Synthesis of 1-[(Aryl)(3-amino-5-oxopyrazolidin-4-ylidene) methyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Derivatives and Their Breast Anticancer Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
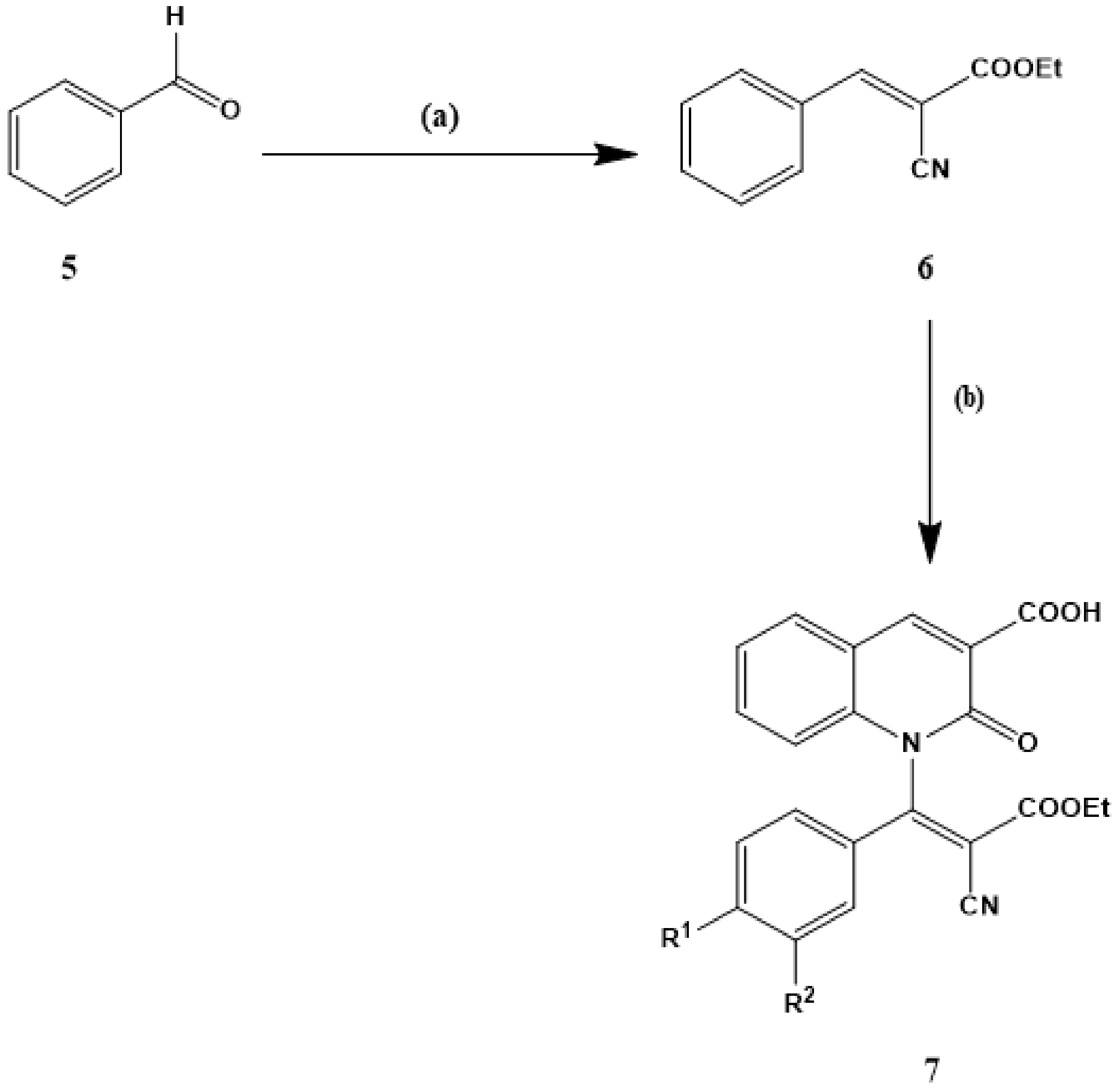
2.1.1. Synthesis of the Starting Materials (2)
2.1.2. Synthesis of 2-Oxo-1,2-dihydroquinoline-3-carboxylic acid (4)
2.1.3. General Procedures for the Preparation of Ethyl 3-aryl-2-cyanoccrylamates (6a–d)
2.1.4. General Procedures for Synthesis of 1-[2-Cyano-2-(ethoxy) carbonyl-1-arylvinyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic acid (7a–d)
2.1.5. General Procedures for Preparation of 1-[(Aryl)(3-amino-5-oxopyrazolidine-4-ylidene)methyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic acid (8a–d)
2.2. Biological Assay
2.2.1. In vitro Anticancer Effect of the Compounds 7a–d and 8a–d Against MCF-7 Cell Line
2.2.2. Cell-Cycle Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. In Vitro Cytotoxic Activity Against MCF-7 Cell Line
3.3. Cell-Cycle Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardason, J.T. Analysis of the Structure Diversity, Substitution Patterns, and Frequency of nitrogen heterocycles among U.S. FDA Approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257. [Google Scholar] [CrossRef] [PubMed]
- Lautz, T.B.; Jie, C.; Clark, S.; Naiditch, J.A.; Jafari, N.; Qiu, Y.Y.; Zheng, X.; Chu, F.; Madonna, M.B. The effect of vorinostat on the development of resistance to doxorubicin in neuroblastoma. PLoS ONE 2012, 7, e40816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Choy, M.L.; Marks, P.A. Mechanisms of Resistance to Histone Deacetylase Inhibitors. In Advances in Cancer Research; Steven, G., Ed.; Academic Press: San Diego, CA, USA, 2012; Volume 116, pp. 39–86. [Google Scholar]
- Richon, V.M. Cancer biology: Mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br. J. Cancer 2006, 95, 2–6. [Google Scholar] [CrossRef]
- Basu, H.S.; Mahlum, A.; Mehraein-Ghomi, F.; Kegel, S.J.; Guo, S.; Peters, N.R.; Wilding, G. Pre-treatment with anti-oxidants sensitizes oxidatively stressed human cancer cells to growth inhibitory effect of suberoylanilide hydroxamic acid (SAHA). Cancer Chemother. Pharmacol. 2011, 67, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, B.S.; Johnson, J.R.; He, K.; Sridhara, R.; Abraham, S.; Booth, B.P.; Verbois, L.; Morse, D.E.; Jee, J.M.; Pope, S.; et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin. Cancer Res. 2007, 13, 2318–2322. [Google Scholar] [CrossRef] [Green Version]
- Kelly, W.K.; O1Connor, O.A.; Krug, L.M.; Chiao, J.H.; Heaney, M.; Curley, T.; MacGregore-Cortelli, B.; Tong, W.; Secrist, J.P.; Schwartz, L.; et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 3923–3931. [Google Scholar] [CrossRef]
- Rundall, B.K.; Denlinger, C.E.; Jones, D.R. Combined histone deacetylase and NF-kB inhibition sensitizes non-small cell lung cancer to cell death. Surgery 2005, 138, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Chakroborty, S. Navigating the Synthesis of Quinoline Hybrid Molecules as Promising Anticancer Agents. ChemistrySelect 2020, 5, 10187–10199. [Google Scholar] [CrossRef]
- Jain, S.; Chandra, V.; Jain, P.K.; Pathak, K.; Pathak, D.; Vaidya, A. Comprehensive review on current developments of quinoline-based anticancer agents. Arab. J. Chem. 2019, 12, 4920–4946. [Google Scholar] [CrossRef] [Green Version]
- Matveeva, M.D.; Purgatorio, R.; Voskressensky, L.G.; Altomare, C.D. Pyrrolo[2,1-a]isoquinoline scaffold in drug discovery: Advances in synthesis and medicinal chemistry. Future Med. Chem. 2019, 11, 2735–2755. [Google Scholar] [CrossRef]
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 19 May 2019).
- Chen, Y.L.; Huang, C.J.; Huang, Z.Y.; Tseng, C.H.; Chang, F.S.; Yang, S.H.; Lin, S.R.; Tzeng, C.C. Synthesis and antiproliferative evaluation of certain 4-anilino-8-methoxy-2-phenylquinoline and 4-anilino-8-hydroxy-2-phenylquinoline derivatives. Bioorg. Med. Chem. 2006, 14, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Zhao, Y.L.; Lu, C.M.; Tzeng, C.C.; Wang, J.P. Synthesis, cytotoxicity, and anti-inflammatory evaluation of 2-(furan-2-yl)-4-(phenoxy)quinoline derivatives. Bioorg. Med. Chem. 2006, 14, 4373–4378. [Google Scholar] [CrossRef]
- Feng, Y.; Lau, E.; Scortegagna, M.; Ruller, C.; De, S.K.; Barile, E.; Krajewski, S.; Aza-Blanc, P.; Williams, R.; Pinkerton, A.B.; et al. Inhibition of melanoma development in the Nras(Q61K)::Ink4a−/− mouse model by the small molecule BI-69A11. Pigm. Cell Melanoma Res. 2013, 26, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Kumari, R.; Bhat, M.K.; Deshpande, M.V.; Srinivasan, K.V. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5,6,7,8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007, 15, 6705–6715. [Google Scholar] [CrossRef]
- Fakhfakh, M.A.; Fournet, A.; Prina, E.; Mouscadet, J.F.; Franck, X.; Hocquemiler, R.; Figadere, B. Synthesis and biological evaluation of substituted quinolines: Potential treatment of protozoal and retroviral co-infection. Bioorg. Med. Chem. 2003, 11, 5013. [Google Scholar] [CrossRef]
- Srivastava, B.K.R.; Joharapurkar, A.; Raval, S.; Patel, J.Z.; Soni, R.; Raval, P.; Gite, A.; Goswami, A.; Sadhwani, N.; Gandhi, N.; et al. Biarylpyrazole inverse Agonists at the Cannabinoid CBI Receptor: Importance of the C-3 Carboxamide oxygen/Lysine3.28(192) interaction. J. Med. Chem. 2007, 50, 5951. [Google Scholar]
- Kim, M.; Park, S.B. An improved synthesis of pyrimidine- and pyrazole-based acyclo-C-nucleosides as carbohydrides. Tetrahed. Lett. 2008, 49, 5080. [Google Scholar]
- Prekupec, S.; Makuc, D.; Plavec, J.; Suman, L.; Kral, M.J.; Pavelic, K.; Balzarin, J.I.; Clercq, E.D.; Mintas, M.; Malic, S. Novel C-6 fluorinated acyclic side chain pyrimidine derivatives: Synthesis, 1H and 13C NMR conformation studies, and antiviral and cytostatic evaluations. J. Med. Chem. 2007, 50, 3037. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Srivastava, k.; Puri, S.K.; Chauhan, P.M.S. Synthesis of 2,4,6-trisubstituted pyrimidinesas antimalarial agents. Bioorg. Med. Chem. Lett. 2005, 13, 4645. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Vobbalareddy, S.; Shivaramakrishna, S.; Krishnamaraju, A.; Rajjak, S.A.; Gasturi, S.R.; Akhilaband, Y.V.; Raoa, K. Methanesulfonamide group at position-4 of the C-5-phenyl ring of 1,5-diarylpyrazole affords a potent class of cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 1683. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.R.S.; Salvador, R.R.S. Pyrazole carbohydrazide derivatives of pharmaceutical interest. Pharmaceuticals 2012, 5, 317. [Google Scholar] [CrossRef] [Green Version]
- Koca, I.; Ozgur, A.; Coskun, K.A.; Tutar, A. Synthesis and anticancer activity of acylthioureas bearing pyrazole moiety. Bioorg. Med. Chem. 2013, 21, 3859. [Google Scholar] [CrossRef]
- Selim, M.; Zahran, M.; Belal, A.; Samir, M.; Shedid, S.; Mehany, A.; Elhagali, G.; Ammar, Y. Hybridized Quinoline Derivatives as Anticancer Agents: Designe, Synthesis, biological Evaluation and Molecular Docking. Anti Cancer Agents Med. Chem. 2019, 19, 439. [Google Scholar] [CrossRef]
- Pirol, S.C.; Caliskan, B.; Sahin, I.D.; Banoglu, E. Synthesis and preliminary mechanistic evaluation of 5-(p-tolyl)-1-(quinolin-2-yl)pyrazole-3-carboxylic acid amides with potent antiproliferative activity on human cancer cell lines. Eur. J. Med. Chem. 2014, 87C, 140–149. [Google Scholar] [CrossRef]
- Bekhit, A.A.; El-Sayed, O.A.; Aboul-Enein, H.Y.; Siddiqui, Y.M.; Al-Ahdal, M.N. Synthesis of aldehyde-sugar derivatives of pyrazoloquinoline as inhibitors of herpes simplex virus type 1 replication. J. Enzyme. Inhib. Med. Chem. 2004, 19, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Z.; Claassen, G.; Grogran-Grundy, C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery and structure-activity relationship of N-phenyl-1H-pyrazolo[3,4-b]quinoline-4-amines as a new series of potent apoptosis inducers. Bioorg. Med. Chem. 2008, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.; Ein Oon, C.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223. [Google Scholar] [PubMed] [Green Version]
- Zeydi, M.M.; Kalantarian, S.J.; Kazeminejad, Z. Overview on developed synthesis procedures of coumarin heterocycles. J. Iran. Chem. Soc. 2020, 17, 3031–3094. [Google Scholar] [CrossRef]
- Torres, K.; Horwitz, S.B. Mechanisms of taxol-induced cell death are concentration dependent. Cancer Res. 1998, 58, 3620–3626. [Google Scholar] [PubMed]
- Murray, A.W. Recycling the cell cycle: Cyclins revisited. Cell 2004, 116, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Zaki, I.; Abdelhameid, M.K.; El-Deen, I.M.; Abdel Wahab, A.H.A.; Ashmawy, A.M.; Mohamed, K.O. Design, synthesis and screening of 1,2,4-triazinone derivatives as potential antitumor agents with apoptosis inducing activity on MCF-7 breast cancer cell line. Eur. J. Med. Chem. 2018, 56, 563. [Google Scholar] [CrossRef] [PubMed]



| Compound | IC50 Values (μM)/MCF-7 |
|---|---|
| 7a | >50 |
| 7b | 3.87 ± 0.33 c |
| 7c | 1.73 ± 0.27 a |
| 7d | >50 |
| 8a | 17.32 ± 0.44 e |
| 8b | 5.67 ± 0.21 d |
| 8c | 4.03 ± 0.60 c |
| 8d | >50 |
| Dox | 2.82 ± 0.07 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaber, A.; Alsanie, W.F.; Alhomrani, M.; Alamri, A.S.; El-Deen, I.M.; Refat, M.S. Synthesis of 1-[(Aryl)(3-amino-5-oxopyrazolidin-4-ylidene) methyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Derivatives and Their Breast Anticancer Activity. Crystals 2021, 11, 571. https://doi.org/10.3390/cryst11050571
Gaber A, Alsanie WF, Alhomrani M, Alamri AS, El-Deen IM, Refat MS. Synthesis of 1-[(Aryl)(3-amino-5-oxopyrazolidin-4-ylidene) methyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Derivatives and Their Breast Anticancer Activity. Crystals. 2021; 11(5):571. https://doi.org/10.3390/cryst11050571
Chicago/Turabian StyleGaber, Ahmed, Walaa F. Alsanie, Majid Alhomrani, Abdulhakeem S. Alamri, Ibrahim M. El-Deen, and Moamen S. Refat. 2021. "Synthesis of 1-[(Aryl)(3-amino-5-oxopyrazolidin-4-ylidene) methyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Derivatives and Their Breast Anticancer Activity" Crystals 11, no. 5: 571. https://doi.org/10.3390/cryst11050571
APA StyleGaber, A., Alsanie, W. F., Alhomrani, M., Alamri, A. S., El-Deen, I. M., & Refat, M. S. (2021). Synthesis of 1-[(Aryl)(3-amino-5-oxopyrazolidin-4-ylidene) methyl]-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Derivatives and Their Breast Anticancer Activity. Crystals, 11(5), 571. https://doi.org/10.3390/cryst11050571








