Bonding SiCp/Al Composites via Laser-Induced Exothermic Reactions
Abstract
1. Introduction
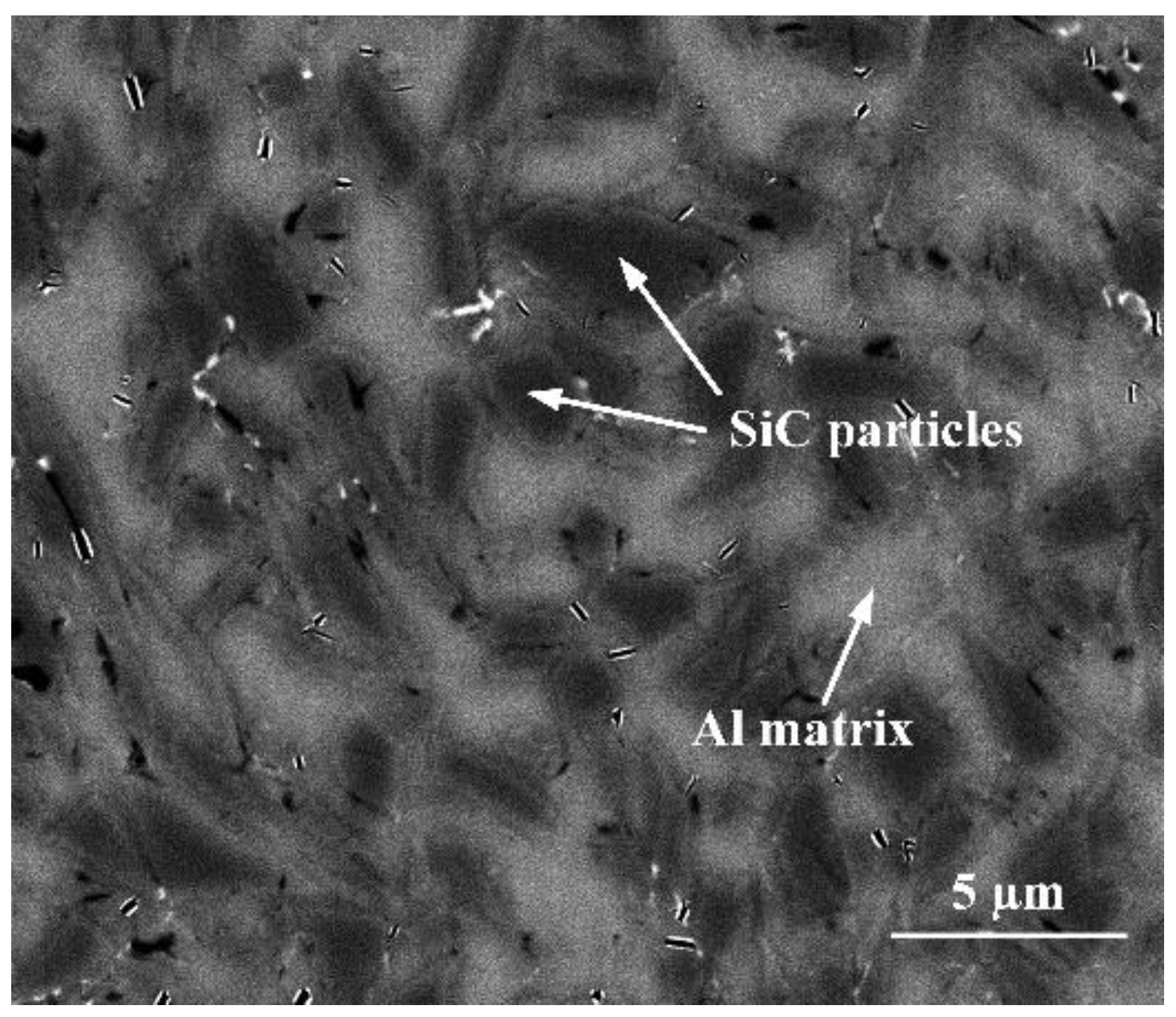
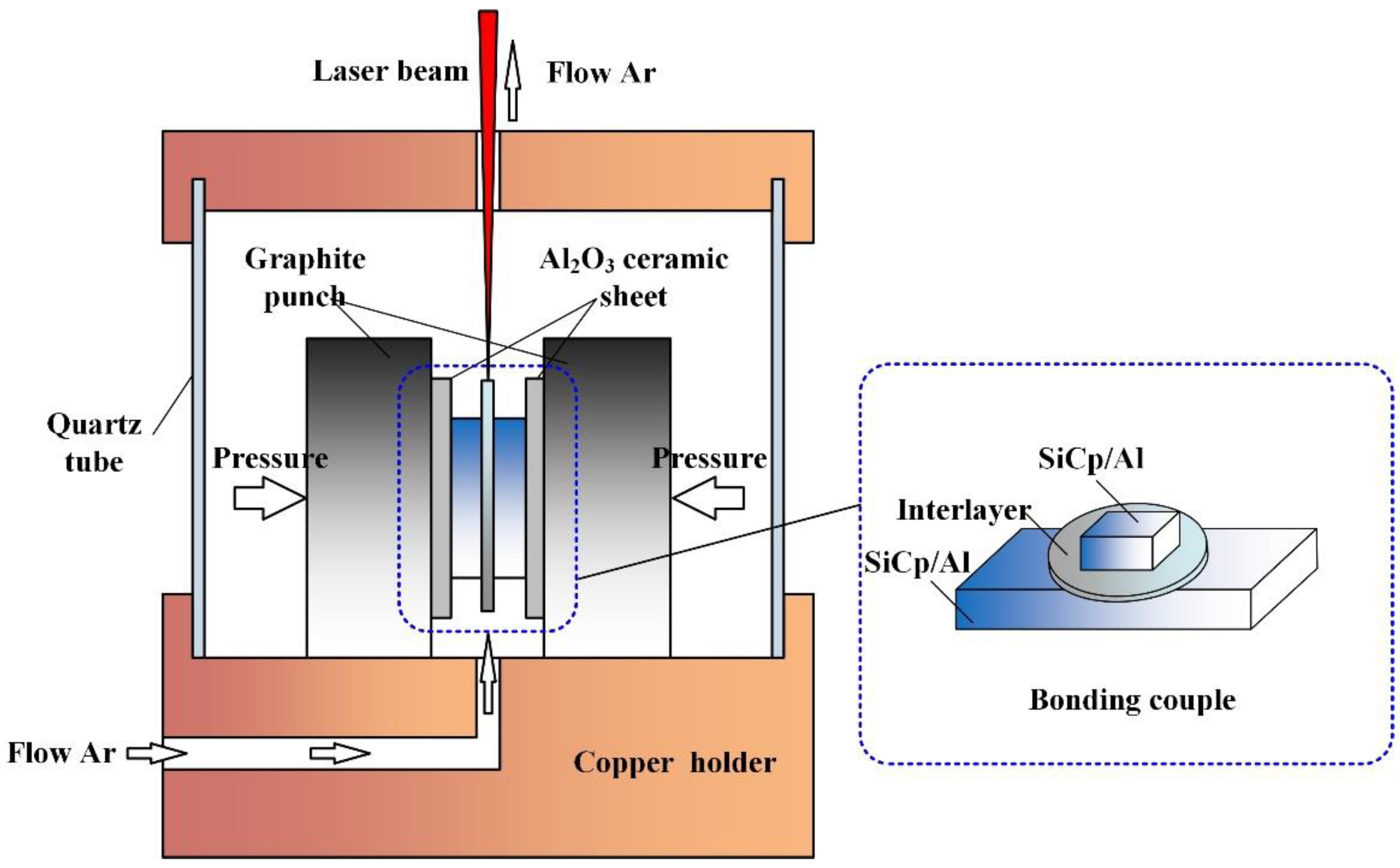
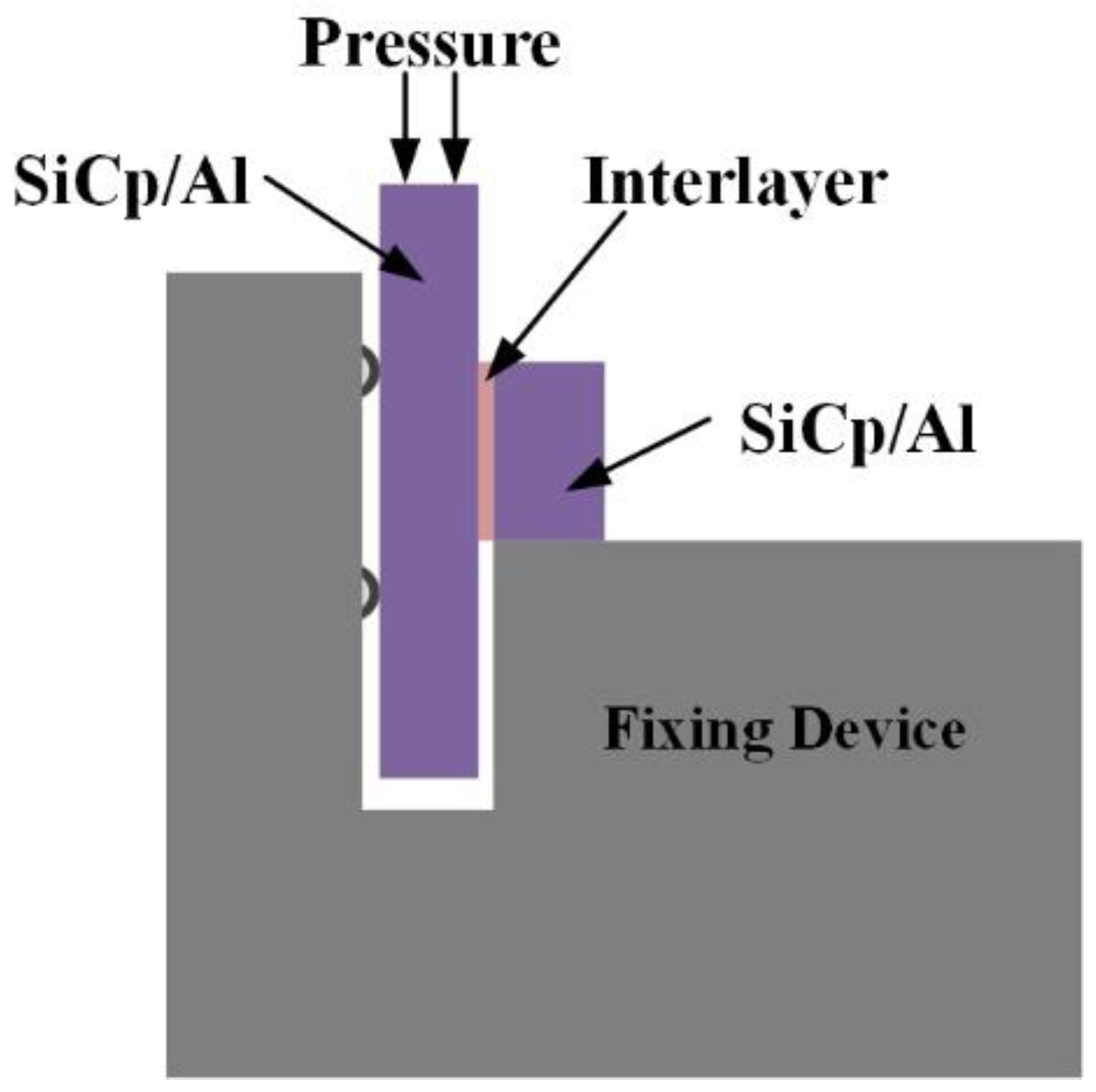
2. Experimental Procedures
3. Results and Discussion
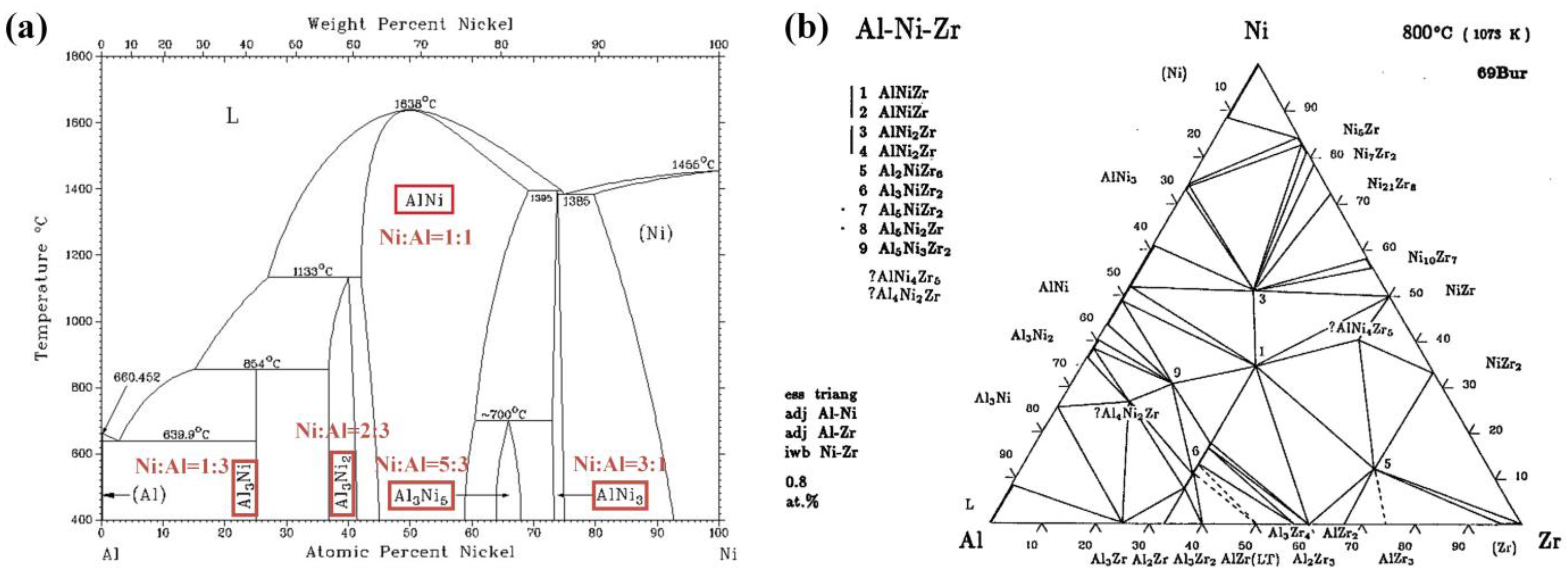
3.1. Design of the Exothermic Interlayer
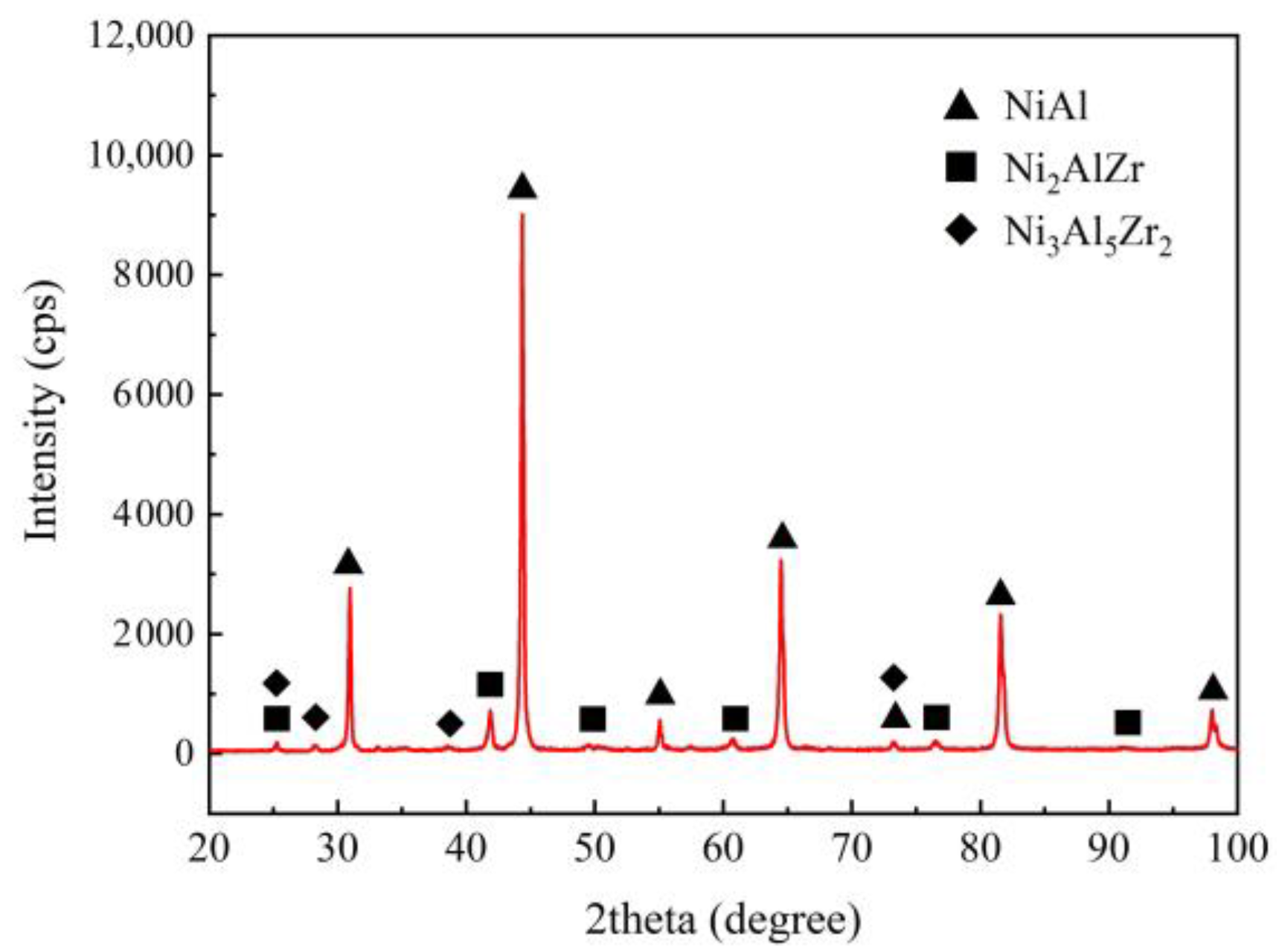
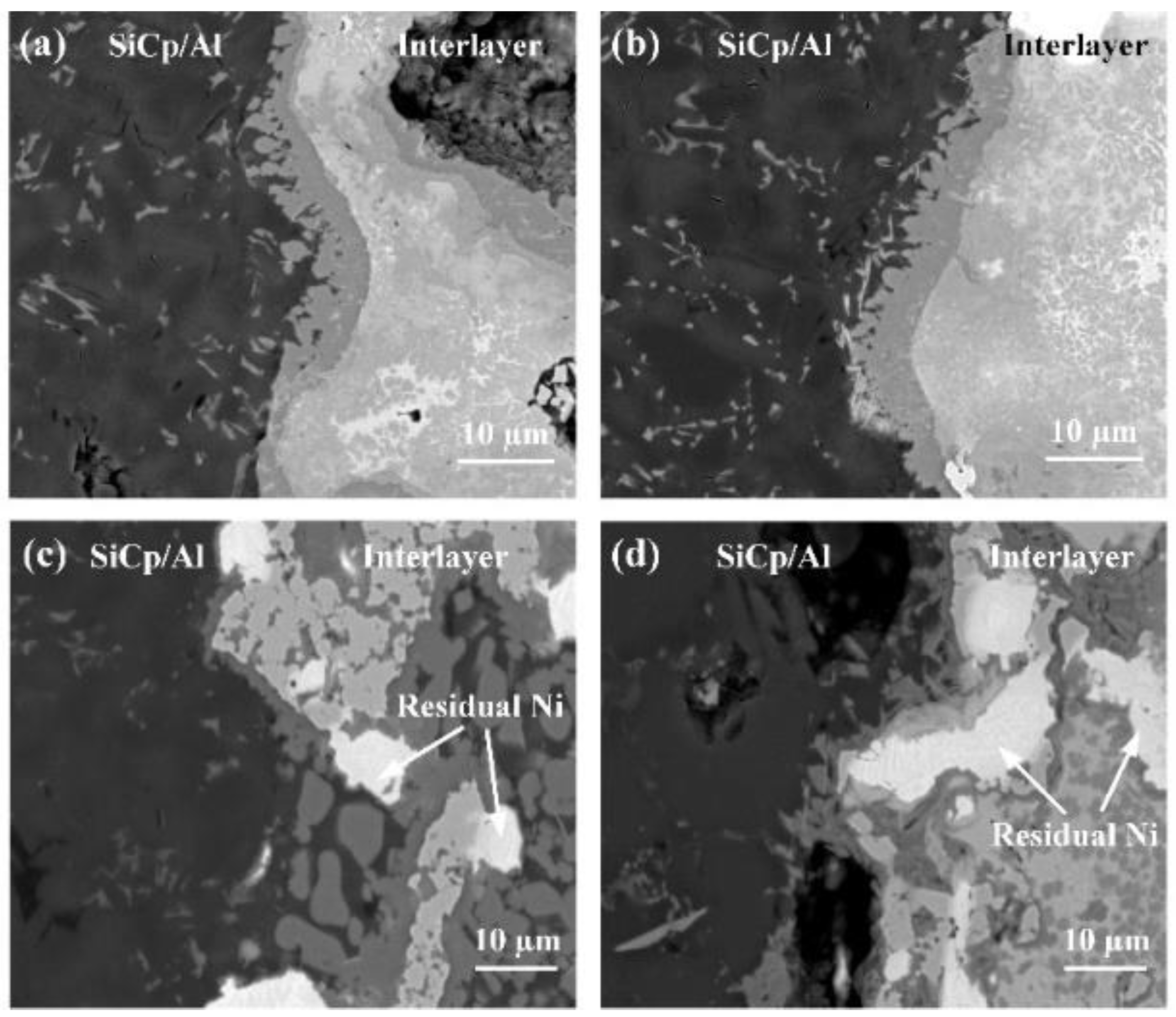
3.2. Joint Microstructure
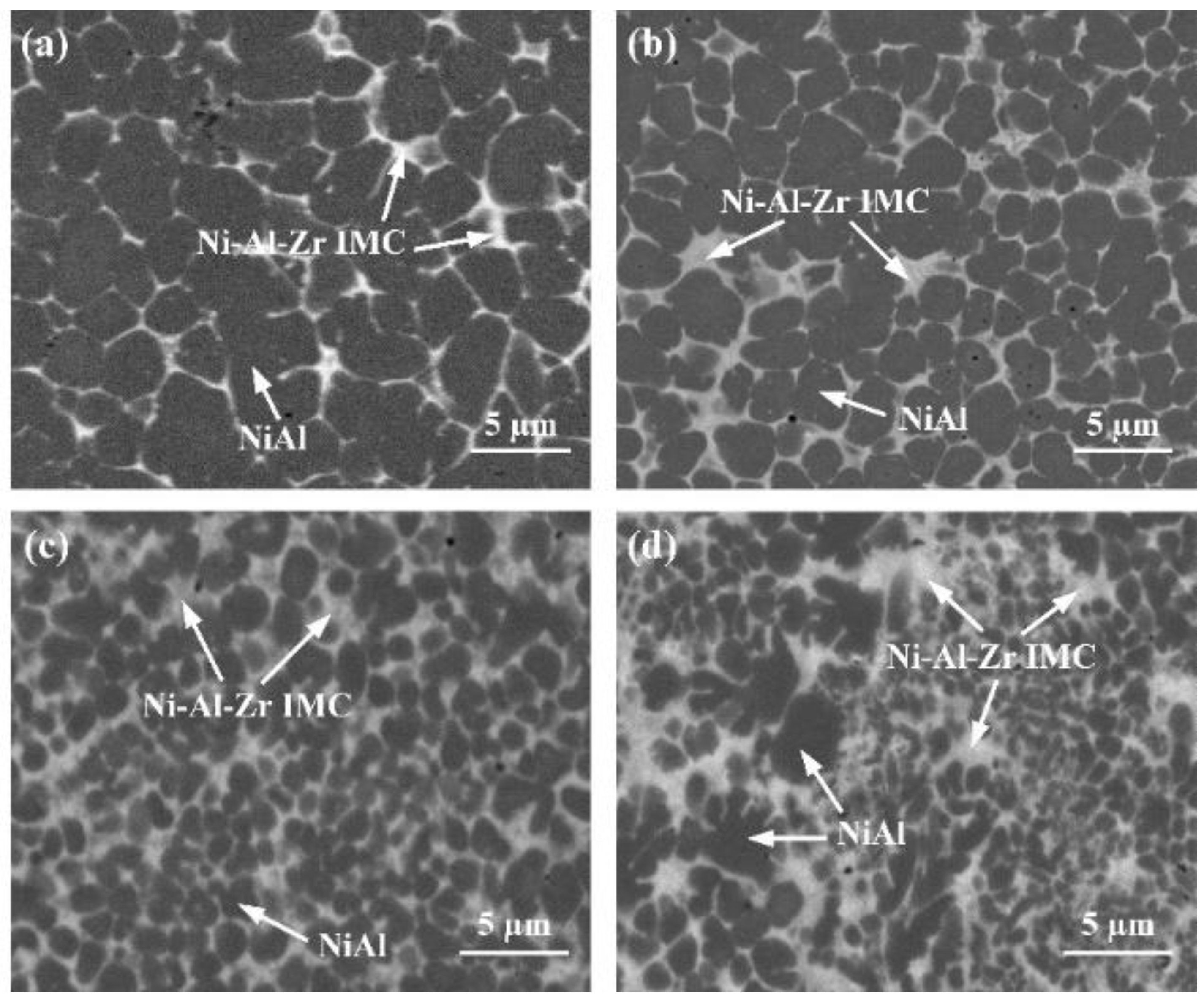
3.3. Influence of the Interlayer Chemical Composition on the Joint Microstructure
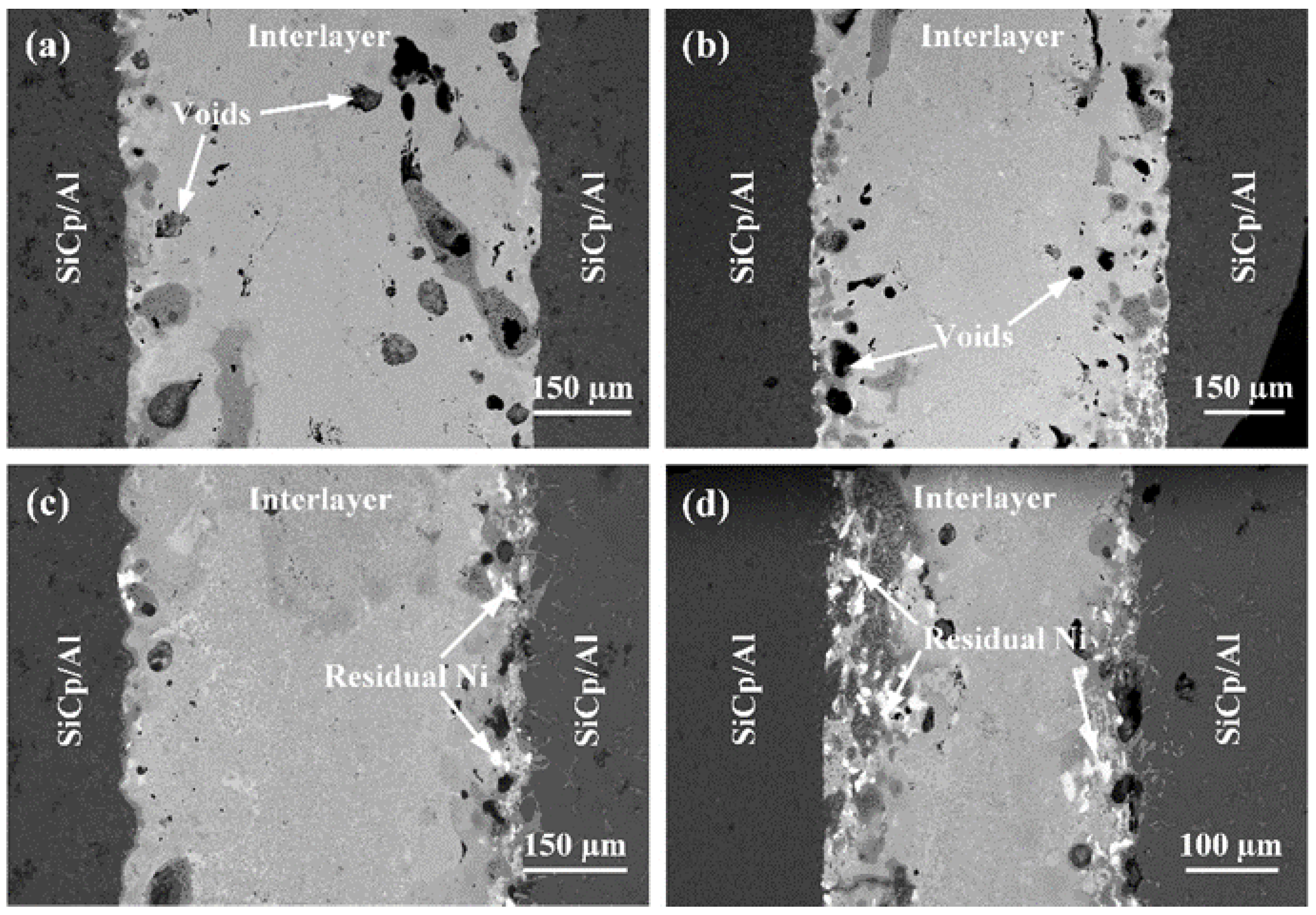
3.4. Influence of the Bonding Pressure on the Joint Microstructure
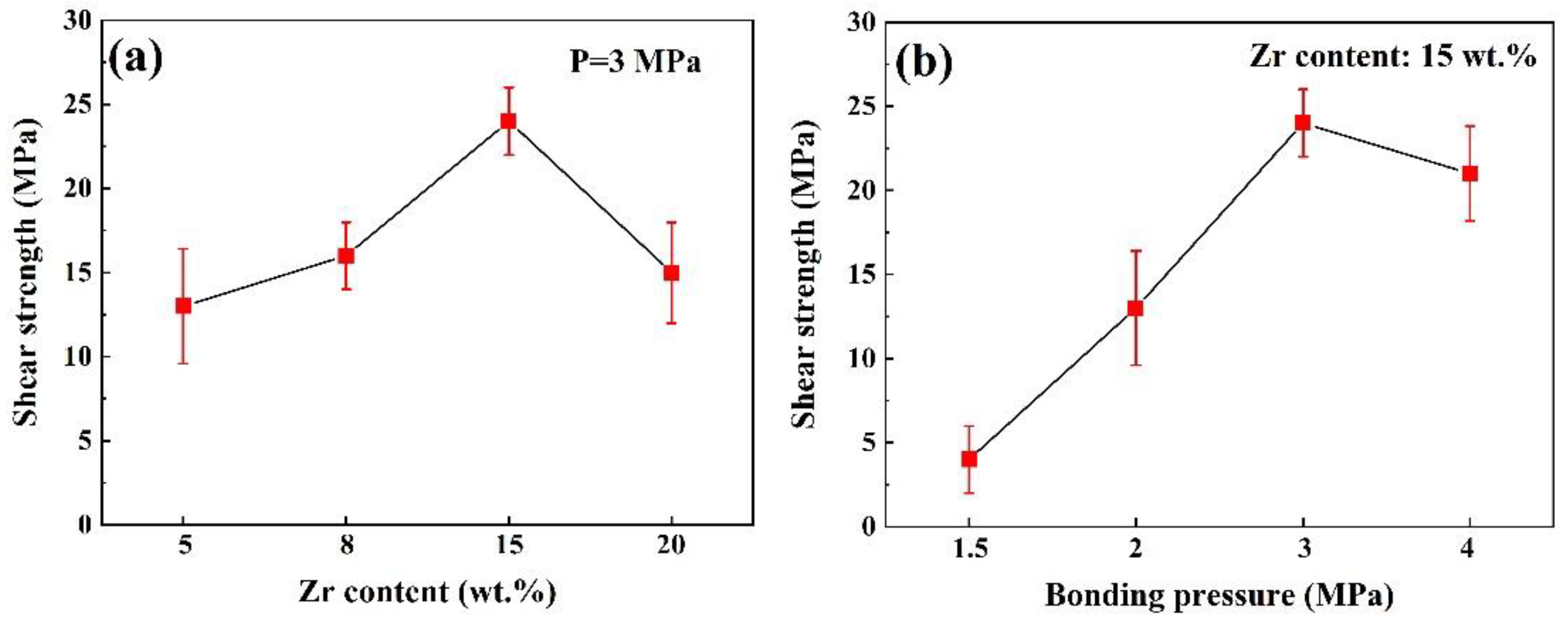
3.5. Joint Shear Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.F.; Zhao, Y.H.; Shen, Z.X.; Dai, L.G.; Zhang, X.L.; Zhu, R. Study on the Joint Strength of SiCp/ Al Metal Matrix Composite by Magnetron Sputtering Method. Mater. Sci. Forum 2009, 628–629, 569–574. [Google Scholar] [CrossRef]
- Venkatachalam, A.; Anurag, P.V.S.; Sadanand, T.D.; Nachimuthu, R. Optimization of the milling parameters for an Al/Si3N4 functionally graded composite using grey relational analysis. Mater. Test 2018, 60, 215–221. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Ding, D. A first-principles investigation of interfacial properties and electronic structure of SiO2/Al interface. Comput. Mater. Sci. 2021, 188. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Ma, T.; Zhu, D.; Dong, D.; Yang, X.; Liu, L.; Wang, G. Evolution of primary and eutectic Si phase and mechanical properties of Al2O3/Al-20Si composites under high pressure. Crystals 2021, 11, 364. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Zhang, Y.; Cui, Y.; Sun, L.; Chen, D. Improving the mechanical properties of B4C/Al composites by solid-state interfacial reaction. J. Alloy. Compd. 2020, 829. [Google Scholar] [CrossRef]
- Duygulu, O. High-resolution transmission electron microscopy investigation of in situ TiC/Al composites. Met. Mater. 2018, 56, 265–275. [Google Scholar] [CrossRef]
- Qiao, Q.; Su, Y.; Cao, H.; Zhang, D.; Ouyang, Q. Effect of post-weld heat treatment on double-sided friction stir welded joint of 120 mm ultra-thick SiCp/Al composite plates. Mater. Charact. 2020, 169, 110668. [Google Scholar] [CrossRef]
- Adebisi, A.A.; Maleque, M.A.; Ali, M.Y.; Bello, K.A. Effect of variable particle size reinforcement on mechanical and wear properties of 6061Al-SiCp composite. Compos. Interface 2016, 23, 533–547. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, Z.; Wu, B.; Feng, H.; Shi, Z.; Zhao, B. Effect of Ultrasonic Vibration Tensile on the Mechanical Properties of High-Volume Fraction SiCp/Al Composite. Int. J. Precis. Eng. Man. 2020, 21, 2051–2066. [Google Scholar] [CrossRef]
- Huang, S.T.; Zhou, L.; Chen, J.; Xu, L.F. Drilling of SiCp/Al Metal Matrix Composites with Polycrystalline Diamond (PCD) Tools. Mater. Manuf. Process. 2012, 27, 1090–1094. [Google Scholar] [CrossRef]
- Kwok, Y. Effects of pulse-impact on the welding of SiCP/Al-6061 aluminium matrix composites. Mater. Sci. Technol. 2017, 33, 2298–2304. [Google Scholar] [CrossRef]
- Long, J.; Zhang, L.J.; Zhang, L.L.; Wang, X.; Zhang, G.F.; Zhang, J.X.; Na, S.J. Effects of minor Zr addition on the microstructure and mechanical properties of laser welded joint of Al/SiCp metal-matrix composite. J. Manuf. Process. 2020, 49, 373–384. [Google Scholar] [CrossRef]
- Xi-He, W.; Ji-Tai, N.; Shao-Kang, G.; Le-Jun, W.; Dong-Feng, C. Investigation on TIG welding of SiCp-reinforced aluminum-matrix composite using mixed shielding gas and Al-Si filler. Mater. Sci. Eng. A 2009, 499, 106–110. [Google Scholar] [CrossRef]
- Wang, P.; Gao, Z.; Niu, J. Micro-nano filler metal foil on vacuum brazing of SiCp/Al composites. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Chen, H.; Nai, X.; Zhao, S.; Lu, D.; Shen, Z.; Li, W.; Cao, J. Improvement for interfacial microstructure and mechanical properties of Ti3SiC2/Cu joint brazed by Ag-Cu-Ti filler with copper mesh. Crystals 2021, 11, 401. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, H.; Feng, J.; Ji, F.; Niu, J.; Brnic, J. Flux-Free Diffusion Joining of SiCp/6063 Al Matrix Composites Using Liquid Gallium with Nano-Copper Particles in Atmosphere Environment. Nanomaterials 2020, 10, 437. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Zhou, Z.; Wang, Y. Joining of Cf/Al composites and TiAl intermetallics by laser-induced self-propagating high-temperature synthesis using the Ni-Al-Zr interlayer. Mater. Des. 2016, 110, 130–137. [Google Scholar] [CrossRef]
- Trenkle, J.C.; Koerner, L.J.; Tate, M.W.; Gruner, S.M.; Weihs, T.P.; Hufnagel, T.C. Phase transformations during rapid heating of Al/Ni multilayer foils. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Pascal, C.; Marin-Ayral, R.M.; Tedenac, J.C. Joining of nickel monoaluminide to a superalloy substrate by high pressure self-propagating high-temperature synthesis. J. Alloy. Compd. 2002, 337, 221–225. [Google Scholar] [CrossRef]
- Swiston, A.J.; Hufnagel, T.C.; Weihs, T.P. Joining bulk metallic glass using reactive multilayer foils. Scripta Mater. 2003, 48, 1575–1580. [Google Scholar] [CrossRef]
- Lin, Y.C.; Nepapushev, A.A.; Mcginn, P.J.; Rogachev, A.S.; Mukasyan, A.S. Combustion joinining of carbon/carbon composites by a reactive mixture of titanium and mechanically activated nickel/aluminum powders. Ceram. Int. 2013, 39, 7499–7505. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Jacob, R.J.; Yang, Y.; Wang, Y.; Zhou, Z.; Sekulic, D.P.; Zachariah, M.R. Laser-induced exothermic bonding of carbon fiber/Al composites and TiAl alloys. Mater. Des. 2017, 126, 197–206. [Google Scholar] [CrossRef]
- Royal, T.E.; Namjoshi, S.; Thadhani, N.N. Mechanistic processes influencing shock chemistry in powder mixtures of the Ti-Si, Ti-Al, and Ti-B systems. Met. Mater. Trans. A 1996, 27, 1761–1771. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Lin, Y.; Rouvimov, S.; McGinn, P.J.; Mukasyan, A.S. Microstructure-reactivity relationship of Ti plus C reactive nanomaterials. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Ligachev, A.E.; Potemkin, G.V.; Lepakova, O.K.; Zhidkov, M.V.; Teresov, A.D.; Golobokov, N.N.; Maksimov, Y.M.; Kolobov, Y.R.; Koval, N.N. Ignition of a Ti-Al-C System by an Electron Beam. Combust. Explo Shock+ 2018, 54, 158–164. [Google Scholar] [CrossRef]
- Epshteyn, A.; Weismiller, M.R.; Huba, Z.J.; Maling, E.L.; Chaimowitz, A.S. Optimization of a High-Energy Ti-Al-B Nanopowder Fuel. Energy Fuels 2017, 31, 1811–1819. [Google Scholar] [CrossRef]
- Umbrajkar, S.M.; Schoenitz, M.; Dreizin, E.L. Exothermic reactions in Al-CuO nanocomposites. Thermochim. Acta 2006, 451, 34–43. [Google Scholar] [CrossRef]
- Song, J.; Guo, T.; Yao, M.; Chen, J.; Ding, W.; Bei, F.; Mao, Y.; Yu, Z.; Huang, J.; Zhang, X.; et al. A comparative study of thermal kinetics and combustion performance of Al/CuO, Al/Fe2O3 and Al/MnO2 nanothermites. Vacuum 2020, 176. [Google Scholar] [CrossRef]
- Shteinberg, A.S.; Lin, Y.; Son, S.F.; Mukasyan, A.S. Kinetics of High Temperature Reaction in Ni-Al System: Influence of Mechanical Activation. J. Phys. Chem. A 2010, 114, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Feng, J.C.; Li, Z.R. Joining of TiAl intermetallic by self-propagating high-temperature synthesis. J. Mater. Sci. 2006, 41, 4720–4724. [Google Scholar] [CrossRef]
- Morris, C.J.; Mary, B.; Zakar, E.; Barron, S.; Fritz, G.; Knio, O.; Weihs, T.P.; Hodgin, R.; Wilkins, P.; May, C. Rapid initiation of reactions in Al/Ni multilayers with nanoscale layering. J. Phys. Chem Solids 2010, 71, 84–89. [Google Scholar] [CrossRef]
- Naiborodenko, Y.S.; Lavrenchuk, G.V.; Filatov, V.M. Self-propagating high-temperature synthesis of aluminides I. Thermodynamic analysis. Powder Metall. Met. Ceram. 1982, 21, 909–912. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Zhou, Z.; Yang, Y.; Sekulic, D.P.; Zachariah, M.R. Microstructure and mechanical properties of C-f/Al-TiAl laser-assisted brazed joint. J. Mater. Process. Technol. 2018, 255, 195–203. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Feng, S.; Zhang, W. Microstructure evolution and formation mechanism of laser-ignited SHS joining between Cf/Al composites and TiAl alloys with Ni–Al–Ti interlayer. Rare Metals 2017, 36, 746–752. [Google Scholar] [CrossRef]
- Chérif, A.; Bachaga, T.; Saurina, J.; Suñol, J.J.; Khitouni, M.; Makhlouf, T. Morphology and structure effect of Ti additive on the solid-state reaction between Ni and Al powders during mechanical alloying. Int. J. Adv. Manuf. Technol. 2016, 86, 2937–2943. [Google Scholar] [CrossRef]
- Li, Z.; Feng, G.; Wang, S.; Feng, S. High-efficiency Joining of Cf/Al Composites and TiAl Alloys under the Heat Effect of Laser-ignited Self-propagating High-temperature Synthesis. J. Mater. Sci. Technol. 2016, 32, 1111–1116. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, J.; Hu, H.; Lu, C. Benefits of Zr additive element in the Ti-24Ni eutectic filler in vacuum brazing of SiC ceramics. Vacuum 2019, 162, 110–113. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Feng, K.; Cai, Y.; Chen, S. Effect of Brazing Parameters on the Microstructure and Properties of SiC Ceramic Joint with Zr-Cu Filler Metal. Crystals 2020, 10, 93. [Google Scholar] [CrossRef]
- Simoes, S.; Sofia Ramos, A.; Viana, F.; Teresa Vieira, M.; Vieira, M.F. TiAl diffusion bonding using Ni/Ti multilayers. Weld. World 2017, 61, 1267–1273. [Google Scholar] [CrossRef]

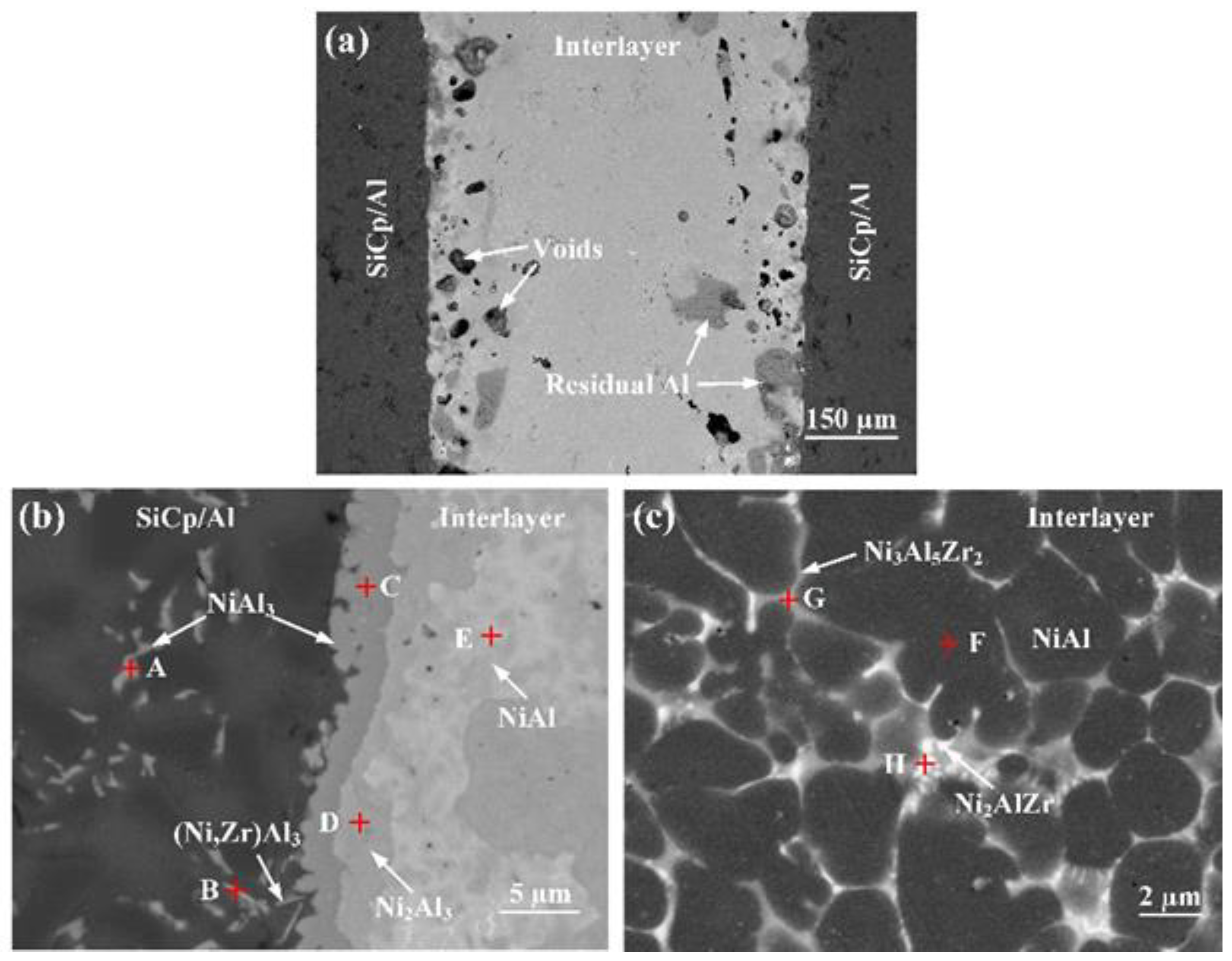

| Zone | Ni | Al | Zr | Possible Phase |
|---|---|---|---|---|
| A | 26.63 | 73.37 | — | NiAl3 |
| B | 6.03 | 84.95 | 9.02 | (Ni,Zr)Al3 |
| C | 28.07 | 71.67 | 0.26 | NiAl3 |
| D | 42.73 | 57.08 | 0.19 | Ni2Al3 |
| E | 60.33 | 39.67 | — | NiAl |
| F | 50.45 | 47.51 | 2.04 | NiAl |
| G | 37.93 | 42.17 | 19.9 | Ni3Al5Zr2 |
| H | 53.03 | 25.39 | 21.59 | Ni2AlZr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, G.; Wei, Y.; Hu, B.; Deng, D. Bonding SiCp/Al Composites via Laser-Induced Exothermic Reactions. Crystals 2021, 11, 535. https://doi.org/10.3390/cryst11050535
Wang Y, Feng G, Wei Y, Hu B, Deng D. Bonding SiCp/Al Composites via Laser-Induced Exothermic Reactions. Crystals. 2021; 11(5):535. https://doi.org/10.3390/cryst11050535
Chicago/Turabian StyleWang, Yifeng, Guangjie Feng, Yan Wei, Bingxu Hu, and Dean Deng. 2021. "Bonding SiCp/Al Composites via Laser-Induced Exothermic Reactions" Crystals 11, no. 5: 535. https://doi.org/10.3390/cryst11050535
APA StyleWang, Y., Feng, G., Wei, Y., Hu, B., & Deng, D. (2021). Bonding SiCp/Al Composites via Laser-Induced Exothermic Reactions. Crystals, 11(5), 535. https://doi.org/10.3390/cryst11050535








