Wetting Behavior of the Ag-5CuO Brazing Alloy on ZTA Composite Ceramic with/without CuO Coating in Air
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
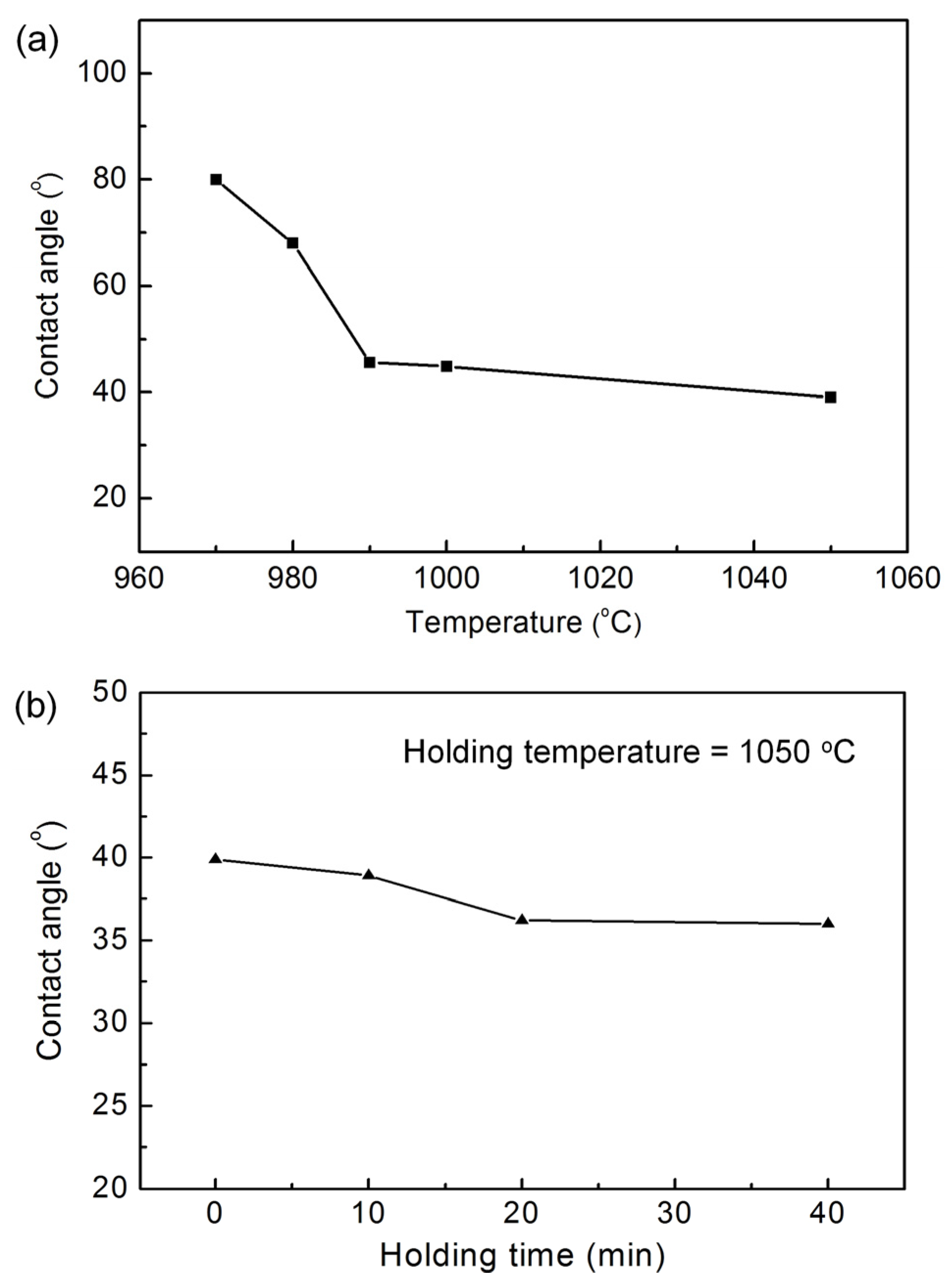
3.1. Wettability of Ag-5CuO Alloy on ZTA Ceramic
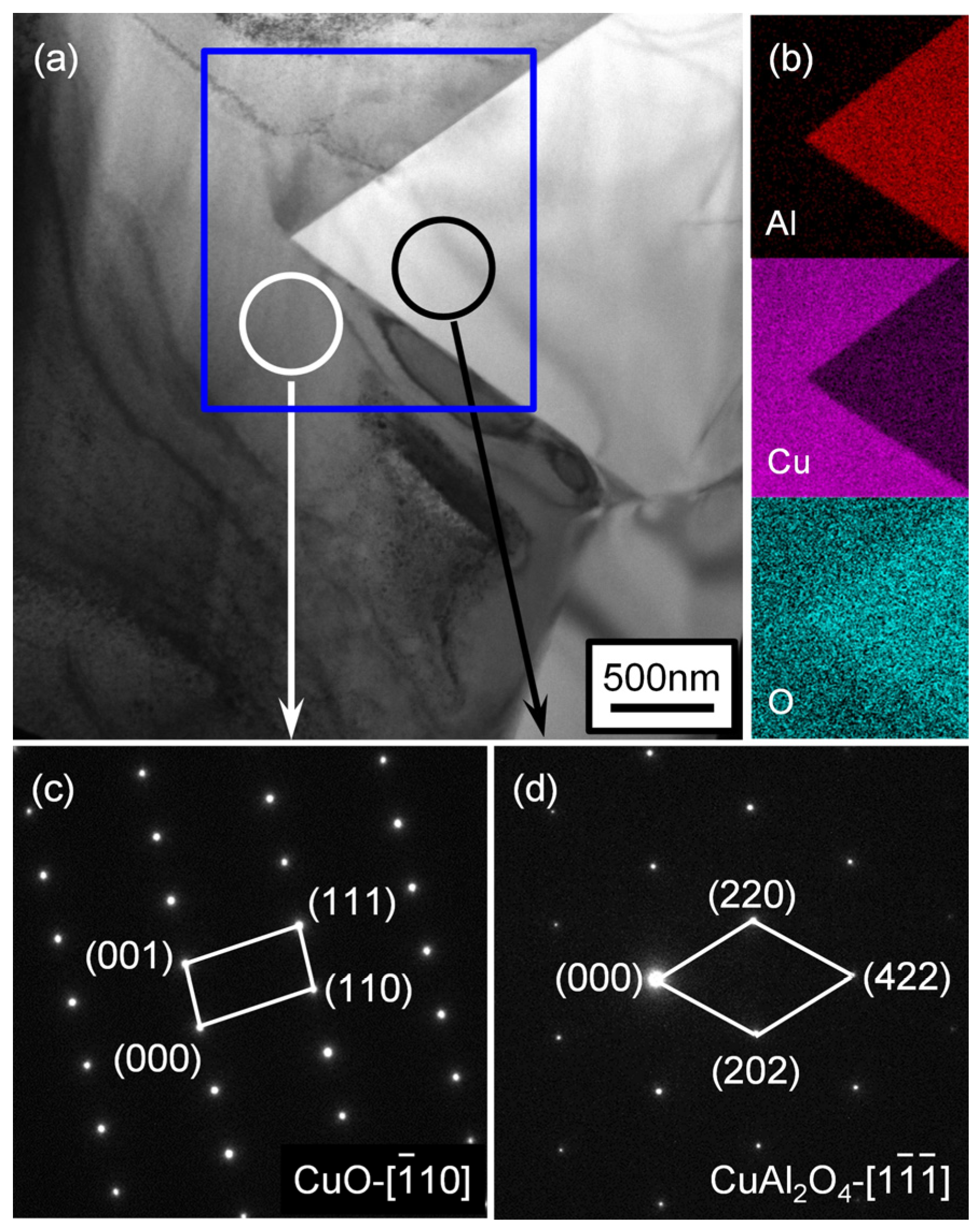
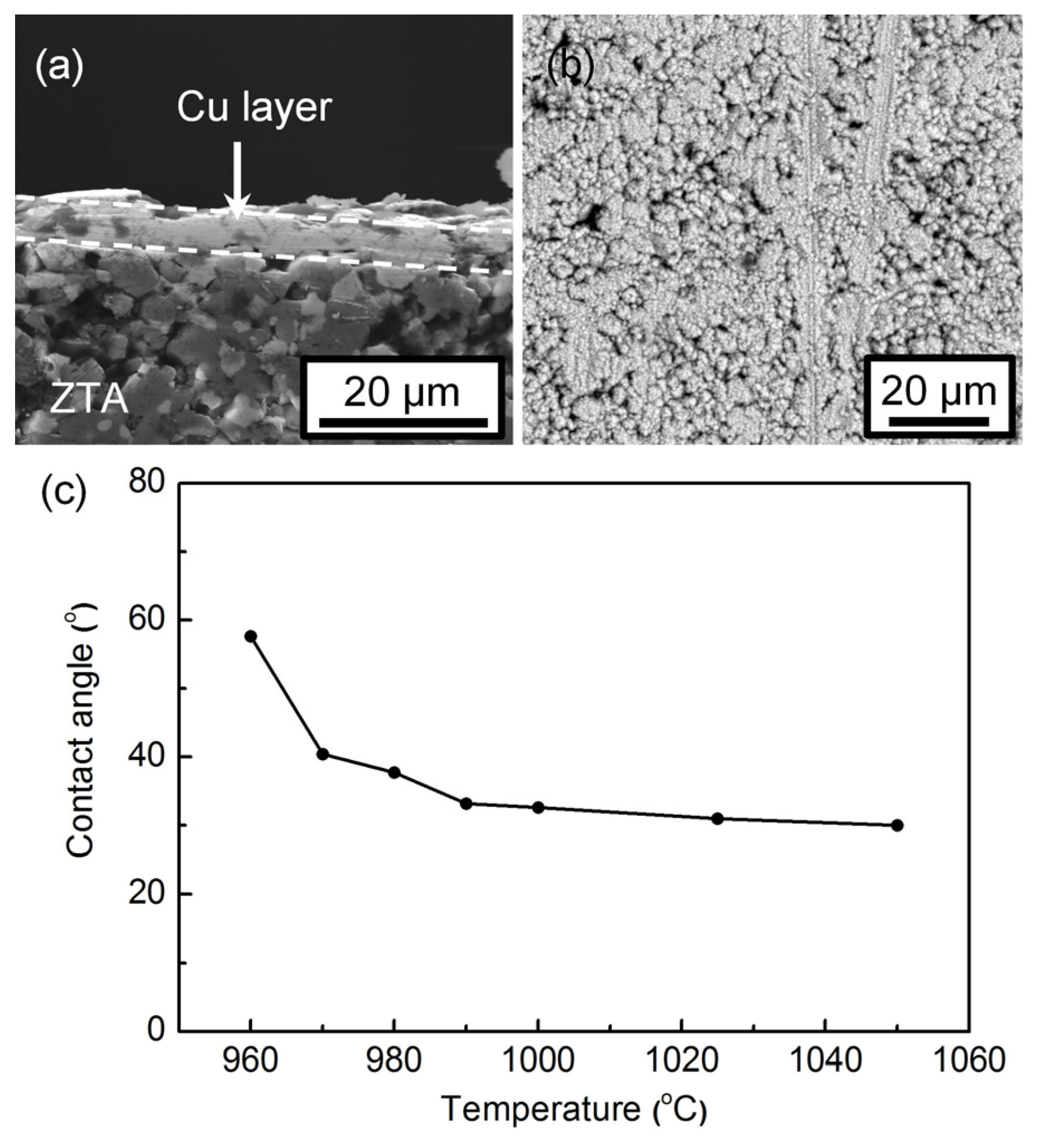
3.2. Microstructural Investigation of the Wetting Specimens
3.3. Possible Wetting Mechanism of Ag-5CuO/ZTA System
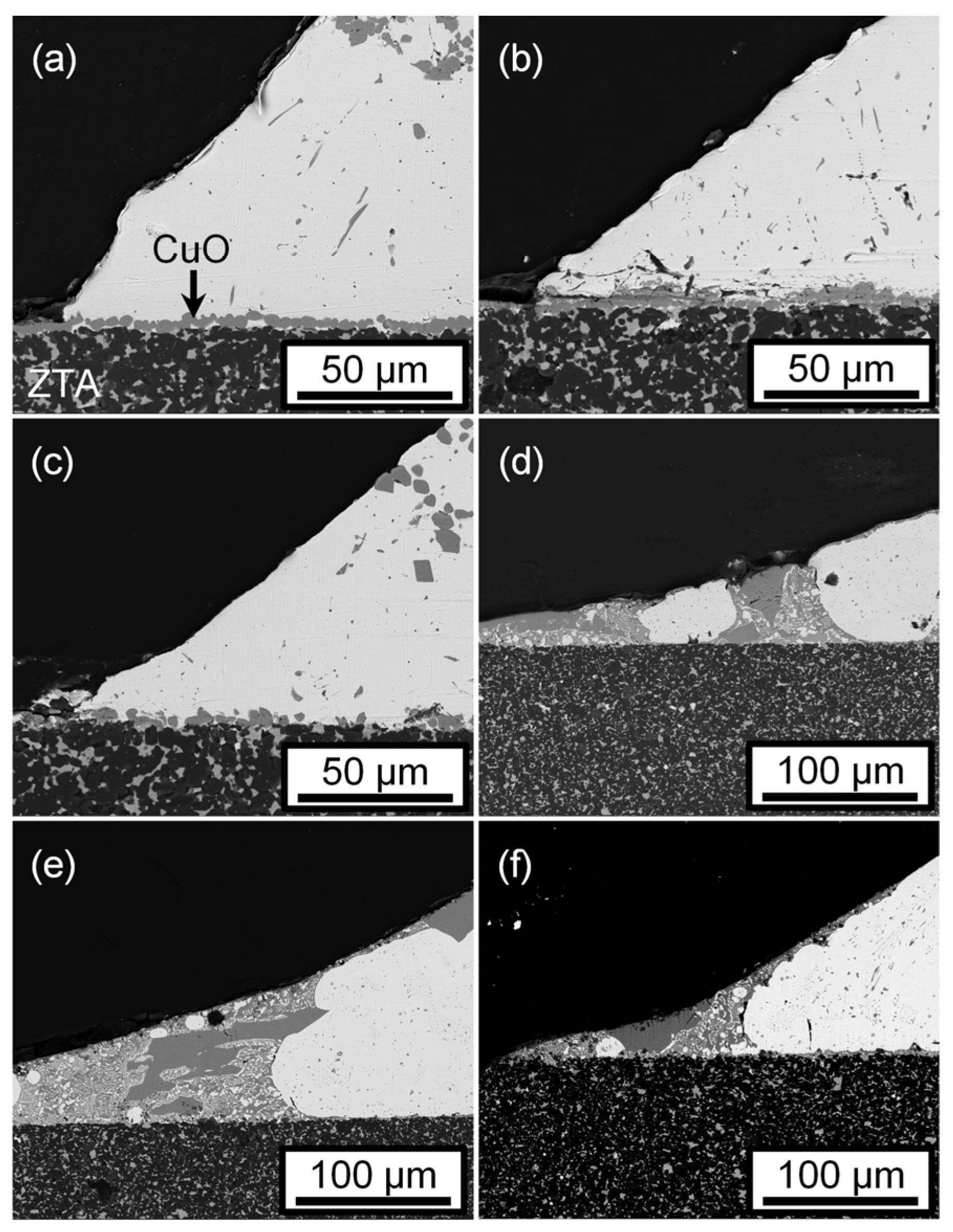
3.4. Wetting of Ag-5CuO Alloy on CuO-Coated ZTA Ceramic
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Deng, C.; Ding, J.; Zhu, H.; Yu, C. Fabrication and microstructures characterization of AlN-Al2O3 porous ceramic by nitridation of Al4O4C. Mater. Charact. 2020, 161, 110159. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, T.; Li, W. Towards structural design and thermal management of advanced 2D CMCs. Ceram. Int. 2021, 47, 4845–4853. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, W.; Sun, J.; Liu, J.; Bai, J. Development and evaluation of Al2O3-ZrO2 composite processed by digital light 3D printing. Ceram. Int. 2020, 46, 8682–8688. [Google Scholar] [CrossRef]
- Lopes, A.C.O.; Coelho, P.G.; Witek, L.; Benalcázar Jalkh, E.B.; Gênova, L.A.; Monteiro, K.N.; Cesar, P.F.; Lisboa-Filho, P.N.; Bergamo, E.T.P.; Ramalho, I.S.; et al. Microstructural, mechanical, and optical characterization of an experimental aging-resistant zirconia-toughened alumina (ZTA) composite. Dent. Mater. 2020, 36, e365–e374. [Google Scholar] [CrossRef]
- Tebaldo, V.; Gautier, G. Influences of evaluation methods and testing load on microhardness and Young’s modulus of ZTA and ATZ ceramics. Ceram. Int. 2013, 39, 2683–2693. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Microstructural evolution and characterisation of interfacial phases in Al2O3/Ag-Cu-Ti/Al2O3 braze joints. Acta Mater. 2015, 96, 143–158. [Google Scholar] [CrossRef]
- Zhou, B.F.; Wang, J.F.; Feng, K.Q.; Cai, Y.C.; Chen, S.T. Effect of brazing parameters on the microstructure and properties of SiC ceramic joint with Zr-Cu filler metal. Crystals 2020, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Nai, X.; Zhao, S.; Lu, D.; Shen, Z.; Li, W.; Cao, J. Improvement for interfacial microstructure and mechanical properties of Ti3SiC2/Cu joint brazed by Ag-Cu-Ti Filler with copper mesh. Crystals 2021, 11, 401. [Google Scholar] [CrossRef]
- Weil, K.S.; Kim, J.Y.; Hardy, J.S. Reactive air brazing: A novel method of sealing SOFCs and other solid-state electrochemical devices. Electrochem. Solid-State Lett. 2005, 8, A133–A136. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Si, X.; Li, W.; Song, X.; Feng, J. Reactive air brazing of YSZ-electrolyte and Al2O3-substrate for gas sensor sealing: Interfacial microstructure and mechanical properties. Int. J. Hydrogen Energy 2017, 42, 10683–10694. [Google Scholar] [CrossRef]
- Si, X.; Cao, J.; Ritucci, I.; Talic, B.; Feng, J.; Kiebach, R. Enhancing the long-term stability of Ag based seals for solid oxide fuel/electrolysis applications by simple interconnect aluminization. Int. J. Hydrogen Energy 2019, 44, 3063–3074. [Google Scholar] [CrossRef]
- Si, X.; Cao, J.; Song, X.; Qu, Y.; Feng, J. Reactive air brazing of YSZ ceramic with novel Al2O3 nanoparticles reinforced Ag-CuO-Al2O3 composite filler: Microstructure and joint properties. Mater. Des. 2017, 114, 176–184. [Google Scholar] [CrossRef]
- Si, X.; Cao, J.; Song, X.; Wang, Z.; Wang, Z.; Feng, J. Evolution behavior of Al2O3 nanoparticles reinforcements during reactive air brazing and its role in improving the joint strength. Mater. Des. 2017, 132, 96–104. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hardy, J.S.; Weil, K.S. Effects of CuO content on the wetting behavior and mechanical properties of a Ag-CuO braze for ceramic joining. J. Am. Ceram. Soc. 2005, 88, 2521–2527. [Google Scholar] [CrossRef]
- Joshi, V.V.; Meier, A.; Darsell, J.; Weil, K.S.; Bowden, M. Trends in wetting behavior for Ag-CuO braze alloys on Ba0.5Sr0.5Co0.8Fe0.2O(3-δ) at elevated temperatures in air. J. Mater. Sci. 2013, 48, 7153–7161. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Guo, W.; Liu, T.; Wu, C.; Ding, W.; Lu, X. Interfacial reaction and microstructural evolution of Ag-Cu braze on BaCo0.7Fe0.2Nb0.1O3-δ at high temperature in air. Ceram. Int. 2017, 43, 810–819. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, W.; Shen, Y.; Sui, Q.; Liu, Y.; Ran, X. Joining ZTA ceramic by using Dy2O3-Al2O3-SiO2 glass ceramic filler. J. Eur. Ceram. Soc. 2020, 40, 5819–5828. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, K.; Lv, S.; Chen, G.; Fu, X.; Zhou, W. Interfacial microstructure and mechanical properties of the superplastically joined TiO2-doped ZTA ceramics. Mater. Sci. Eng. A 2020, 779, 139121. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Si, X.; Qi, J.; Feng, J.; Cao, J. Brazing ZTA ceramic to TC4 alloy using the Cu foam as interlayer. Vacuum 2018, 155, 7–15. [Google Scholar] [CrossRef]
- Prevost, E.; DeMarco, A.J.; MacMichael, B.; Joshi, V.V.; Meier, A.; Hoffman, J.W.; Walker, W.J. Microstructural development and mechanical properties for reactive air brazing of ZTA to Ni alloys using Ag-CuO braze alloys. Adv. Eng. Mater. 2014, 16, 1448–1455. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhang, H.; Wen, Z.; Chang, M.; Feng, G.; Deng, D. Fabrication of reliable ZTA composite/Ti6Al4V alloy joints via vacuum brazing method: Microstructural evolution, mechanical properties and residual stress prediction. J. Eur. Ceram. Soc. 2021, 41, 4273–4283. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xu, W.; Zhang, Y.; Nie, H.Y. Quantitative calibration of conductive pattern growth via electroless copper plating at nano-resolution. Surf. Topogr. Metrol. Prop. 2020, 8, 035003. [Google Scholar] [CrossRef]
- Weil, K.; Darsell, J.; Kim, J. Evidence of a high-temperature wetting transition between Ag-CuO liquid alloys and yttria-stabilized zirconia substrates. In Interfaces in Heterogeneous Ceramic Systems: Ceramics Transactions Series; Cook, L.P., Tanaka, S.-I., Wong-Ng, W., Schwartz, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 45–54. [Google Scholar]
- Sinnamon, K.E.; Meier, A.M.; Joshi, V.V. Wetting and mechanical performance of zirconia brazed with silver/copper oxide and silver/vanadium oxide alloys. Adv. Eng. Mater. 2014, 16, 1482–1489. [Google Scholar] [CrossRef]
- Phongpreecha, T.; Nicholas, J.D.; Bieler, T.R.; Qi, Y. Computational design of metal oxides to enhance the wetting and adhesion of silver-based brazes on yttria-stabilized-zirconia. Acta Mater. 2018, 152, 229–238. [Google Scholar] [CrossRef]
- Saiz, E.; Cannon, R.M.; Tomsia, A.P. High-temperature wetting and the work of adhesion in metal/oxide systems. Annu. Rev. Mater. Res. 2008, 38, 197–226. [Google Scholar] [CrossRef]
- Muolo, M.L.; Valenza, F.; Passerone, A.; Passerone, D. Oxygen influence on ceramics wettability by liquid metals: Ag/α-Al2O3—Experiments and modelling. Mater. Sci. Eng. A 2008, 495, 153–158. [Google Scholar] [CrossRef]
- Shao, Z.B.; Liu, K.R.; Liu, L.Q.; Liu, H.K.; Dou, S. Equilibrium phase diagrams in the systems PbO-Ag and CuO-Ag. J. Am. Ceram. Soc. 1993, 76, 2663–2664. [Google Scholar] [CrossRef]
- Gauckler, L.J. Experimental phase diagram in the Ag-Cu2O-CuO System. J. Am. Ceram. Soc. 1998, 87, 2181–2187. [Google Scholar]
- Donnan, F.G.; Shaw, T.W.A. The solubility of oxygen in molten silver. J. Soc. Chem. Ind. Rev. 1910, 29, 987–989. [Google Scholar] [CrossRef] [Green Version]
- Voytovych, R.; Robaut, F.; Eustathopoulos, N. The relation between wetting and interfacial chemistry in the CuAgTi/alumina system. Acta Mater. 2006, 54, 2205–2214. [Google Scholar] [CrossRef]
- Kozlova, O.; Voytovych, R.; Eustathopoulos, N. Initial stages of wetting of alumina by reactive CuAgTi alloys. Scr. Mater. 2011, 65, 13–16. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Liu, M.; Liu, Y.; Jin, Z.; Wang, Y.; Deng, D. Wetting Behavior of the Ag-5CuO Brazing Alloy on ZTA Composite Ceramic with/without CuO Coating in Air. Crystals 2021, 11, 609. https://doi.org/10.3390/cryst11060609
Feng G, Liu M, Liu Y, Jin Z, Wang Y, Deng D. Wetting Behavior of the Ag-5CuO Brazing Alloy on ZTA Composite Ceramic with/without CuO Coating in Air. Crystals. 2021; 11(6):609. https://doi.org/10.3390/cryst11060609
Chicago/Turabian StyleFeng, Guangjie, Manqin Liu, Yalei Liu, Zhouxin Jin, Yifeng Wang, and Dean Deng. 2021. "Wetting Behavior of the Ag-5CuO Brazing Alloy on ZTA Composite Ceramic with/without CuO Coating in Air" Crystals 11, no. 6: 609. https://doi.org/10.3390/cryst11060609




