when there is a need to develop fibrous scaffolds that can mimic the extracellular matrix of native tissue. Such fibers are prepared by electrospinning a precursor mixture containing ions (i.e., calcium ions in Ca(NO3)2·4H2O and phosphate ions in (C2H5O)3PO) and polymer additive, followed by thermal treatment. | [17,18] |
| Self-assembling | This is a self-aggregation process that involves the spontaneous aggregation to form the target composites. During the self-assembling process, the organic phase (i.e., collagen) is made to interact with the mineral phase (i.e., hydroxyapatite) via the use of suitable precursors (i.e., Ca(OH)2 for Ca2+ and H3PO4 for PO43−). | [12] |
| Chemical vapor deposition | In this method, the film is deposited on the surface of the substrate through chemical reaction from gas-phase or vapor-phase precursor (i.e., Fe2O3/HA + H2 as carrier gas). | [13] |
| Hydrothermal | In this approach, a mixture composed of suitable precursors containing calcium ions (i.e., calcium nitrate tetrahydrate) and phosphate ions (diammonium hydrogen phosphate solutions) is used in dispersing the composite candidate material (i.e., graphene) at a high temperature condition (i.e., 180 °C).The hydrothermal method is also employed in the fabrication of three-dimensional reduced graphene oxide/hydroxyapatite (HA)/gelatin scaffolds. | [19,20]. |
| Solvothermal Synthesis | HA nanoparticles are crystalized via a two-state solvothermal method at the high temperature of 180 °C. Calcium nitrate tetrahydrate and diammonium hydrogen phosphate are used as calcium and phosphate precursors, respectively. | [21] |
| Materials | Cell Type | Outcome | Techniques | Sources |
|---|---|---|---|---|
| HA/β-TCP | Osteoblasts from femora and tibiae of male Lewis rats | Combination of this scaffold with primary osteoblasts and BMP-2 yielded significant amounts of newly formed bone in heterotopic locations and physiological gene expression patterns. | Inkjet-based 3D printing | [38] |
| HA | The preosteoblastic cell line MC3T3-E1, derived from mouse calvariae | The osteoblast-like cells were found to be present on the external and internal surface of the scaffold; they were embedded in collagenous extracellular matrix. | SLA-based 3D printing | [48] |
| Silk/HA | human bone marrow derived mesenchymal stem cells (hMSCs) and human mammary microvascular endothelial cells (hMMECs) | By combining HA, a good matrix for hMSCs osteogenesis, with silk to promote endothelial cell growth, migration was observed. The created scaffolds were capable of supporting both stem cell and endothelial cell functions to allow for new tissue formation and bone remodeling with vascular inputs within a single construct environment. | Extrusion-based 3D printing | [58] |
| HA | Human bone marrow stromal cells | Cells were tightly anchored to the surfaces of all scaffolds and had begun to spread | Laser-assisted 3D printing | [66] |
| HA/TCP | Rabbit Bone Marrow Stromal Cells (BMSCs) | the phosphoric acid scaffolds with a HA/β-TCP weight ratios of 60:40 may be the best candidate for bone TE applications. | Inkjet-based 3D printing | [68] |
| HA | Human osteoblast cells (HOBS) | The HOBS are attached to the surface of HA scaffolds and have high cellular activity. | SLA-based 3D printing | [47] |
| HA | The preosteoblastic cell line MC3T3-E1 | The osteoblast-like cells were found to cover the whole external and internal surface of the scaffold, and they were embedded in collagenous extracellular matrix. | SLA-based 3D printing | [48] |
| HA | L929 cells and rabbit osteoblast cells | The rabbits had no adverse physiological reactions such as infection, and the wafer formed a strong bone connection with the defect, indicating that the final HAP samples have good biosafety in vivo. | SLA-based 3D printing | [49] |
| HA/TCP | Osteoblast-like MG-63 cells | The histological analysis did not indicate evidence of inflammation but highlighted close contacts between newly formed bone and the experimental biomaterials, revealing an excellent scaffold osseointegration. | SLA-based 3D printing | [50] |
| HA | The preosteoblastic cell line MC3T3-E1, derived from mouse calvariae | The osteoblast-like cells were found to be present on the external and internal surface of the scaffold; they were embedded in a collagenous extracellular matrix. | SLA-based 3D printing | [48] |
| Silk/HA | Human bone marrow derived mesenchymal stem cells (hMSCs) and human mammary microvascular endothelial cells (hMMECs) | By combining HA, a good matrix for hMSCs osteogenesis, with silk to promote endothelial cell growth, migration was observed. The created scaffolds could support both stem cell and endothelial cell functions to allow for new tissue formation and bone remodeling with vascular inputs within a single construct environment. | Extrusion-based 3D printing | [58] |
| CHA | Rabbit Bone Marrow Stromal Cells (BMSCs) | The printed CHA scaffolds had the advantages of promoting BMSCs proliferation and differentiation and promoting defect repair compared to the nonprinted CHA scaffolds | Extrusion-based 3D printing | [69] |
| Coll/HA | Vero cells | It was demonstrated that Coll/HA can be 3D printed, that the scaffold is conducive to cell proliferation, and that it is suitable for biomedical applications. | Extrusion-based 3D printing | [70] |
| HA/SF | Human bone marrow-derived mesenchymal stem cells (hBMSCs) | Cell attachment and penetration into scaffolds were supported by all the groups. Increased content of SF/HA led to better cell proliferation and enhanced ALP activity. | Extrusion-based 3D printing | [71] |
| HA/SA | Mouse bone mesenchymal stem cells (mBMSCs) | The sustainable drug release function of the porous scaffolds aided mouse bone mesenchymal stem cells (mBMSCs) being cultured on the porous scaffolds. | Extrusion-based 3D printing | [72] |
| HA/CH | MC3T3-E1 cells | The 3D 10% HAp/CH scaffolds etched with N2 plasma significantly improved cell proliferation. The 3D 20% HAp/CH scaffolds etched with O2 plasma showed the highest osteoblastic differentiation. | Extrusion-based 3D printing | [73] |
| PLA/HA | Human MG-63 osteoblast-like cell | PLA-HA scaffolds have proved to be an excellent composite material with enhanced surface activity due to the coating of HA nanoparticles. | Extrusion-based 3D printing | [74] |
| PCL/PLGA/HA | Rat bone marrow stem cells (rBMSCs) | 3D printable ink made of PCL/PLGA/HAp can be a highly useful material for 3D printing of bone tissue constructs. | Extrusion-based 3D printing | [75] |
| PMMA/CNT/HA | L929 cells | Biocompatibility analysis indicates that introducing both HAp and CNT particles improves cell viability and growth. | Extrusion-based 3D printing | [76] |
| CH/PVA/HA | Mesenchymal stem cells (ATCC) | The scaffolds have high elastic modulus and good biocompatibility. | Extrusion-based 3D printing | [77] |
| PCL/GEL/BC/HA | Human osteoblast cells (ATCC) | The PCL/GEL/BC/0.25%HA scaffold demonstrated good cell viability and cell adhesion. | Extrusion-based 3D printing | [78] |
| PLA/HA/Silk | MC3T3 osteoblast precursor cells | 3D printed PLA, PLA/HA, and PLA/HA/Silk composite bone clips were successfully developed. | Extrusion-based 3D printing | [79] |
| PCL/HA/TCP | Saos-2 cells | the fabricated hybrid scaffold had high porosity and excellent microstructural interconnectivity, and superior cell proliferation and alkaline phosphatase assay results for the hybrid scaffold. | Extrusion-based 3D printing | [80] |
| Sr/HA | MC3T3-E1 cells | Sr5-HA promoted cell proliferation, osteogenic differentiation, and cellular mineralization more efficiently compared with the other scaffolds. | Extrusion-based 3D printing | [81] |
| GEL/HA | Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) | The scaffold supports the adhesion, growth, and proliferation of hUCB-MSCs and induces their chondrogenic differentiation in vitro. | Extrusion-based 3D printing | [82] |
| Printing Technique | Parameters to Optimize Technique | Challenges |
|---|---|---|
| Inkjet-based 3D printing | Nozzle/extrusion temperature, printing speed, and layer thickness [83]. Specifically for materials such as ceramics, the solid loading and formulations of the ink also need to be considered [84] | The technique requires high temperatures which may preclude the incorporation of temperature sensitive bioactive molecules during the 3D printing process of HA based composites [85]. Additionally, when employed in printing of materials such as ceramics, the inks typically have low viscosity (i.e., low solid loading), to enable the use of the biomaterial. However, low viscosities lead to longer drying time and shrinkage. These challenges may also adversely affect the final accuracy of the printed scaffold [86]. Further concerns associated with the aggregation of solid particles due to convective macroscopic flow may lead to the printed structure having defects [86]. |
| SLA 3D printing | Typically, optimization of this techniques involves the consideration of the layer thickness, post curing time and orientation [87]. Due to issues associated with shrinkage, it is also crucial that formulations containing materials such as ceramics are optimized for proper viscosity while also avoiding issues of the solid segregation [86]. | The technology can only use photopolymers with the utilization of a UV light further restricting the incorporation of living cells in the biomaterials [88]. Another challenge is the effect of light scattering due to the presence of ceramic particles in the suspensions since the scattering limits light penetrating. Furthermore, such scattering increases the curing width, leading to unfavorable effects on dimensional accuracy of the printing technique [89]. Furthermore, materials such as ceramics that absorb or refract photopolymerization wavelength are very difficult to process [86,90]. |
| Digital Light Processing (DLP) 3 D printing | Factors to be considered to optimize the technique include the viscosity of the slurry, solid loading, and the specific operating mechanisms (i.e., top-down, bottom-up, method for recoating, etc.) [91]. | According to the authors of [91], this technique is characterized by several challenges, with the major challenge when handling components such as ceramics related to the length/width ratio of the fabricated component. It was suggested that the risk of random fracturing in the fabricated component is enhanced when the length is ≥2 times the width. This challenge is presented when the bottom-up approach is employed. The alternative top-down approach may also present some limitations when employed in fabricating structures with large cross-sectional areas, with 3 mm2 suggested as the preferred upper limit. |
| Extrusion-based 3D printing | To optimize the process, variables such as rod width (i.e., of the fused ceramic composite filament), layer thickness, building orientation, and the infill percentage must be considered [86,92]. | Due to the high melting temperature of biomaterials such as ceramics, its use is not feasible with thermoplastic binders needed to formulate the composites such that the ceramic particles is ~60 vol% [86]. When printing materials, particularly ceramics are used, there is a major concern of there being an offset between the printed layers such that layer marks become distinctly visible (i.e., the staircase effect) [86]. Challenges related to surface roughness of the scaffold have also be highlighted [86]. Other challenges of this technique which also affects materials such as ceramics include the difficulty of biomolecules incorporation and low resolution [88]. |
| Laser-assisted 3D printing | For this technique, it may be necessary to optimize the formulation, fabrication parameters (layer thickness, infill percentage, and extruder temperature [93]), position, and orientation for optimal printing processes [86] | When using materials such as ceramics, there are challenges of high shrinkage, high porosity, and the thermal-gradient-induced problem. Additionally, challenges such as low resolution, poor surface finish, and porous microstructures within the fabricated parts also persist when SLS is used [86]. It must also be stated that generic issues of high cost, difficulty in printing cells, and long processing times also negatively affect this technique [88]. |
| FDM 3D printing | The optimization of this technique depends on several process parameters such as the rod width of the fused ceramic/polymer filament, layer thickness, building orientation, and raster angle [86] | This technique presents the challenge of the staircase effect when employed in printing ceramic composites ceramic parts. Significant concerns related to surface roughness also exist [86]. |
| 3D Printing Technology | Binder | Some Notes | Source |
|---|---|---|---|
| DIW writing | Polymethylsilsesquioxane | This binder has been shown to be viable in the fabrication of ceramic matrix composite. In the study, polymethylsilsesquioxane and ceramics were used in the preparation of a preceramic polymer. Using this binder and 3D printing technology, complex ceramic matrix composite structures with porosity and compressive strength of ~75% and ~4 MPa were fabricated. | [248] |
| Inkjet-based 3D printing | Polymethylsilsesquioxane | This binder was employed in the 3D printing with β-TCP and a polysiloxane to manufacture bulk β-TCP with a silica coating. The mechanical strength of the final sintered porous structures was within the range of that of trabecular bones, in the order of 0.1–16 MPa. | [249] |
| Inkjet-based 3D printing | Colloidal silica | In this study, the focus was to demonstrate and assess the possibility of using the inkjet-based 3D printing technique and the colloidal silica binder in the fabrication of porous ceramic-based composite parts. Information regarding the mechanical strength of the composite was however not presented. | [250] |
| DLP 3D printing | Silicon resin | In this work, a DLP-based 3D printing technique was used in fabricating a ceramic composite while also employing silicon resin as the binder. The study showed that the compressive strength and elastic modulus values 3D-structured ceramic based lattice were 5.12 and 2.1 MPa, respectively. | [251] |
| Extrusion-based 3D printing | PVA | In this study, PVA was employed as a binder in the fabrication of structures of HA composites. The study showed that, at 7–14% of the polymer, HA composites are well extruded and presented a mechanical strength of ~4 MPa after hardening. | [252] |
| Selective laser sintering | Schelofix, Polymeric binder | In this study, water soluble Schelofix was employed as a binder in the fabrication of HA based composited for 3D printing of scaffolds. A structure with mechanical strength of 22 MPa via the printing technique was achieved. | [101] |
| Selective laser sintering | Polyvinyl alcohol | In this study, water-soluble PVA was employed as a binder, in the fabrication of ceramic based composites. The study showed that, by using the binder in conjunction with the selective laser sintering, the resulting structure has an average flexural strength of 363.5 MPa and a relative density of 98%. | [253] |
| SLA based 3D printing | Photopolymer binder such as (meth)acrylate monomer/oligomers | In the study [254] 1,6-hexanediol diacrylate was used as an acrylate-based monomer as the photopolymer binder with a ceramic content of 50 vol% to enable the fabrication of structures with high relative density of 99.95% and high flexural strength of 1008.5 MPa. | [255] |
| Materials | Pore Size | Porosity | Compressive Strength | Some Notes | Sources |
|---|---|---|---|---|---|
| HA/chitosan | 200–400 µm | No access | No access | - | [53] |
| Silk/HA | 200–750 µm | 50–80% | - | - | [58] |
| HA | 300–600 µm | 49.8% | 15.25 MPa | - | [99] |
| HA/SF | 400 µm | 70% | 6 MPa | Good in vitro biomineralization activity | [71] |
| HA/silk | 200–750 µm | 50–80% | - | Enhanced the osteogenesis and vasculogenesis | [58] |
| HA/bacterial cellulose | 300 µm | - | - | Induces the orderly deposition of HA crystals | [141] |
| HA/chitosan/SiO2 | 200 µm | 53.57 ± 0.35% | 10–13 MPa | Exactly comparable to human trabecular bone | [333] |
| HA/PCL | 600–800 µm | 78.54–70.31% | 1.38–3.17 MPa | Satisfies basic requirements of bone TE scaffolds | [66] |
| HA | 350 µm | 52.26% | 16.77 ± 0.38 MPa | Can be readily integrated with the native bone | [334] |
| HA | 500 µm | 50% | - | Promotes cell proliferation | [48] |
| HA | 500 µm | 31–33.5% | - | formed a strong bone connection | [49] |
| HA | 450–570 µm | - | 22 MPa | - | [101] |
| CHA | 400 µm | 71.8–82.9% | 20 MPa | - | [69] |
| HA/SF | 400 µm | 70% | 6 MPa | Good in vitro biomineralization activity | [71] |
| PLA/HA | - | 47–69% | 16–53 MPa | - | [74] |
| PCL/PLGA/HA | 500 µm | - | 15.9–20.9 MPa | - | [75] |
| HA/TCP | 800 µm | 50% | 2.6 MPa | - | [68] |
| HA/TCP | 500 µm | 70% | 23 MPa | - | [257] |
| Sr/HA | 300–500 µm | - | 3.8–4.2 MPa | Good osteogenesis | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals 2021, 11, 353. https://doi.org/10.3390/cryst11040353
Han Y, Wei Q, Chang P, Hu K, Okoro OV, Shavandi A, Nie L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals. 2021; 11(4):353. https://doi.org/10.3390/cryst11040353
Chicago/Turabian StyleHan, Yanting, Qianqian Wei, Pengbo Chang, Kehui Hu, Oseweuba Valentine Okoro, Amin Shavandi, and Lei Nie. 2021. "Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application" Crystals 11, no. 4: 353. https://doi.org/10.3390/cryst11040353
APA StyleHan, Y., Wei, Q., Chang, P., Hu, K., Okoro, O. V., Shavandi, A., & Nie, L. (2021). Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals, 11(4), 353. https://doi.org/10.3390/cryst11040353










