Facile Charge Transfer between Barbituric Acid and Chloranilic Acid over g-C3N4: Synthesis, Characterization and DFT Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Preparation of Solid Complexes and Nanocomposite
2.3. Computational Details
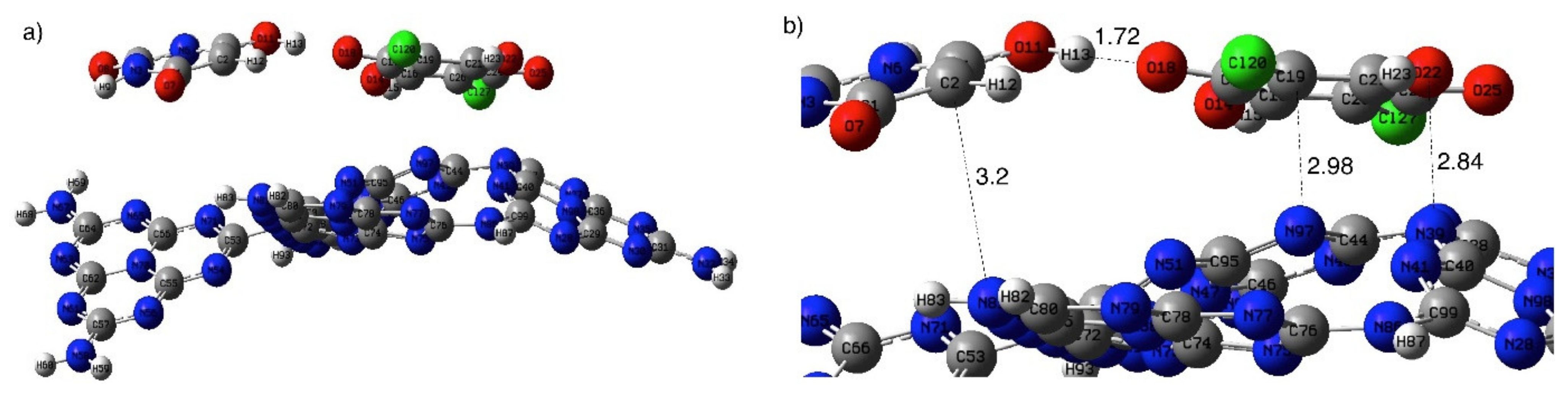
3. Results and Discussion
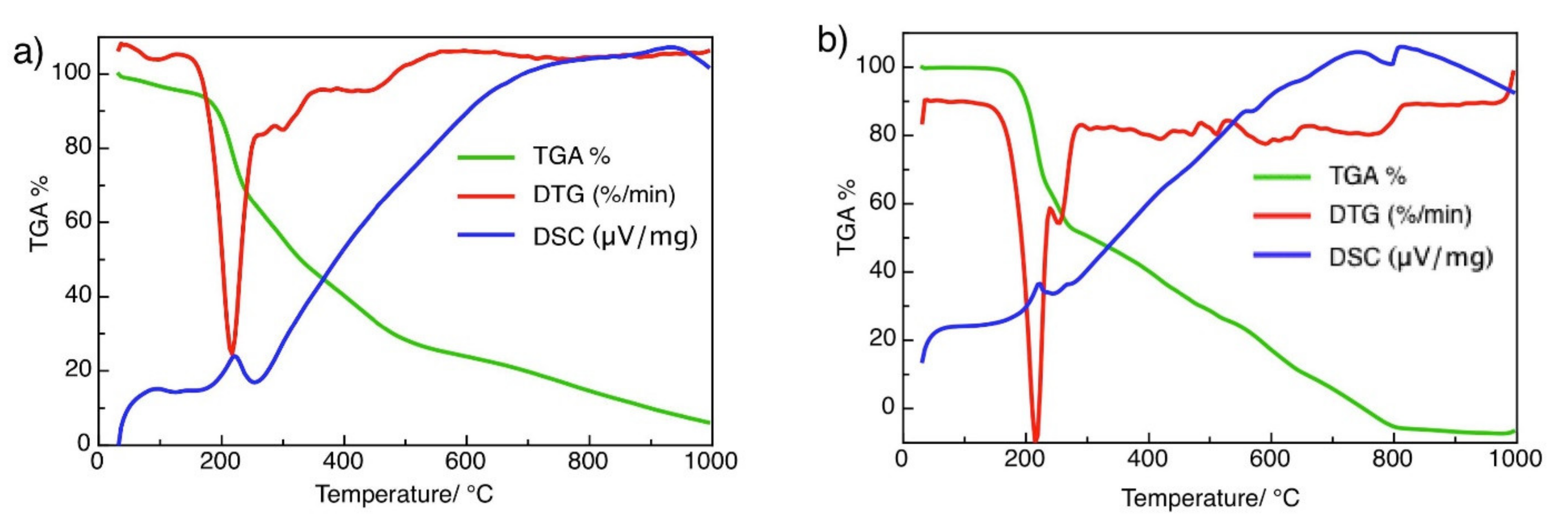
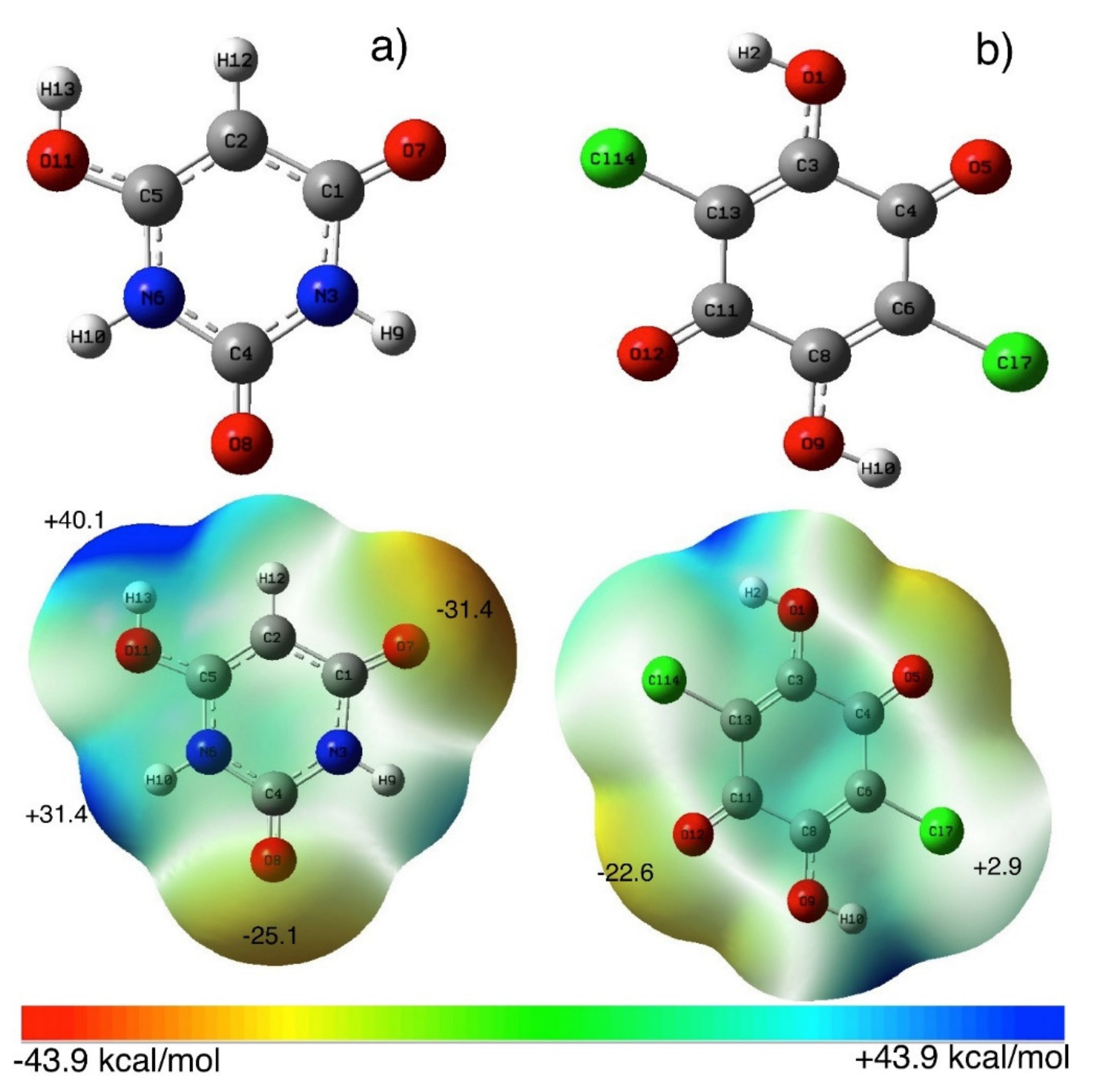
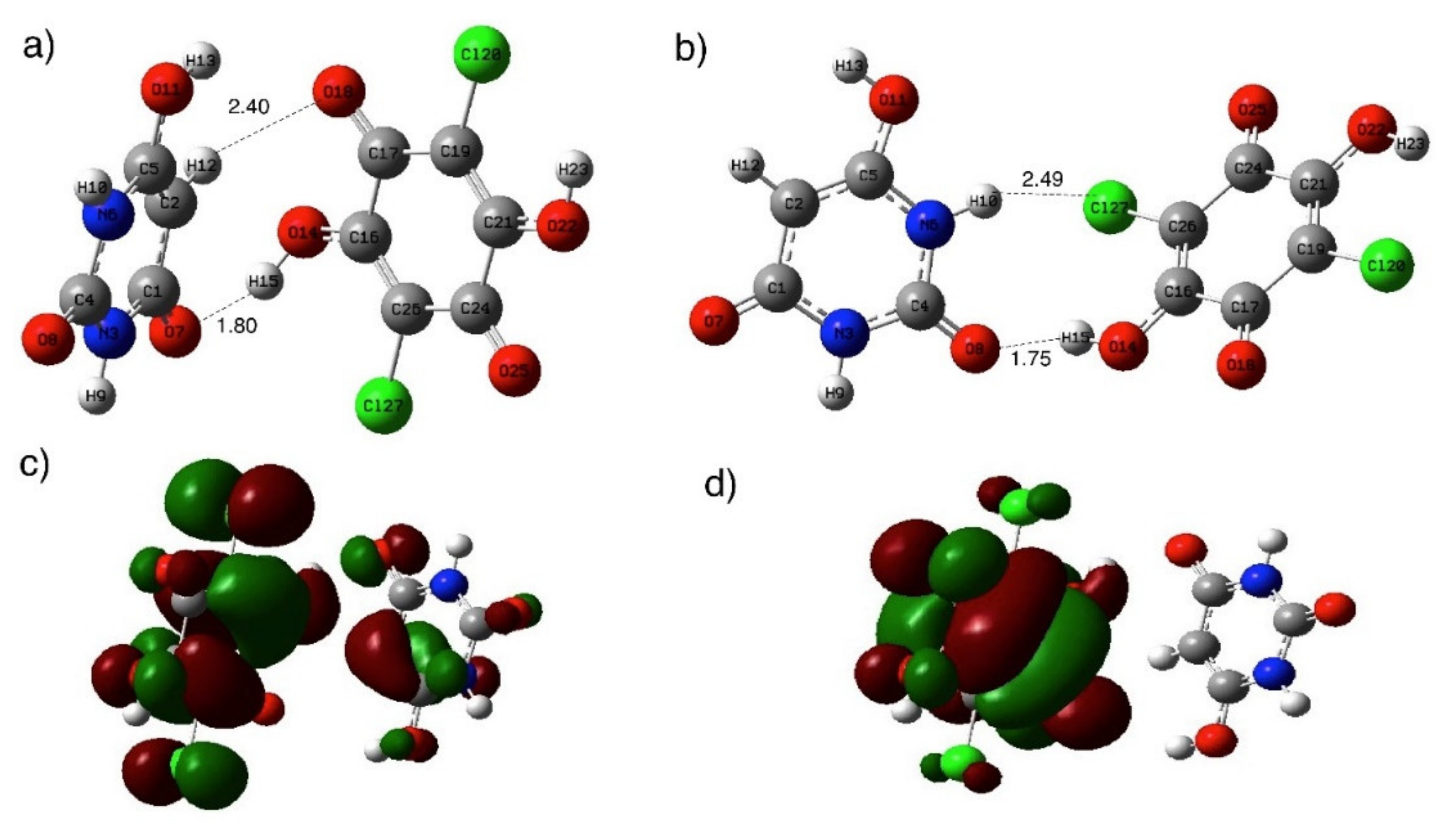
3.1. Spectroscopic Properties and Structure Characterization of BUChA
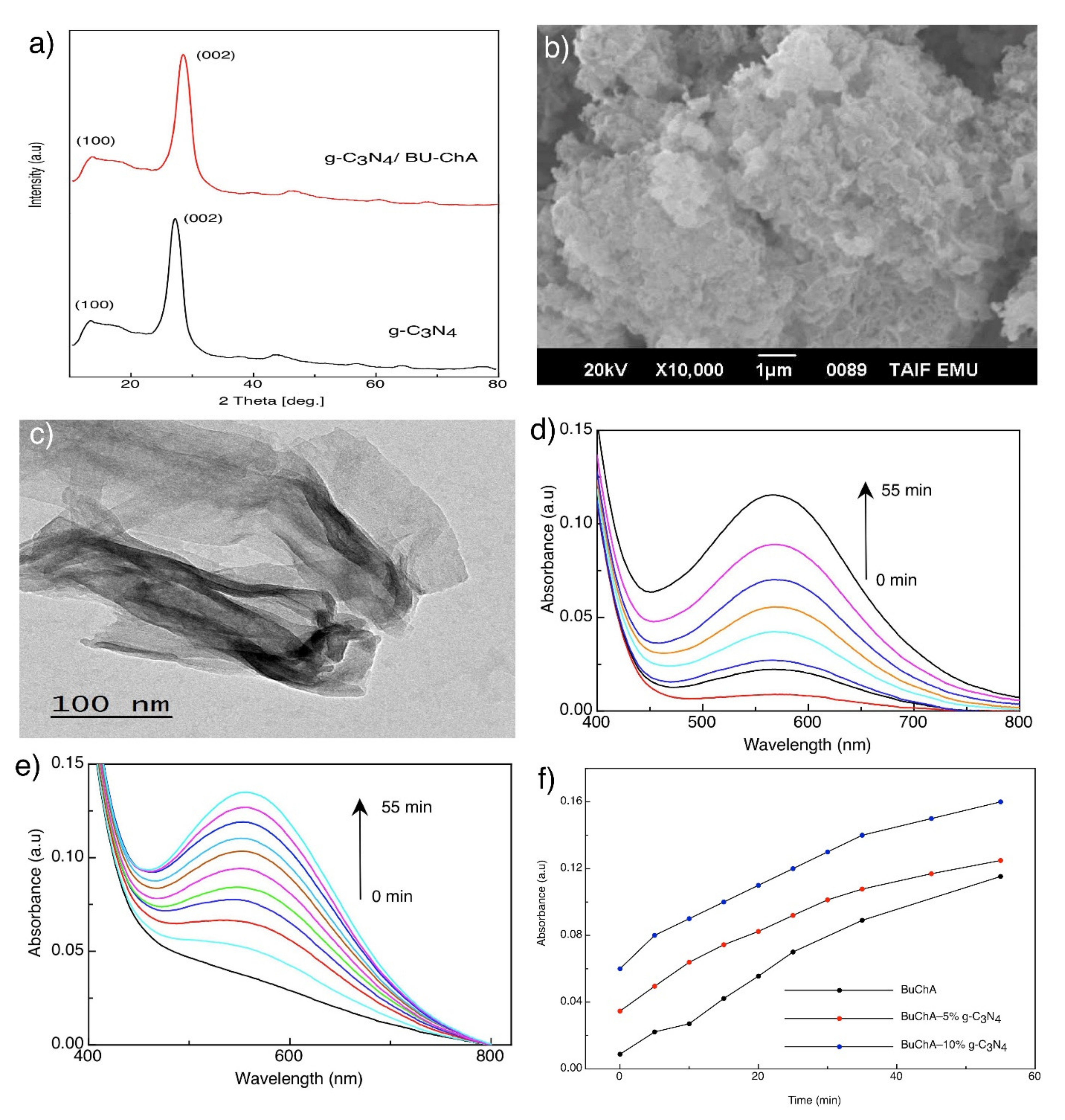
3.2. Immobilization of BUChA over g-C3N4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uekusa, T.; Sato, R.; Yoo, D.; Kawamoto, T.; Mori, T. Transistor Characteristics of Charge-Transfer Complexes Observed across a Neutral–Ionic Transition. ACS Appl. Mater. Interfaces 2020, 12, 24174–24183. [Google Scholar] [CrossRef]
- Gélvez-Rueda, M.C.; Van Gompel, W.T.M.; Herckens, R.; Lutsen, L.; Vanderzande, D.; Grozema, F.C. Inducing Charge Separation in Solid-State Two-Dimensional Hybrid Perovskites through the Incorporation of Organic Charge-Transfer Complexes. J. Phys. Chem. Lett. 2020, 11, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Barrett, B.J.; Saund, S.S.; Dziatko, R.A.; Clark-Winters, T.L.; Katz, H.E.; Bragg, A.E. Spectroscopic Studies of Charge-Transfer Character and Photoresponses of F4TCNQ-Based Donor–Acceptor Complexes. J. Phys. Chem. C 2020, 124, 9191–9202. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Chan, A.K.-W.; Hong, E.Y.-H. Charge-transfer processes in metal complexes enable luminescence and memory functions. Nat. Rev. Chem. 2020, 4, 528–541. [Google Scholar] [CrossRef]
- Liu, Y.; Atanassov, P. Charge transfer at biotic/abiotic interfaces in biological electrocatalysis. Curr. Opin. Electrochem. 2020, 19, 175–183. [Google Scholar] [CrossRef]
- Poli, E.; Jong, K.H.; Hassanali, A. Charge transfer as a ubiquitous mechanism in determining the negative charge at hydrophobic interfaces. Nat. Commun. 2020, 11, 901. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chu, M.; Fan, J.-X.; Lau, T.-K.; Ren, A.-M.; Lu, X.; Miao, Q. Crystal engineering of biphenylene-containing acenes for high-mobility organic semiconductors. J. Am. Chem. Soc. 2019, 141, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Yang, F.; Zhang, X.; Hu, W. Cocrystal engineering: A collaborative strategy toward functional materials. Adv. Mater. 2019, 31, 1902328. [Google Scholar] [CrossRef] [PubMed]
- Goetz, K.P.; Vermeulen, D.; Payne, M.E.; Kloc, C.; McNeil, L.E.; Jurchescu, O.D. Charge-transfer complexes: New perspectives on an old class of compounds. J. Mater. Chem. C 2014, 2, 3065–3076. [Google Scholar] [CrossRef]
- Bender, C.J. Theoretical models of charge-transfer complexes. Chem. Soc. Rev. 1986, 15, 475–502. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Ibrahim, M.M.; Aly, M.R.E.; El-Kemary, M. Spectroscopic and structure investigation of the molecular complexes of tris (2-aminoethyl) amine with π-acceptors. J. Mol. Liq. 2016, 213, 82–91. [Google Scholar] [CrossRef]
- Karmakar, A.; Singh, B. Spectroscopic analysis and theoretical investigation of hydrogen bonding interaction of quercetin with different acceptor molecules. J. Mol. Struct. 2019, 1180, 698–707. [Google Scholar] [CrossRef]
- Al-Ahmary, K.M.; Habeeb, M.M.; Aljahdali, S.H. Synthesis, spectroscopic characterization and DFT/TD-DFT computations of a novel charge transfer complex via hydrogen bonding between 3-amino-1, 5-dimethylpyrazole with chloranilic acid in different solvents. J. Mol. Struct. 2019, 1181, 48–60. [Google Scholar] [CrossRef]
- Karmakar, A.; Banerjee, S.; Singh, B.; Mandal, N.C. Study of hydrogen bonding interaction of acridine orange with different acceptor molecules by spectroscopic, theoretical, and antimicrobial studies. J. Mol. Struct. 2019, 1177, 418–429. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Ibrahim, M.M.; El-Mehasseb, I.; El-Kemary, M. Spectrochim Acta. A Mol. Biomol. Spectrosc. 2015, 143, 120–127. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, H.S.; Salman, H.M.A.; El-Kemary, M. Orthogonal hydrogen/halogen bonding in 1-(2-methoxyphenyl)-1H-imidazole-2 (3H)-thione-I2 adduct: An experimental and theoretical study. Spectrochim Acta. A Mol. Biomol. Spectrosc. 2015, 137, 442–449. [Google Scholar] [CrossRef]
- Borley, W.; Watson, B.; Nizhnik, Y.P.; Zeller, M.; Rosokha, S.V. Complexes of Diiodine with Heteroaromatic N-Oxides: Effects of Halogen-Bond Acceptors in Halogen Bonding. J. Phys. Chem. A 2019, 123, 7113–7123. [Google Scholar] [CrossRef]
- Sharada, D.; Saha, A.; Saha, B.K. Charge transfer complexes as colour changing and disappearing–reappearing colour materials. New J. Chem. 2019, 43, 7562–7566. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, L.-K.; Zhang, Q.; He, J.-W.; Feng, H.-J.; Würthner, F.; Yang, X.-J.; Wu, B. Anion-Coordination-Assisted Assembly of Supramolecular Charge-Transfer Complexes Based on Tris (urea) Ligands. Chem. A Eur. J. 2020, 26, 1414–1421. [Google Scholar] [CrossRef]
- El-Dissouky, A.; Khalil, T.E.; Elbadawy, H.A.; El-Sayed, D.S.; Attia, A.A.; Foro, S. X-ray crystal structure, spectroscopic and DFT computational studies of H-bonded charge transfer complexes of tris (hydroxymethyl) aminomethane (THAM) with chloranilic acid (CLA). J. Mol. Struct. 2020, 1200, 127066. [Google Scholar] [CrossRef]
- Basha, M.T.; Alghanmi, R.M.; Soliman, S.M.; Alharby, W.J. Synthesis, spectroscopic, thermal, structural characterization and DFT/TD-DFT computational studies for charge transfer complexes of 2, 4-diamino pyrimidine with some benzoquinone acceptors. J. Mol. Liq. 2020, 309, 113210. [Google Scholar] [CrossRef]
- Cheng, T.; Shen, D.X.; Meng, M.; Mallick, S.; Cao, L.; Patmore, N.J.; Zhang, H.L.; Zou, S.F.; Chen, H.W.; Qin, Y.; et al. Efficient electron transfer across hydrogen bond interfaces by proton-coupled and-uncoupled pathways. Nat. Commun. 2019, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Amdursky, N.; Lin, Y.; Aho, N.; Groenhof, G. Exploring fast proton transfer events associated with lateral proton diffusion on the surface of membranes. Proc. Natl. Acad. Sci. USA 2019, 116, 2443–2451. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J.P. Proton circuits in biological energy interconversions. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.Y.; Heberle, J.; Cherepanov, D.A. Protons@ interfaces: Implications for biological energy conversion. Biochim. Biophys. Acta 2006, 1757, 913–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Stahl, S.S. Electrochemical oxidation of organic molecules at lower overpotential: Accessing broader functional group compatibility with electron− proton transfer mediators. Acc. Chem. Res. 2020, 53, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Mo, F.; Yang, Q.; Huang, Z.; Li, X.; Wang, D.; Liu, Z.; Li, H.; Zhang, Q.; Zhi, C. Commencing an acidic battery based on a copper anode with ultrafast proton-regulated kinetics and superior dendrite-free property. Adv. Mater. 2019, 31, 1905873. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Wang, F.; Yu, H. Effect of K2NbF7 on the hydrogen release behaviour of NaAlH4. Appl. Catal. B Environ. 2019, 247, 70–77. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y. A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Li, R.; Xian, T. Design of ternary CaTiO3/g-C3N4/AgBr Z-scheme heterostructured photocatalysts and their application for dye photodegradation. Solid State Sci. 2020, 100, 106102. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, H.; Yu, Y.; Chen, Y.; Yan, J.; Li, X.; Zhang, H. Trithiocyanuric acid derived g–C3N4 for anchoring the polysulfide in Li–S batteries application. J. Energy Chem. 2020, 43, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Li, X.; Yin, C.; Wang, J.; Aslam, M.S.; Qi, H.; Cao, Y.; Jin, J.; Cui, L. Three-dimensional mesoporous sandwich-like g-C3N4-interconnected CuCo2O4 nanowires arrays as ultrastable anode for fast lithium storage. J. Colloid Interface Sci. 2019, 554, 269–277. [Google Scholar] [CrossRef]
- Luan, X.; Wang, C.; Wang, C.; Gu, X.; Yang, J.; Qian, Y. Stable lithium deposition enabled by an acid-treated g-C3N4 interface layer for a lithium metal anode. ACS Appl. Mater. Interfaces 2020, 12, 11265–11272. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, J.; Zhang, G. Ag-bridged Z-scheme 2D/2D Bi5FeTi3O15/g-C3N4 heterojunction for enhanced photocatalysis: Mediator-induced interfacial charge transfer and mechanism insights. ACS Appl. Mater. Interfaces 2019, 11, 27686–27696. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Li, J.; Zhang, G. Boosting interfacial charge separation of Ba5Nb4O15/g-C3N4 photocatalysts by 2D/2D nanojunction towards efficient visible-light driven H2 generation. Appl. Catal. B Environ. 2020, 263, 117730. [Google Scholar] [CrossRef]
- Gobouri, A.A.; Altalhi, T.; Alkhaldi, H.H.; El-Shishtawy, R.M.; Ibrahim, M.M.; El-Sheshtawy, H.S. Orthogonal hydrogen and halogen bonding facilitate intermolecular charge transfer between barbaturic acid and molecular halogens over g-C3N4 nanosheet: A comparative experimental and DFT calculations. J. Mol. Struct. 2021, 1223, 129211. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.; Guo, S.; Cheng, K.; Xu, J.; Zhang, N. Interfacial charge-transfer transitions enhanced photocatalytic activity of TCNAQ/g-C3N4 organic hybrid material. Mater. Lett. 2019, 255, 126546. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Anusuyadevi, P.R.; Riazanova, A.V.; Hedenqvist, M.S.; Svagan, A.J. Floating Photocatalysts for Effluent Refinement Based on Stable Pickering Cellulose Foams and Graphitic Carbon Nitride (g-C3N4). ACS Omega 2020, 5, 22411–22419. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Wang, Z.; Zhu, Y. Enhanced visible photocatalytic oxidation activity of perylene diimide/g-C3N4 nn heterojunction via π-π interaction and interfacial charge separation. Appl. Catal. B Environ. 2020, 271, 118933. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Liu, Y.; Yang, P. Effect of nonmetal element dopants on photo-and electro-chemistry performance of ultrathin g-C3N4 nanosheets. Int. J. Hydrogen Energy 2020, 45, 16519–16527. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M.; et al. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09. 2009. Available online: https://gaussian.com/g09citation/ (accessed on 19 April 2021).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. Comput. J. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, J.; Jiang, C.; Cheng, B.; Yu, J. First principle investigation of halogen-doped monolayer g-C3N4 photocatalyst. Appl. Catal. B Environ. 2017, 207, 27–34. [Google Scholar] [CrossRef]
- Chen, Q.; Dou, H.; Zheng, S.; Rao, X.; Zhang, Y. Photocatalytic H2 evolution and MB degradation over nickel-doped graphitic carbon nitride microwires under visible light irradiation. J. Photochem. Photobiol. A Chem. 2019, 382, 111931. [Google Scholar] [CrossRef]
- AlRabiah, H.; Abdel-Aziz, H.A.; Mostafa, G.A.E. Charge transfer complexes of brucine with chloranilic acid, 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone and tetracyanoquinodimethane: Synthesis, spectroscopic characterization and antimicrobial activity. J. Mol. Liq. 2019, 286, 110754. [Google Scholar] [CrossRef]
- Alghanmi, R.M.; Soliman, S.M.; Basha, M.T.; Habeeb, M.M. Electronic spectral studies and DFT computational analysis of hydrogen bonded charge transfer complexes between chloranilic acid and 2, 5-dihydroxy-p-benzoquinone with 2-amino-4-methylbenzothiazole in methanol. J. Mol. Liq. 2018, 256, 433–444. [Google Scholar] [CrossRef]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chim. Phys. 1928, 9, 113–203. [Google Scholar]
- Abu-Eittah, R.; El-Kourashy, A. Intermolecular charge-transfer studies. Thioamide-iodine system. J. Phys. Chem. 1972, 76, 2405–2409. [Google Scholar] [CrossRef]
- Refat, M.S.; Saad, H.A.; Adam, A.M.A. Proton transfer complexes based on some π-acceptors having acidic protons with 3-amino-6-[2-(2-thienyl) vinyl]-1, 2, 4-triazin-5 (4H)-one donor: Synthesis and spectroscopic characterizations. J. Mol. Struct. 2011, 995, 116–124. [Google Scholar] [CrossRef]
- Soayed, A.A.; Refaat, L.; Sinha, H.M. Syntheses, structural elucidation, thermal properties, theoretical quantum chemical studies (DFT) and biological studies of barbituric–hydrazone complexes. J. Saudi Chem. Soc. 2015, 19, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.U.; Brüning, J.; Glinnemann, J.; Hützler, M.W.; Mörschel, P.; Ivashevskaya, S.N.; van de Streek, J.; Braga, D.; Maini, L.; Chierotti, M.R.; et al. The thermodynamically stable form of solid barbituric acid: The enol tautomer. Angew. Chem. Int. Ed. 2011, 50, 7924–7926. [Google Scholar] [CrossRef]
- Jamadar, A.; Das, A. A pH-responsive graftable supramolecular polymer with tailorable surface functionality by orthogonal halogen bonding and hydrogen bonding. Polym. Chem. 2020, 11, 385–392. [Google Scholar] [CrossRef]
- He, Y.; Cai, J.; Li, T.; Wu, Y.; Lin, H.; Zhao, L.; Luo, M. Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chem. Eng. J. 2013, 215–216, 721–730. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; El-Hosainy, H.M.; Shoueir, K.R.; El-Mehasseb, I.M.; El-Kemary, M. Facile immobilization of Ag nanoparticles on g-C3N4/V2O5 surface for enhancement of post-illumination, catalytic, and photocatalytic activity removal of organic and inorganic pollutants. Appl. Surf. Sci. 2019, 467–468, 268–276. [Google Scholar] [CrossRef]
- Ma, Z.; Cui, Z.; Lv, Y.; Sa, R.; Wu, K.; Li, Q. Three-in-One: Opened Charge-transfer channel, positively shifted oxidation potential, and enhanced visible light response of g-C3N4 photocatalyst through K and S Co-doping. Int. J. Hydrogen Energy 2020, 45, 4534–4544. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yu, Z.; Jiang, X.; Chen, J.; Tao, L.; Wang, M.; Shen, Y. Artificial photosynthesis of ethanol using type-II g-C3N4/ZnTe heterojunction in photoelectrochemical CO2 reduction system. Nano Energy 2019, 60, 827–835. [Google Scholar] [CrossRef]


| System | KCT | εCT | EHOMO | ELUMO | Egap | C/e | |
|---|---|---|---|---|---|---|---|
| BUChA-I | BUChA-II | ||||||
| BU | – | – | −4.04 | −1.05 | 2.99 | 0.0 | 0.0 |
| BUChA | 4.2 × 103 | 3.5 × 105 | −4.10 | −1.96 | 2.36 | +0.21 | +0.13 |
| BUChA-g-C3N4 | – | – | −5.13 | −2.9 | 2.23 | +0.18 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mersal, G.A.M.; Ibrahim, M.M.; Amin, M.A.; Mezni, A.; Mostafa, N.Y.; Alharthi, S.; Boukherroub, R.; El-Sheshtawy, H.S. Facile Charge Transfer between Barbituric Acid and Chloranilic Acid over g-C3N4: Synthesis, Characterization and DFT Study. Crystals 2021, 11, 636. https://doi.org/10.3390/cryst11060636
Mersal GAM, Ibrahim MM, Amin MA, Mezni A, Mostafa NY, Alharthi S, Boukherroub R, El-Sheshtawy HS. Facile Charge Transfer between Barbituric Acid and Chloranilic Acid over g-C3N4: Synthesis, Characterization and DFT Study. Crystals. 2021; 11(6):636. https://doi.org/10.3390/cryst11060636
Chicago/Turabian StyleMersal, Gaber A. M., Mohamed M. Ibrahim, Mohammed A. Amin, Amine Mezni, Nasser Y. Mostafa, Sarah Alharthi, Rabah Boukherroub, and Hamdy S. El-Sheshtawy. 2021. "Facile Charge Transfer between Barbituric Acid and Chloranilic Acid over g-C3N4: Synthesis, Characterization and DFT Study" Crystals 11, no. 6: 636. https://doi.org/10.3390/cryst11060636
APA StyleMersal, G. A. M., Ibrahim, M. M., Amin, M. A., Mezni, A., Mostafa, N. Y., Alharthi, S., Boukherroub, R., & El-Sheshtawy, H. S. (2021). Facile Charge Transfer between Barbituric Acid and Chloranilic Acid over g-C3N4: Synthesis, Characterization and DFT Study. Crystals, 11(6), 636. https://doi.org/10.3390/cryst11060636





