Preparation of Magnesia Partially Stabilized Zirconia Nanomaterials from Zirconium Hydroxide and Magnesium Carbonate Precursors Using PEG as a Template
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MSZ
2.3. Characterization
3. Results
3.1. Characteristics of the Local Zircon-Based Zirconium Hydroxide Precursor
3.2. FT-IR Spectra Characteristics of the As-Synthesized MSZ Samples
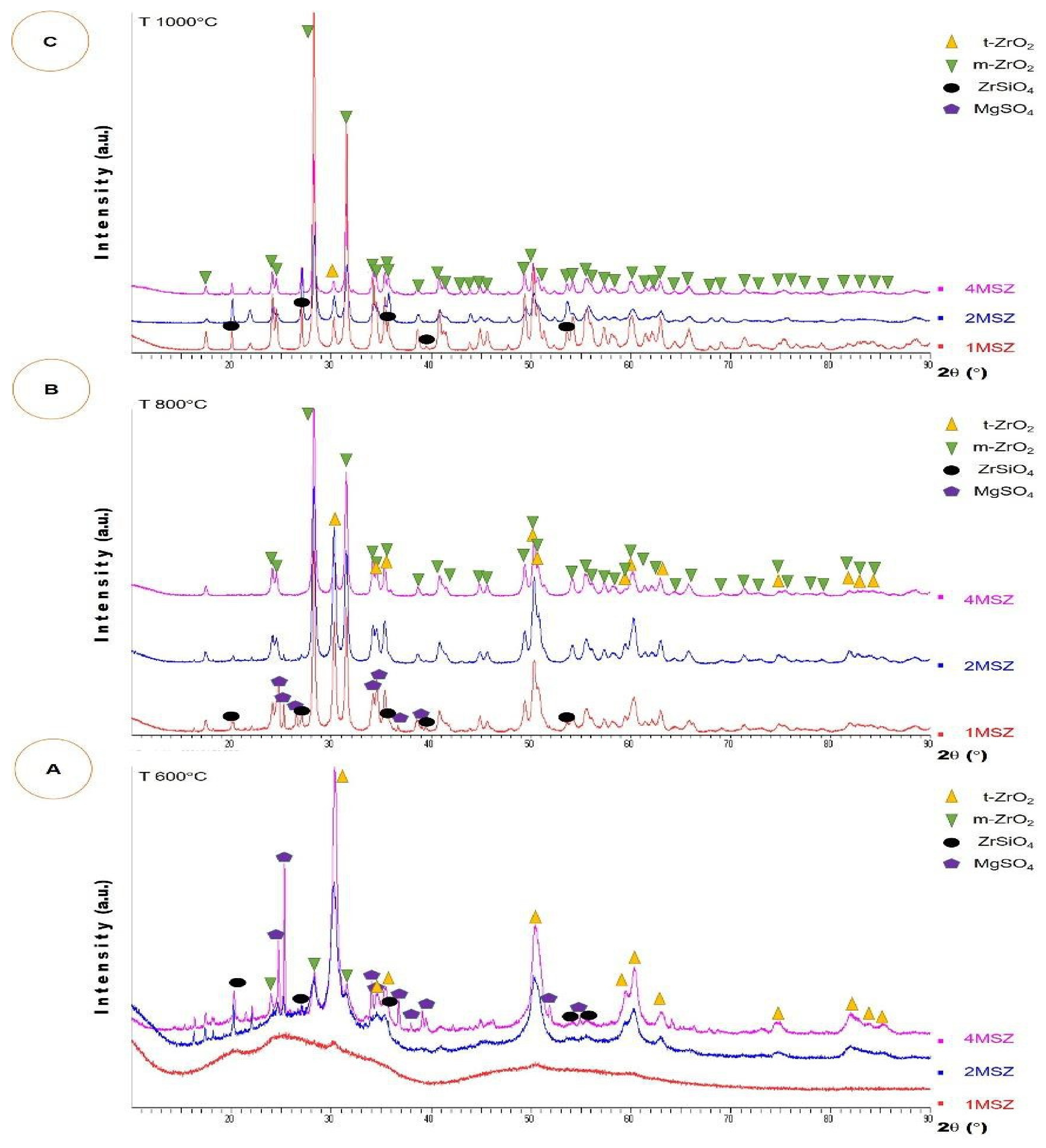
3.3. Phase Transformation of ZrO2 in a MSZ System
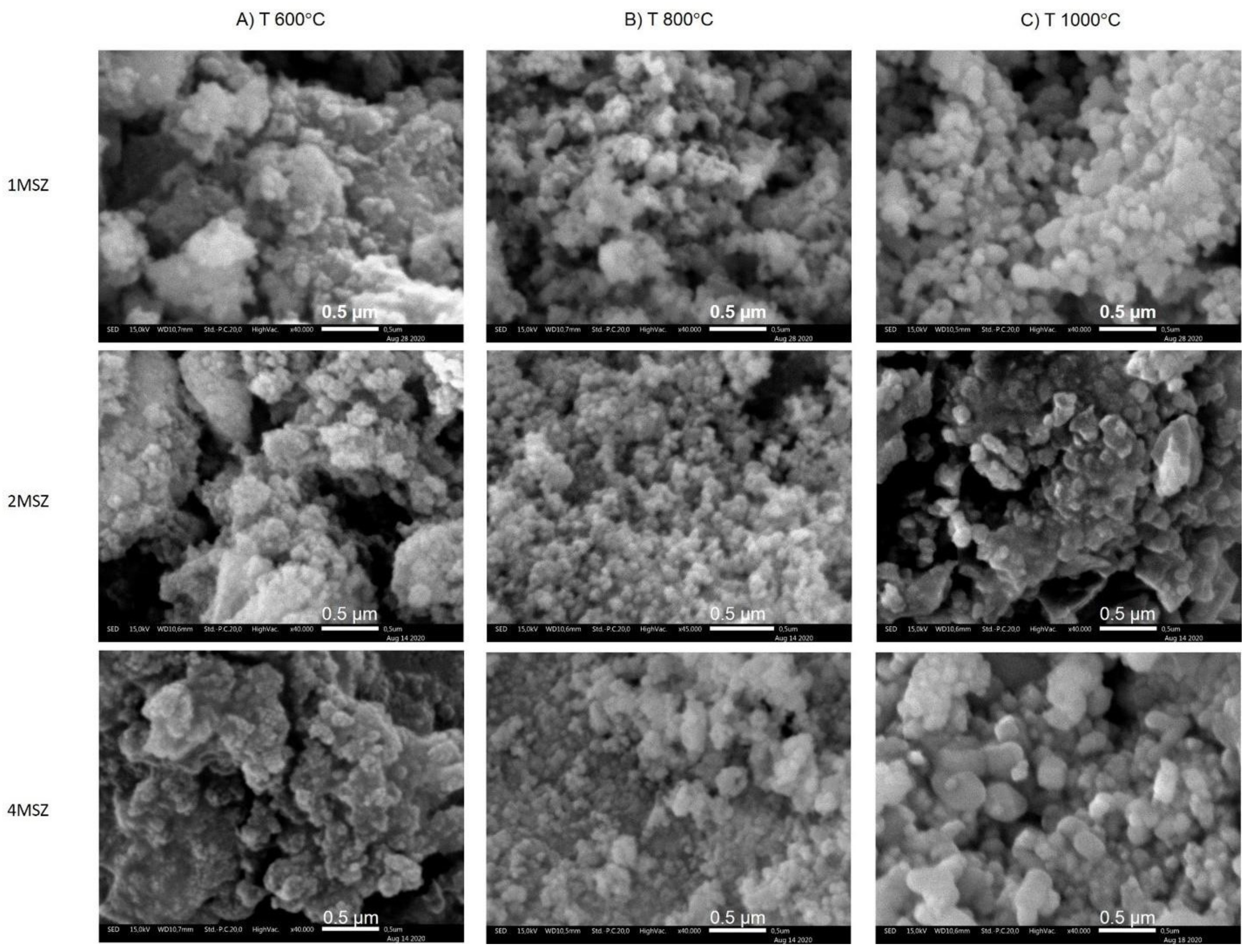
3.4. Microstructure Analysis by SEM and TEM
3.5. The Characteristics of the Sintered MSZ Samples at 1500 °C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gankhuyag, S.; Bae, D.S. Effect of pH Values on the Formation of Magnesia Doped Zirconia by Ball-Mill Assisted Co-precipitation Method. J. Phys. Sci. Appl. 2016, 6, 31–36. [Google Scholar]
- Ali, S.A.; Karthigeyan, S.; Deivanai, M.; Mani, R. Zirconia: Properties and Application—a Review. Pak. Oral Dent. J. 2014, 34, 178–183. [Google Scholar]
- Gauna, M.R.; Conconi, M.S.; Gomez, S.; Suárez, G.; Aglietti, E.F.; Rendtorff, N.M. Monoclinic-tetragonal Zirconia Quantification of Commercial Nanopowder Mixtures by XRD and DTA. Ceram. Silikaty 2015, 50, 318–325. [Google Scholar]
- Shukla, S.; Seal, S. Mechanisms of Room Temperature Metastable Tetragonal Phase Stabilisation in Zirconia. Int. Mater. Rev. 2005, 50, 45–64. [Google Scholar] [CrossRef]
- Maridurai, T.; Balaji, D.; Sagadevan, S. Synthesis and Characterization of Yttrium Stabilized Zirconia Nanoparticles. Mater. Res. 2016, 19, 812–816. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Ghani, Z.A.; Ismail, Y.M.B.; Syariff, K.A.; Mat, A.N.C.; Ariffin, Z.; Liszen, T.; Noor, A.F.M. Non-aqueous sol-gel derived calcia partially stabilized zirconia: Synthesis and characterizations. Malays. J. Microsc. 2020, 16, 67–74. [Google Scholar]
- Davar, F.; Shayan, N.; Najafabadi, A.H.; Sabaghi, V.; Hasani, S. Development of ZrO2-MgO Nanocomposite powders by the modified sol-gel method. Int. J. Appl. Ceram. Technol. 2017, 14, 211–219. [Google Scholar] [CrossRef]
- Septawendar, R.; Sunendar, B.; Suhanda, S. Penyiapan Nanopartikel Zirkonia Terstabilkan Magnesia (Mg-PSZ) pada Suhu Kalsinasi Rendah dengan Metode Gel Koloidal. J. Keramik dan Gelas Indones. 2012, 21, 44–59. [Google Scholar]
- Narayanan, K.; Sakthivel, C.; Prabha, I. MgO–ZrO2 Mixed Nanocomposites: Fabrication Methods and Applications. Mater. Today Sustain. 2019, 3, 100007. [Google Scholar]
- Judes, J.; Kamaraj, V. Study on Process Development and Property Evaluation of Sol-Gel Derived Magnesia Stabilized Zirconia Minispheres. Mater. Sci. Pol. 2014, 32, 145–156. [Google Scholar] [CrossRef]
- Xia, J.; Li, C.; Wang, Z.; Jiang, D.; Li, Q. Preparation of MgO-Partially Stabilized Zirconia Nano-powder by Coprecipitation Pressing. Key Eng. Mater. 2012, 492, 89–92. [Google Scholar] [CrossRef]
- Mirtaleb, A.H.; Mamoory, R.S. Enhanced Thermoluminescence of Magnesia Doped Zirconia Nanoparticles Expose to UV/beta Irradiation. Nanotechnology 2020, 31, 115601. [Google Scholar] [CrossRef]
- Abi, C.B.E.; Yazici, E.G. Effects of Magnesium Chloride Addition on Stabilization Zirconia. Acad. J. Sci. 2014, 3, 177–185. [Google Scholar]
- Zhao, L.; Yao, S.; Li, Y.; Zhao, Z.; Xue, Q. Effects of Calcium Oxide and Magnesium Oxide Stabilizing Agents on the Critical Transformation Size of Tetragonal Zirconia. Mater. Sci. Forum. 2019, 980, 15–24. [Google Scholar] [CrossRef]
- Septawendar, R.; Nurrudin, A.; Maryani, E.; Suhanda, S. Synthesis of Zirconia 1-D Nanomaterials from Local Zircon Based Zr(OH)4 Mediated by PEG-6000. Res. J. Chem. Environ. 2018, 22, 163–171. [Google Scholar]
- Riaz, S.; Bashir, M.; Naseem, S. Synthesis of Stabilized Zirconia Hollow Nanoparticles: Sugar as a Template. J. Sol Gel Sci. Technol. 2015, 74, 275–280. [Google Scholar] [CrossRef]
- Septawendar, R.; Purwasasmita, B.S.; Suhanda, S. Effect of Hydrolysis Catalyst NH4OH on the Preparation of Calcia Stabilized Zirconia with Sugar as a Masking Agent at Low Temperature. J. Aust. Ceram. Soc. 2013, 49, 101–108. [Google Scholar]
- Sofiyaningsih, N.; Suhanda, S.; Septawendar, R. Pengaruh Jenis Prekursor terhadap Karakteristik Mineralogi dan Mikrostruktur Zirkonia Nano dengan Metode Templat. J. Keramik dan Gelas Indones. 2020, 29, 45–56. [Google Scholar] [CrossRef]
- Septawendar, R.; Nuruddin, A.; Suhanda, S.; Asri, L.A.T.W.; Maryani, E.; Setiawan, A.R.; Purwasasmita, B.S. Synthesis of one-dimensional ZrO2 nanomaterials from Zr(OH)4 precursors assisted by glycols through a facile precursor-templating method. Mater. Res. Express. 2019, 6, 065037. [Google Scholar] [CrossRef]
- Heuer, C.; Storti, E.; Graule, T.; Aneziris, C.G. Electrospinning of Y2O3- and MgO-stabilized Zirconia Nanofibers and Characterization of the Evolving Phase Composition and Morphology During Thermal Treatment. Ceram. Int. 2020, 46, 12001–12008. [Google Scholar] [CrossRef]
- Septawendar, R.; Sutardi, S.; Karsono, U.; Sofiyaningsih, N. A Low-Cost, Facile Method on Production of Nano Zirconia and Silica from Local Zircon in a Large Scale Using a Sodium Carbonate Sintering Technology. J. Aust. Ceram. Soc. 2016, 52, 92–102. [Google Scholar]
- Septawendar, R.; Nuruddin, A.; Sutardi, S.; Maryani, E.; Asri, L.A.T.W.; Purwasasmita, B.S. Low-temperature metastable tetragonal zirconia nanoparticles (NpMTZ) synthesized from local zircon by a modified sodium carbonate sintering method. J. Aust. Ceram. Soc. 2018, 54, 643–654. [Google Scholar] [CrossRef]
- Pramono, E.; Utomo, S.B.; Wulandari, V.; Clegg, F. FTIR studies on The Effect of Concentration of Polyethylene Glycol on Polimerization of Shellac. J. Phys. Conf. Ser. 2016, 776, 776. [Google Scholar] [CrossRef]
- Pilarska, A.; Wysokowski, M.; Markiewicz, E.; Jesionowski, T. Synthesis of magnesium hydroxide and its calcinates by a precipitation method with the use of magnesium sulfate and poly(ethylene glycols). Powder Technol. 2013, 235, 148–157. [Google Scholar] [CrossRef]
- Ovalles, F.; Gallignani, M.; Rondón, R.; Brunetto, M.R.; Luna, R. Determination of Sulphate for Measuring Magnesium Sulphate in Pharmaceuticals by Flow Analysis-Fourier Transforms Infrared Spectroscopy. Lat. Am. J. Pharm. 2009, 28, 173–182. [Google Scholar]
- Zahir, M.H.; Rahman, M.M.; Irshad, K.; Rahman, M.M. Shape-Stabilized Phase Change Materials for Solar Energy Storage: MgO and Mg(OH)2 Mixed with Polyethylene Glycol. Nanomaterials 2019, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Skoda, D.; Styskalik, A.; Moravec, Z.; Bezdicka, P.; Pinkas, J. Templated Non-Hydrolytic Synthesis of Mesoporous Zirconium Silicates and Their Catalytic Properties. J. Mater. Sci. 2015, 50, 3371–3382. [Google Scholar] [CrossRef]




| Compound | Zirconium Hydroxides at 1000 °C | |
|---|---|---|
| wt% | Std. Err | |
| ZrO2 | 89.52 | 0.15 |
| SiO2 | 3.26 | 0.09 |
| Al2O3 | 1.34 | 0.06 |
| Na2O | 1.27 | 0.06 |
| HfO2 | 1.18 | 0.05 |
| Fe2O3 | 0.755 | 0.038 |
| MoO3 | 0.429 | 0.025 |
| SO3 | 0.319 | 0.016 |
| CaO | 0.224 | 0.011 |
| Y2O3 | 0.187 | 0.009 |
| P2O5 | 0.126 | 0.017 |
| MgO | 0.111 | 0.006 |
| TiO2 | 0.0951 | 0.0047 |
| ThO2 | 0.065 | 0.026 |
| Yb2O3 | 0.0441 | 0.0034 |
| U3O8 | 0.0335 | 0.0056 |
| Cr2O3 | 0.0243 | 0.0018 |
| Er2O3 | 0.0242 | 0.0039 |
| La2O3 | 0.0218 | 0.0035 |
| K2O | 0.024 | 0.0017 |
| ZnO | 0.0178 | 0.0014 |
| Sc2O3 | 0.0158 | 0.0017 |
| Rest = 0.83 LOI | ||
| Vibration Modes | 1MSA | 2MSZ | 4MSZ |
|---|---|---|---|
| Wavenumber (1/cm) | |||
| Zr–O, Mg–O (overlapped peaks) | 463, 416 | 463, 424 | 470, 440 |
| Mg–OH | 3750 | 3750 | 3754 |
| ν Si–O–Zr | 949 | 949 | 949 |
| δ SO42- | 601 | 601 | 601 |
| δ CH PEG-6000 | 1396, 1350 | 1350 | 1350 |
| δ CH2–PEG-6000 | 1466 | 1466 | 1474 |
| ν CH2–PEG-6000 | 2924, 2857 | 2924, 2857 | 2924, 2857 |
| ν C–O–C PEG-6000 | 1103 | 1111 | 1111 |
| –OH on surface | 1636 | 1636 | 1628 |
| –O–H2 (hydrogen bonding) | 3426, 3235 | 3426, 3235 | 3426, 3235 |
| Code | Bulk Density, g/cm3 | Water Adsorption, % | Porosity, % |
|---|---|---|---|
| 4MSZ (1500) | 4.14 | 3.45 | 14.29 |
| 2MSZ (1500) | 4.12 | 4.05 | 16.67 |
| 1MSZ (1500) | 3.95 | 4.12 | 16.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijayanti, R.B.; Rosmayanti, I.; Wahyudi, K.; Maryani, E.; Hernawan, H.; Septawendar, R. Preparation of Magnesia Partially Stabilized Zirconia Nanomaterials from Zirconium Hydroxide and Magnesium Carbonate Precursors Using PEG as a Template. Crystals 2021, 11, 635. https://doi.org/10.3390/cryst11060635
Wijayanti RB, Rosmayanti I, Wahyudi K, Maryani E, Hernawan H, Septawendar R. Preparation of Magnesia Partially Stabilized Zirconia Nanomaterials from Zirconium Hydroxide and Magnesium Carbonate Precursors Using PEG as a Template. Crystals. 2021; 11(6):635. https://doi.org/10.3390/cryst11060635
Chicago/Turabian StyleWijayanti, Rizky Berliana, Irna Rosmayanti, Kristanto Wahyudi, Eneng Maryani, Hernawan Hernawan, and Rifki Septawendar. 2021. "Preparation of Magnesia Partially Stabilized Zirconia Nanomaterials from Zirconium Hydroxide and Magnesium Carbonate Precursors Using PEG as a Template" Crystals 11, no. 6: 635. https://doi.org/10.3390/cryst11060635
APA StyleWijayanti, R. B., Rosmayanti, I., Wahyudi, K., Maryani, E., Hernawan, H., & Septawendar, R. (2021). Preparation of Magnesia Partially Stabilized Zirconia Nanomaterials from Zirconium Hydroxide and Magnesium Carbonate Precursors Using PEG as a Template. Crystals, 11(6), 635. https://doi.org/10.3390/cryst11060635







