A Computational Validation of Water Molecules Adsorption on an NaCl Surface
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Adsorption of Water Monomer on an NaCl(001) Surface
3.2. Adsorption of Water Tetramer on an NaCl(001) Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaelides, A.; Ranea, V.A.; de Andres, P.L.; King, D.A. First-principles study of H2O diffusion on a metal surface: H2O on Al{100}. Phys. Rev. Lett. 2003, 90, 216102. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Xu, L.F.; Wang, E.G.; Gao, S. Vibrational Recognition of Hydrogen-Bonded Water Networks on a Metal Surface. Phys. Rev. Lett. 2002, 89, 176104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.; Wang, E.G.; Gao, S. A molecular picture of hydrophilic and hydrophobic interactions from ab initio density functional theory calculations. J. Chem. Phys. 2003, 119, 7617. [Google Scholar] [CrossRef]
- Feibelman, P.J. Partial Dissociation of Water on Ru(0001). Science 2002, 295, 99. [Google Scholar] [CrossRef]
- Morgenstern, K.; Nieminen, J. Intermolecular Bond Length of Ice on Ag(111). Phys. Rev. Lett. 2002, 88, 066102. [Google Scholar] [CrossRef]
- Ogasawara, H.; Brena, B.; Nordlund, D.; Nyberg, M.; Pelmenschikov, A.; Pettersson, L.G.M.; Nilsson, A. Structure and Bonding of Water on Pt(111). Phys. Rev. Lett. 2002, 89, 276102. [Google Scholar] [CrossRef]
- Wassermann, B.; Mirbt, S.; Reif, J.; Zink, J.C.; Matthias, E. Clustered water adsorption on the NaCl(100) surface. J. Chem. Phys. 1993, 98, 10049. [Google Scholar] [CrossRef]
- Allouche, A. Water adsorption on NaCl(100): A quantum ab-initio cluster calculation. Surf. Sci. 1998, 406, 279. [Google Scholar] [CrossRef]
- Meyer, H.; Entel, P.; Hafner, J. Physisorption of water on salt surfaces. Surf. Sci. 2001, 488, 177. [Google Scholar] [CrossRef]
- Bruch, L.W.; Glebov, A.; Toennies, J.P.; Weiss, H. A helium atom scattering study of water adsorption on the NaCl(100) single crystal surface. J. Chem. Phys. 2001, 103, 5109. [Google Scholar] [CrossRef]
- Verdaguer, A.; Sacha, G.M.; Luna, M.; Ogletree, D.F.; Salmeron, M. Initial stages of water adsorption on NaCl(100) studied by scanning polarization force microscopy. J. Chem. Phys. 2005, 123, 124703. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Meng, X.; Chen, J.; Peng, J.; Sheng, J.; Li, X.-Z.; Xu, L.; Shi, J.-R.; Wang, E.; Jiang, Y. Real-space imaging of interfacial water with submolecular resolution. Nat. Mater. 2014, 13, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Goniakowski, J.; Suzanne, J. Partial Dissociation of Water Molecules in the (3×2) Water Monolayer Deposited on the MgO (100). Surf. Phys. Rev. Lett. 1998, 81, 1271. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, S.; Wang, E.G. Water adsorption on a NaCl (001) surface: A density functional theory study. Phys. Rev. B 2006, 74, 245409. [Google Scholar] [CrossRef]
- Ma, R.; Cao, D.; Zhu, C.; Tian, Y.; Peng, J.; Guo, J.; Chen, J.; Li, X.Z.; Francisco, J.S.; Zeng, X.C. Atomic imaging of the edge structure and growth of a two-dimensional hexagonal ice. Nature 2020, 577, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Vitek, A.; Kalus, R.; Paidarova, I. Structural changes in the water tetramer. A combined Monte Carlo and DFT study. Phys. Chem. Chem. Phys. PCCP 2010, 12, 13657–13666. [Google Scholar] [CrossRef] [PubMed]
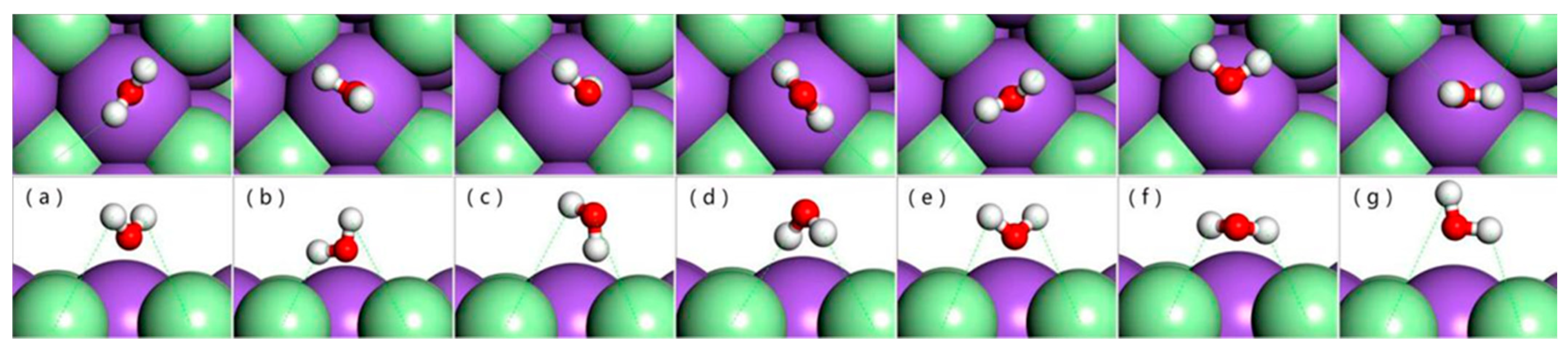
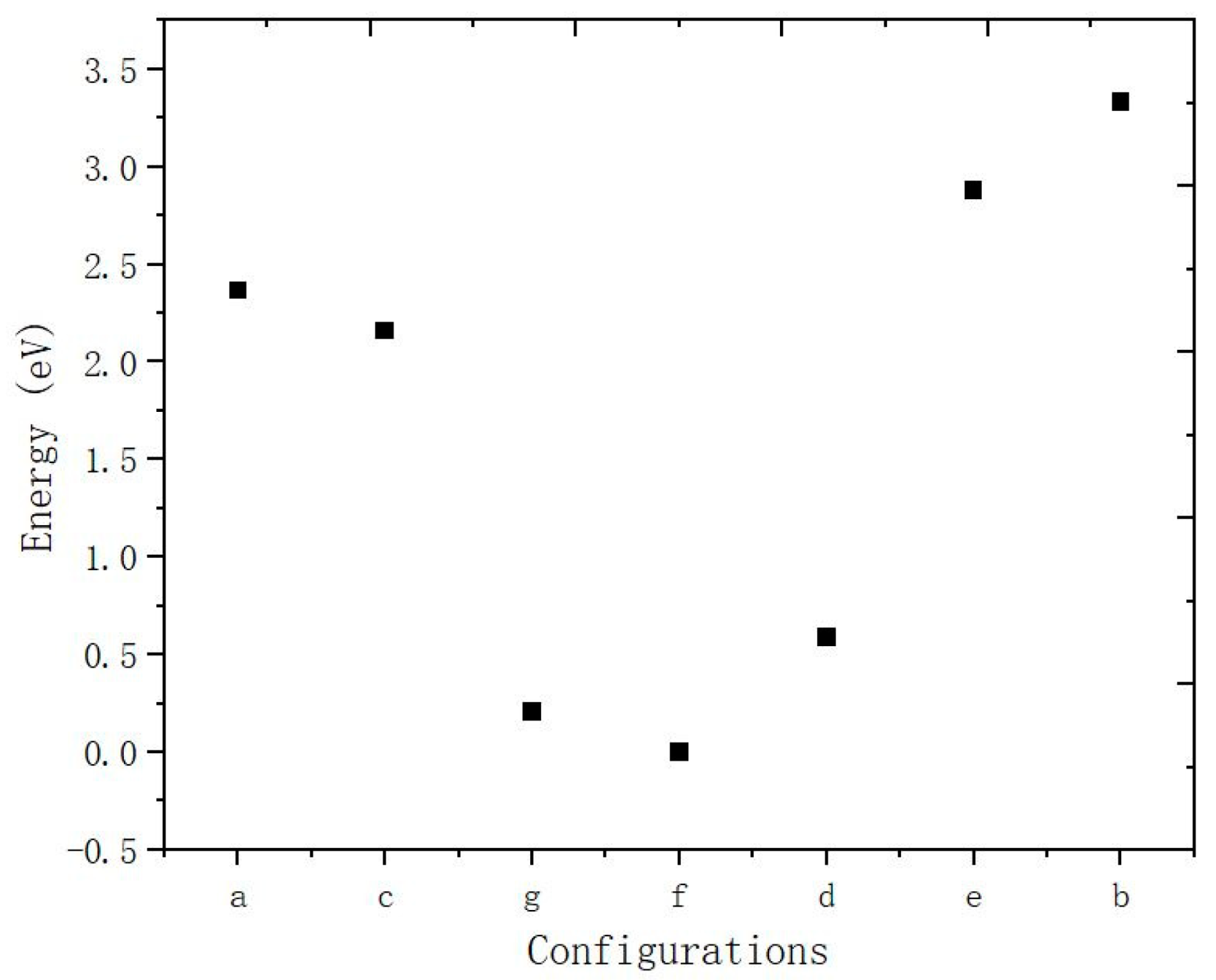

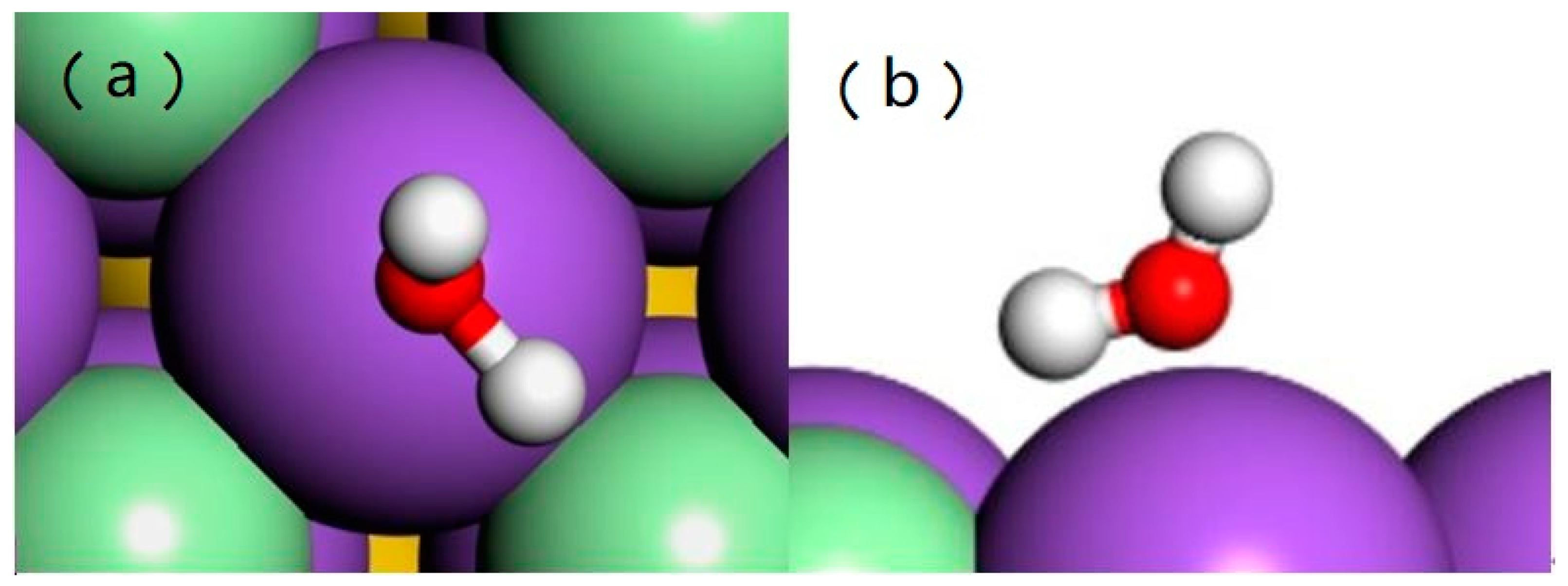

| Models | E (eV) | H1-Cl (Å) | H-Cl (Å) |
|---|---|---|---|
| a | 2.365 | 2.817 | 3.084 |
| b | 3.334 | 2.281 | 3.538 |
| c | 2.158 | 3.120 | 3.372 |
| d | 0.587 | 2.632 | 2.760 |
| e | 2.878 | 2.839 | 3.020 |
| f | 0 | 2.443 | 3.404 |
| g | 0.210 | 3.007 | 4.124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-Y.; Cao, J.-W.; Qin, X.-L.; Zhu, X.-L.; Yu, X.-H.; Wang, X.-C.; Yuan, X.-Q.; Liu, Y.-H.; Wang, Y.; Zhang, P. A Computational Validation of Water Molecules Adsorption on an NaCl Surface. Crystals 2021, 11, 610. https://doi.org/10.3390/cryst11060610
Liu X-Y, Cao J-W, Qin X-L, Zhu X-L, Yu X-H, Wang X-C, Yuan X-Q, Liu Y-H, Wang Y, Zhang P. A Computational Validation of Water Molecules Adsorption on an NaCl Surface. Crystals. 2021; 11(6):610. https://doi.org/10.3390/cryst11060610
Chicago/Turabian StyleLiu, Xiao-Yan, Jing-Wen Cao, Xiao-Ling Qin, Xu-Liang Zhu, Xu-Hao Yu, Xue-Chun Wang, Xiao-Qing Yuan, Yu-He Liu, Yong Wang, and Peng Zhang. 2021. "A Computational Validation of Water Molecules Adsorption on an NaCl Surface" Crystals 11, no. 6: 610. https://doi.org/10.3390/cryst11060610
APA StyleLiu, X. -Y., Cao, J. -W., Qin, X. -L., Zhu, X. -L., Yu, X. -H., Wang, X. -C., Yuan, X. -Q., Liu, Y. -H., Wang, Y., & Zhang, P. (2021). A Computational Validation of Water Molecules Adsorption on an NaCl Surface. Crystals, 11(6), 610. https://doi.org/10.3390/cryst11060610







