Extraction–Pyrolytic Method for TiO2 Polymorphs Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Precursors
2.1.1. Preparation of Aqueous Solution of Titanium (III) Chloride TiCl3
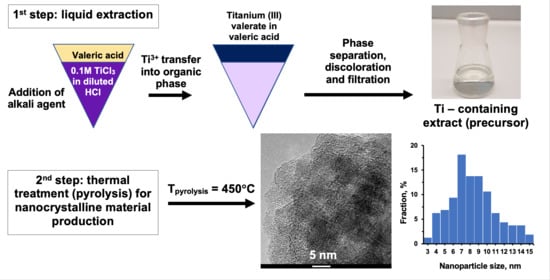
2.1.2. Preparation of Titanium-Containing Precursors (E) via Liquid–Liquid Extraction
2.1.3. Preparation of Titanium-Containing Precursor (P) via Precipitation
2.2. Thermal Treatment of Precursors
2.3. Characterization Methods
3. Results
3.1. Precursors Characterization
3.2. Thermal Behavior of Precursors E1, E2, and P
3.3. XRD Analysis
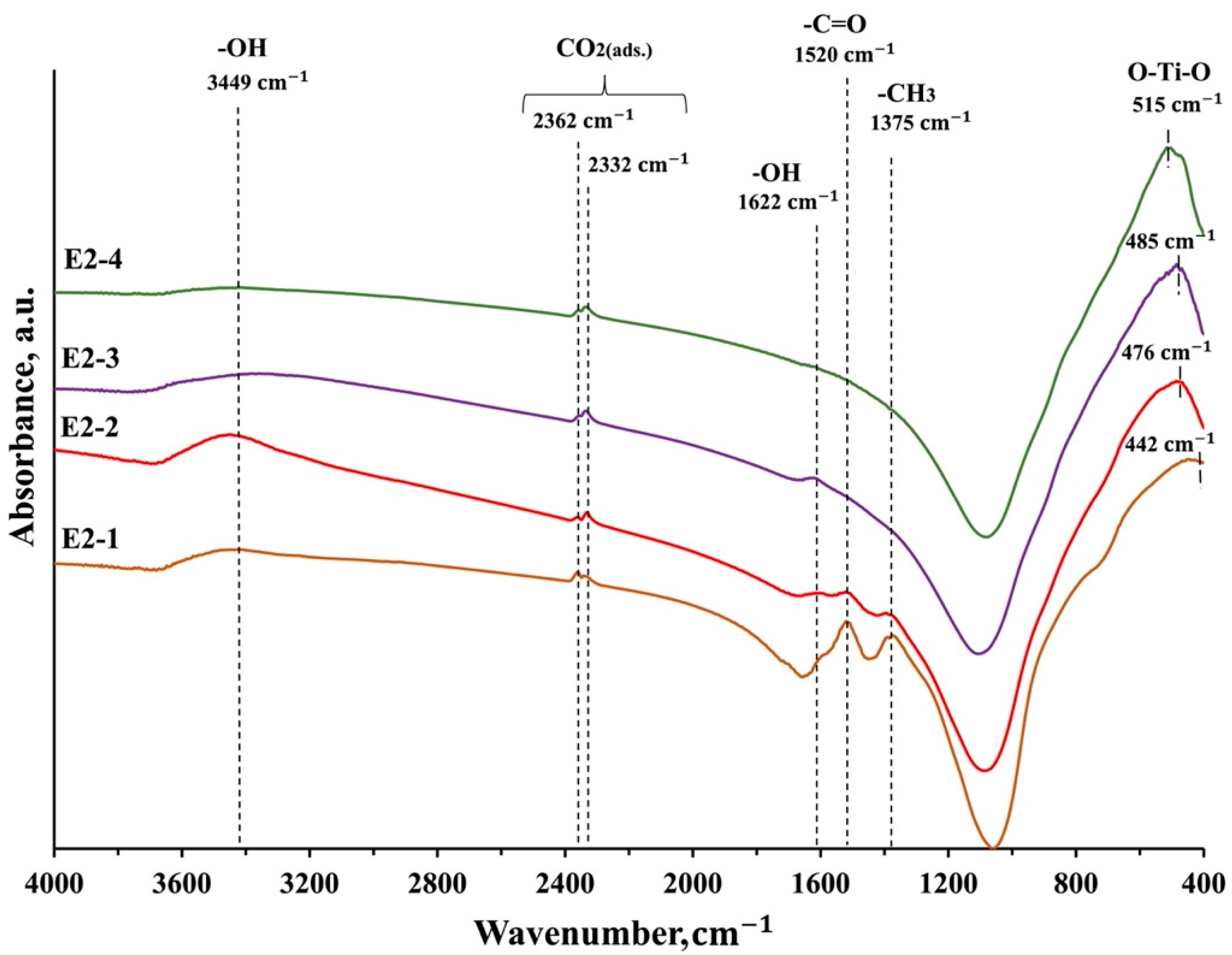
3.4. FTIR Spectroscopy
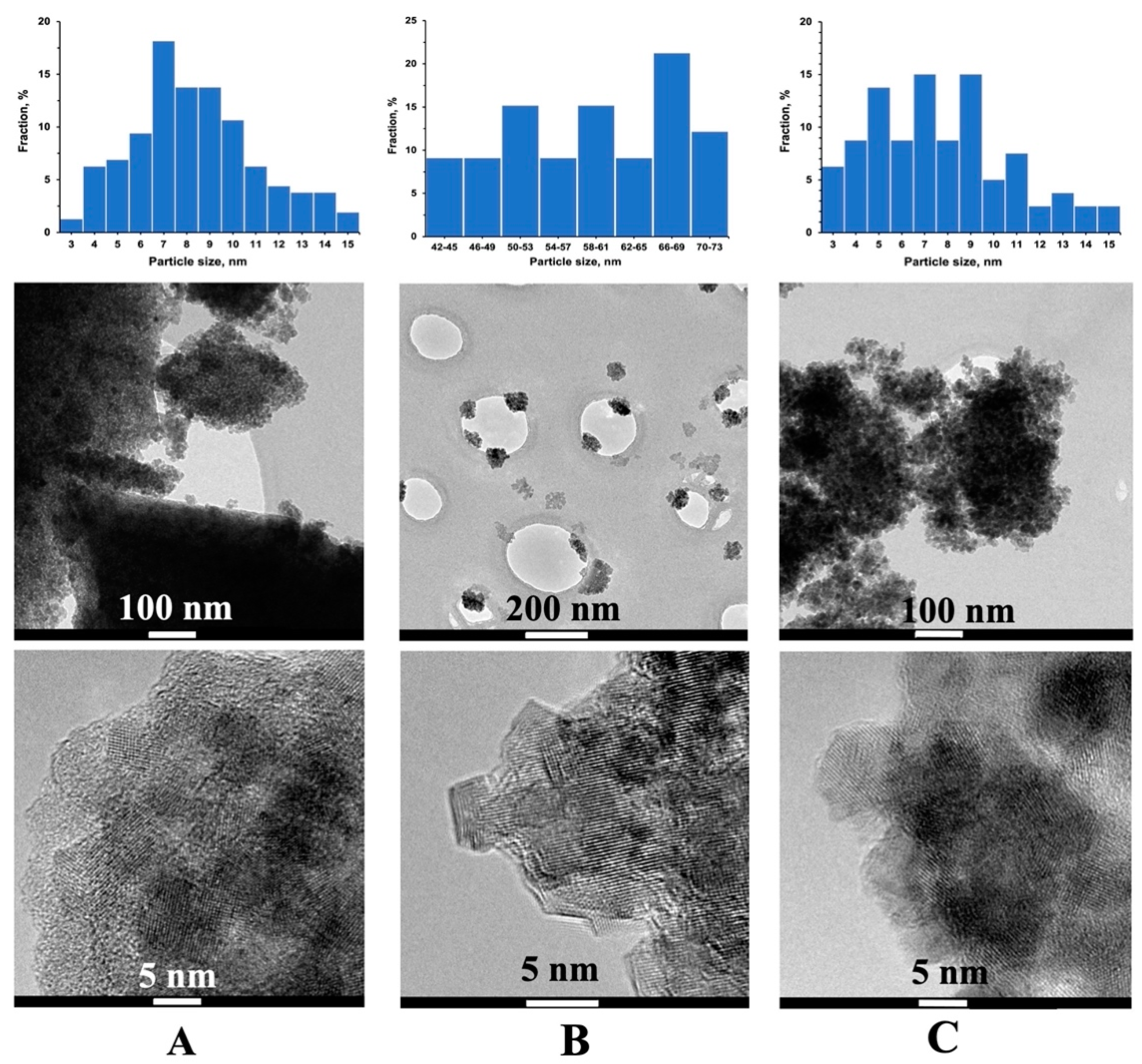
3.5. Transmission Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type synthesis and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, I.; Yadav, H.; Park, J.; Jo, J.W.; Yim, S.; Lee, J.-J. Non-hydrolytic sol-gel route to synthesize TiO2 nanoparticles under ambient condition for highly efficient and stable perovskite solar cells. Sol. Energy 2019, 185, 307–314. [Google Scholar] [CrossRef]
- Lingaraju, K.; Basavaraj, R.B.; Jayanna, K.; Bhavana, S.; Devaraja, S.; Kumar Swamy, H.M.; Nagaraju, G.; Nagabhushan, H.; Raja Naika, H. Biocompatible fabrication of TiO2 nanoparticles: Antimicrobial, anticoagulant, antiplatelet, direct hemolytic and cytotoxicity properties. Inorg. Chem. Commun. 2021, 127, 10850. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Haider, A.J.; AL-Anbari, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential environmental applications of TiO2 nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and characterization of TiO2 nanoparticles for the reduction of water pollutants. Materials 2017, 10, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.C.; Chen, C.C.; Chen, S.H. A review on production, characterization, and photocatalytic applications of TiO2 nanoparticles and nanotubes. Curr. Nanosci. 2017, 13, 373–393. [Google Scholar] [CrossRef]
- Wang, S.; Yu, H.; Yuan, S.; Zhao, Y.; Wang, Z.; Fang, J.; Zhang, M.; Shi, L. Synthesis of triphasic, biphasic, and monophasic TiO2 nanocrystals and their photocatalytic degradation mechanisms. Res. Chem. Intermed. 2016, 42, 3775–3788. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of sensors for the determination of gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Zhukovskii, Y.F.; Piskunov, S.; Lisovski, O.; Bocharov, D.; Evarestov, R.A. Doped 1D nanostructures of transition-metal oxides: First-principles evaluation of photocatalytic suitability. Isr. J. Chem. 2017, 57, 461–476. [Google Scholar] [CrossRef]
- Sidaraviciute, R.; Kavaliunas, V.; Puodziukynas, L.; Guobiene, A.; Martuzevicius, D.; Andrulevicius, M. Enhancement of photocatalytic pollutant decomposition efficiency of surface mounted TiO2 via lithographic surface patterning. Environ. Technol. Innov. 2020, 19, 100983. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Tamm, A.; Seinberg, L.; Kozlova, J.; Link, J.; Pikma, P.; Stern, R.; Kukli, K. Quasicubic α-Fe2O3 nanoparticles embedded in TiO2 thin films grown by atomic layer deposition. Thin Solid Films 2016, 612, 445–449. [Google Scholar] [CrossRef]
- Rempel, A.A.; Kuznetsova, Y.V.; Dorosheva, I.B.; Valeeva, A.A.; Weinstein, I.A.; Kozlova, E.A.; Saraev, A.A.; Selishchev, D.S. High Photocatalytic Activity Under Visible Light of Sandwich Structures Based on Anodic TiO2/CdS Nanoparticles/Sol–Gel TiO2. Top. Catal. 2020, 63, 130–138. [Google Scholar] [CrossRef]
- Tuckute, S.; Varnagiris, S.; Urbonavicius, M.; Lelis, M.; Sakalauskaite, S. Tailoring of TiO2 film crystal texture for higher photocatalysis efficiency. Appl. Surf. Sci. 2019, 489, 576–583. [Google Scholar] [CrossRef]
- Kenmoe, S.; Lisovski, O.; Piskunov, S.; Bocharov, D.; Zhukovskii, Y.F.; Spohr, E. Water adsorption on clean and defective anatase TiO2 (001) nanotube surfaces: A surface science approach. J. Phys. Chem. B 2018, 122, 5432–5440. [Google Scholar] [CrossRef]
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. Electronic properties of Nano-porous TiO2- and ZnO- thin films—Comparison of simulations and experiments. J. Ceram. Process. Res. 2004, 5, 343–354. [Google Scholar]
- Knoks, A.; Kleperis, J.; Grinberga, L. Raman spectral identification of phase distribution in anodic titanium dioxide coating. Proc. Estonian Acad. Sci. 2017, 66, 422–429. [Google Scholar] [CrossRef]
- Brik, M.G.; Antic, Ž.M.; Vukovic, K.; Dramicanin, M.D. Judd-Ofelt analysis of Eu3+ emission in TiO2 anatase nanoparticles. Mater. Trans. 2015, 56, 1416–1418. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, S.; Yanagisawa, K.; Lu, D.; Vequizo, J.J.M.; Yamakata, A.; Kimoto, K.; Inada, M.; Maeda, K. Enhanced water splitting through two-step photoexcitation by sunlight using tantalum/nitrogen-codoped rutile titania as a water oxidation photocatalyst. Sustain. Energy Fuels 2019, 3, 2337–2346. [Google Scholar] [CrossRef] [Green Version]
- Kavaliunas, V.; Krugly, E.; Sriubas, M.; Mimura, H.; Laukaitis, G.; Hatanaka, Y. Influence of Mg, Cu, and Ni dopants on amorphous TiO2 thin films photocatalytic activity. Materials 2020, 13, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Hu, X.; Fan, J.; Sun, T.; Kang, L.; Hou, W.; Zhu, C.; Liu, H. Photocatalytic activity of Ag/TiO2 nanotube arrays enhanced by surface plasmon resonance and application in hydrogen evolution by water splitting. Plasmonics 2013, 8, 501–508. [Google Scholar] [CrossRef]
- Linitis, J.; Kalis, A.; Grinberga, L.; Kleperis, J. Photo-activity research of nano-structured TiO2 layers. IOP Conf. Ser. Mater. Sci. Eng. 2011, 23, 012010. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskiy, A.; Shlimas, D.; Kenzhina, I.; Boretskiy, O.; Zdorovets, M. Study of the effect of low-energy irradiation with O2+ ions on radiation hardening and modification of the properties of thin TiO2 films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 790–801. [Google Scholar] [CrossRef]
- Mattsson, M.S.M.; Azens, A.; Niklasson, G.A.; Granqvist, C.G.; Purans, J. Li intercalation in transparent Ti-Ce oxide films: Energetics and ion dynamics. J. Appl. Phys. 1997, 81, 6432–6437. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Kozlovskiy, A.; Kenzhina, I.; Berguzinov, A.; Zdorovets, M. Study of the effect of irradiation with Fe7+ ions on the structural properties of thin TiO2 foils. Mater. Res. Express 2019, 6, 046309. [Google Scholar] [CrossRef]
- Kiisk, V.; Akulitš, K.; Kodu, M.; Avarmaa, T.; Mändar, H.; Kozlova, J.; Eltermann, M.; Puust, L.; Jaaniso, R. Oxygen-sensitive photoluminescence of rare earth ions in TiO2 thin films. J. Phys. Chem. C 2019, 123, 17908–17914. [Google Scholar] [CrossRef]
- Milovanov, Y.S.; Gavrilchenko, I.V.; Gayvoronsky, V.Y.; Kuznetsov, G.V.; Skryshevsky, V.A. Impact of Nanoporous Metal Oxide Morphology on Electron Transfer Processes in Ti–TiO2–Si Heterostructures. J. Nanoelectron. Optoelectron. 2014, 9, 432–436. [Google Scholar] [CrossRef]
- Reklaitis, I.; Radiunas, E.; Malinauskas, T.; Stanionytė, S.; Juška, G.; Ritasalo, R.; Pilvi, T.; Taeger, S.; Strassburg, M.; Tomašiūnas, R. A comparative study on atomic layer deposited oxide film morphology and their electrical breakdown. Surf. Coat. Technol. 2020, 399, 126123. [Google Scholar] [CrossRef]
- Luchinsky, G.P. Chemistry of the Titanium; Khimija: Moskow, Russia, 1971. (In Russian) [Google Scholar]
- Gribb, A.A.; Banfield, J.F. Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Amer. Miner. 1997, 82, 717–728. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Kharat, A.N.; Fatholahi, L.; Beiranvand, Z.M. A review on synthesis of nano-TiO2 via different methods. J. Nanostruct. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cao, X.; Wu, S.; Liu, C.; Li, G.; Jiang, W.; Wang, H.; Wang, N.; Ding, W. Preparation and characterization of TiO2 nanoparticles by two different precipitation methods. Ceram. Int. 2020, 46, 15333–15341. [Google Scholar] [CrossRef]
- Wategaonkar, S.B.; Pawar, R.P.; Parale, V.G.; Nade, D.P.; Sargar, B.M.; Mane, R.K. Synthesis of rutile TiO2 nanostructures by single step hydrothermal route and its characterization. Mater. Today Proc. 2020, 23, 444–451. [Google Scholar] [CrossRef]
- Kusior, A.; Banas, J.; Trenczek-Zajac, A.; Zubrzycka, P.; Micek-Ilnicka, A.; Radecka, M. Structural properties of TiO2 nanomaterials. J. Mol. Struct. 2018, 1157, 327–336. [Google Scholar] [CrossRef]
- Sharma, A.; Karn, R.K.; Pandiyan, S.K. Synthesis of TiO2 nanoparticles by sol-gel method and their characterization. J. Basic Appl. Eng. Res. 2014, 1, 1–5. [Google Scholar]
- Toygun, S.; Konecoglu, G.; Kalpakli, Y. General principles of sol-gel. J. Eng. Nat. Sci. 2013, 31, 456–476. [Google Scholar]
- Khol’kin, A.I.; Patrusheva, T.N. Extraction-Pyrolytic Method: Fabrication of Functional Oxide Materials; KomKniga: Moskow, Russian, 2006; ISBN 548-400-582-5. (In Russian) [Google Scholar]
- Patrusheva, T.N.; Popov, V.S.; Prabhu, G.; Popov, A.V.; Ryzhenkov, A.V.; Snezhko, N.Y.; Morozchenko, D.A.; Zaikovskii, V.D.; Khol’kin, A.I. Preparation of a photoanode with a multilayer structure for solar cells by extraction pyrolysis. Theor. Found. Chem. Eng. 2014, 48, 454–460. [Google Scholar] [CrossRef]
- Popov, A.I.; Shirmane, L.; Pankratov, V.; Lushchik, A.; Kotlov, A.; Serga, V.E.; Kulikova, L.D.; Chikvaidze, G.; Zimmermann, J. Comparative study of the luminescence properties of macro- and nanocrystalline MgO using synchrotron radiation. Nucl. Instrum. Methods Phys. Res. B 2013, 310, 23–26. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudzius, R.; Zarkov, A.; Kareiva, A.; Popov, A. Impact of gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef] [PubMed]
- Burve, R.; Serga, V.; Krumina, A.; Poplausks, R. Preparation and characterization of nanocrystalline gadolinium oxide powders and films. Key Eng. Mater. 2020, 850, 267–272. [Google Scholar] [CrossRef]
- Gindin, L.M. Extraction Processes and Its Application; Nauka: Moskow, Russia, 1984. (In Russian) [Google Scholar]
- Sharlo, G. Quantitative Analysis of the Inorganic Compounds. In Methods of the Analytical Chemistry; Lur’e, Y.Y., Ed.; Himija: Moskow, Russia, 1969; Volume 2, ISBN 978-544-584-821-9. (In Russian) [Google Scholar]
- Drozdov, A.A.; Zlomanov, G.N.; Mazo, G.N.; Spiridinov, F.M. Chemistry of the Transition Elements. In Inorganic Chemistry; Tretyakov, Y.D., Ed.; Akademija: Moskow, Russia, 2008; Volume 3, Part 1; pp. 56–99. ISBN 576-952-532-0. (In Russian) [Google Scholar]
- Mehrotra, R.C.; Bohra, R. Metal Carboxylates; Academic Press: London, UK, 1983; ISBN 978-012-488-160-0. [Google Scholar]
- Patil, K.C.; Chandrashekhar, G.V.; George, M.V.; Rao, C.N.R. Infrared spectra and thermal decompositions of metal acetates and dicarboxylates. Can. J. Chem. 1968, 46, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C. A process for successful infrared spectral interpretation. Spectroscopy 2016, 31, 14–21. [Google Scholar]
- Stuart, B. Analytical techniques in the sciences. In Infrared Spectroscopy: Fundamentals and Applications; Ando, D.J., Ed.; John Wiley&Sons: Chichester, UK, 2004; ISBN 978-047-085-427-3. [Google Scholar]
- Smith, B.C. The carbonyl group, part V: Carboxylates-coming clean. Spectroscopy 2018, 33, 20–23. [Google Scholar]
- Nyquist, R.A.; Kagel, R.O. Infrared spectra of inorganic compounds. In Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts, 1st ed.; Acad. Press: London, UK, 1971; pp. 1–18. ISBN 978-008-087-852-2. [Google Scholar]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 2 November 2020).
- Ocana, M.; Fornés, V.; García Ramos, J.V.; Serna, C.J. Factors affecting the infrared and raman spectra of rutile powders. J. Solid State Chem. 1988, 75, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Savchyn, P.; Karbovnyk, I.; Vistovskyy, V.; Voloshinovskii, A.; Pankratov, V.; Cestelli Guidi, M.; Mirri, C.; Myahkota, O.; Riabtseva, A.; Mitina, N. Vibrational properties of LaPO4 nanoparticles in mid-and far-infrared domain. J. Appl. Phys. 2012, 112, 124309. [Google Scholar] [CrossRef]
- Balasubramanian, C.; Bellucci, S.; Cinque, G.; Marcelli, A.; Guidi, M.C.; Piccinini, M.; Popov, A.; Soldatov, A.; Onorato, P. Characterization of aluminium nitride nanostructures by XANES and FTIR spectroscopies with synchrotron radiation. J. Phys. Condens. Matter 2006, 18, S2095–S2104. [Google Scholar] [CrossRef]
- Bellucci, S.; Popov, A.I.; Balasubramanian, C.; Cinque, G.; Marcelli, A.; Karbovnyk, I.; Savchyn, V.; Krutyak, N. Luminescence, vibrational and XANES studies of AlN nanomaterials. Radiat. Meas. 2007, 42, 708–711. [Google Scholar] [CrossRef]





| Sample Nr. | Production Conditions | XRD Analysis Results | |||
|---|---|---|---|---|---|
| Precursor | Pyrolysis Temperature T, °C | Phase Composition | d, nm | W, % | |
| E1-1 | E1 | 350 | Amorphous | - | - |
| E2-1 | E2 | ||||
| E1-2 | E1 | 400 | Amorphous | - | - |
| E2-2 | E2 | Anatase | 5 | 100 | |
| E1-3 | E1 | 450 | Anatase | 8 | 100 |
| E2-3 | E2 | Anatase | 9 | 100 | |
| P-1 | P | Anatase | 9 | 100 | |
| E1-4 | E1 | 550 | Anatase | 15 | 100 |
| E2-4 | E2 | Anatase Rutile | 20 ~30 | 87.7 12.3 | |
| P2 | P | Anatase | 10 | 100 | |
| E1-5 | E1 | 650 | Anatase Rutile | 30 ~40 | 80.9 19.1 |
| E2-5 | E2 | Anatase Rutile | ~35 45 | 20.6 79.4 | |
| P3 | P | Anatase Rutile | 14 Discerned | 96.4 3.6 | |
| E1-6 | E1 | 750 | Anatase Rutile | Discerned 65 | 1.1 98.9 |
| E2-6 | E2 | Rutile | 53 | 100 | |
| P-4 | P | Rutile Na2Ti6O13 | 68 - | 100 - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. https://doi.org/10.3390/cryst11040431
Serga V, Burve R, Krumina A, Romanova M, Kotomin EA, Popov AI. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals. 2021; 11(4):431. https://doi.org/10.3390/cryst11040431
Chicago/Turabian StyleSerga, Vera, Regina Burve, Aija Krumina, Marina Romanova, Eugene A. Kotomin, and Anatoli I. Popov. 2021. "Extraction–Pyrolytic Method for TiO2 Polymorphs Production" Crystals 11, no. 4: 431. https://doi.org/10.3390/cryst11040431
APA StyleSerga, V., Burve, R., Krumina, A., Romanova, M., Kotomin, E. A., & Popov, A. I. (2021). Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals, 11(4), 431. https://doi.org/10.3390/cryst11040431