Sintering/Crystallization and Viscosity of Sealing Glass-Ceramics
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Analysis
3.2. Sintering Behaviour
3.3. Influence of the Glass Powder Particle Size and the Heating Rate in the DTA Characteristic Temperatures: Crystallization Mechanism
- (1)
- n = m, for which the KTAB equations have been employed.
- (2)
- n ≠ m, for which the Marseglia and Matusita equations have been employed, the m value has been obtained from this last equation.
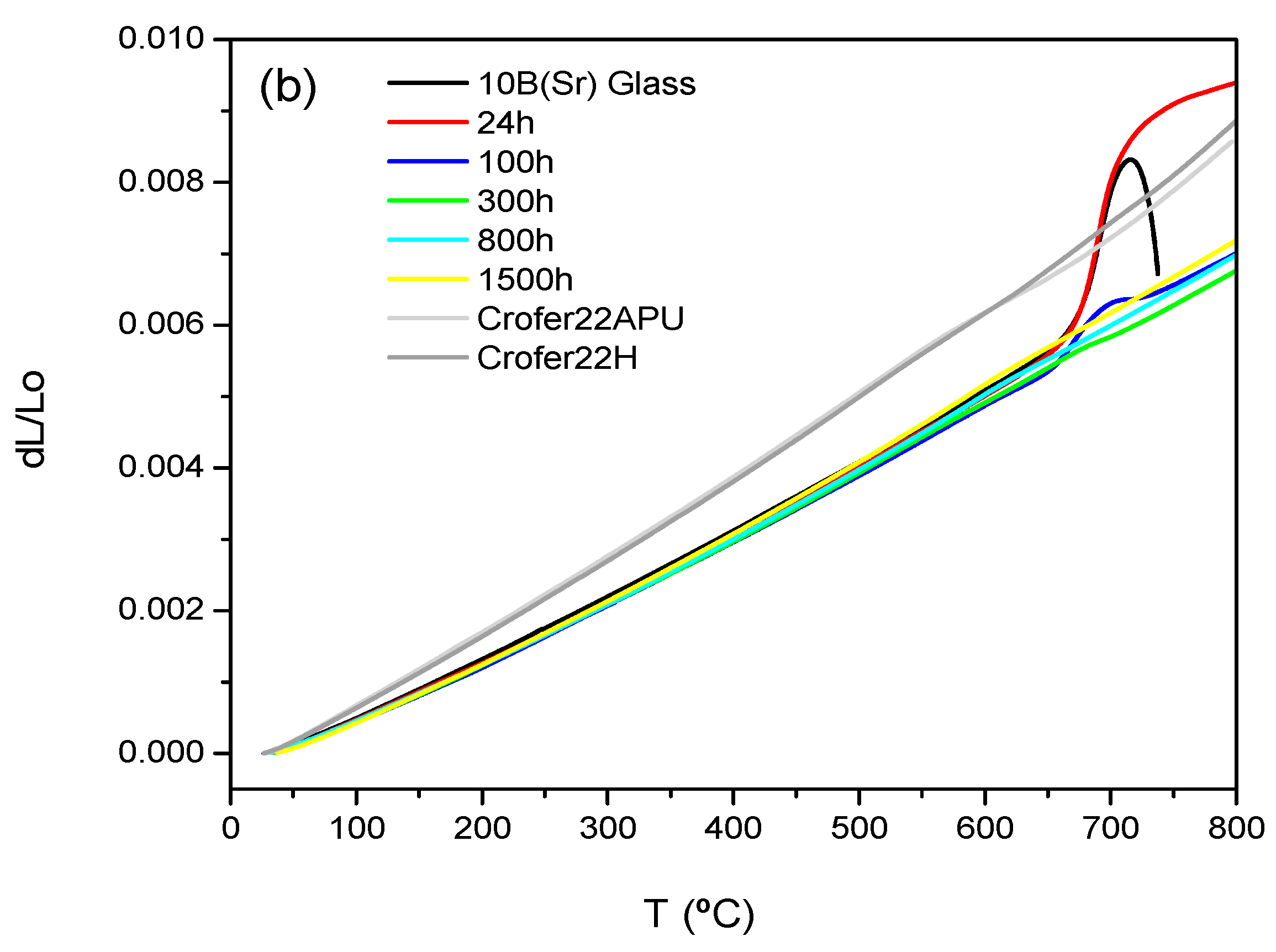
3.4. Thermal Expansion
3.5. Glass-Ceramics Viscosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blum, L.; Buchkremer, H.P.; Gross, S.; Gubner, A. Solid oxide fuel cell development at Forschungszentrum Juelich. Fuel Cells 2007, 7, 204–210. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Marlowe, C.A.; Lee, K.T. Role of solid oxide fuel cells in a balanced energy strategy. Energy Env. Sci. 2012, 5, 5498. [Google Scholar] [CrossRef]
- Weil, K.S. The state-of-the-art in sealing technology for solid oxide fuel cells. J. Miner. Met. Mater. Soc. 2006, 58, 37–44. [Google Scholar] [CrossRef]
- Lessing, P.A. A review of sealing technologies applicable to solid oxide electrolysis cells. J. Mater. Sci. 2007, 42, 10–3465. [Google Scholar] [CrossRef]
- Fergus, J.W. Sealants for solid oxide fuel cells. J. Power Sources 2005, 147, 46–57. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Glass-based seals for solid oxide fuel and electrolyzer cells- A review. Mater. Sci. Eng. Rep. 2010, 67, 65–85. [Google Scholar] [CrossRef]
- Gross, S.M.; Koppitz, T.; Remmel, I.; Reisgen, U.; Verlotski, V.; Conradt, R. Glass-ceramic composite as a new sealing material for SOFCs. Proc. Electrochem. Soc. PV 2005, 2005–2007, 1924–1931. [Google Scholar] [CrossRef]
- Goel, A.; Reddy, A.A.; Pascual, M.J.; Gremillard, L.; Malchere, A.; Ferreira, J.M.F. Sintering behavior of lanthanide-containing glass-ceramic sealants for solid oxide fuel cells. J. Mater. Chem. Chem. 2012, 22, 10042–10054. [Google Scholar] [CrossRef]
- Ertugrul, T.Y.; Celik, S.; Mat, M.D. Optimum processing parameters to improve sealing performance in solid oxide fuel cells. Ceram. Int. 2015, 41, 9834–9842. [Google Scholar] [CrossRef]
- Agea-Blanco, B.; Reinsch, S.; Müller, R. Sintering and foaming of barium silicate glass powder compacts. Front. Mater. 2016, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.D.; Rodrigues, A.M.; Rodrigues, A.C.M.; Pascual, M.J.; Durán, A.; Cabral, A.A. Sintering and crystallization of SrO-CaO-B2O3-SiO2 glass-ceramics with different TiO2 contents. J. Non-Cryst. Solids 2017, 473, 33–40. [Google Scholar] [CrossRef]
- Javed, H.; Sabato, A.G.; Herbrig, K.; Ferrero, D.; Walter, C.; Salvo, M.; Smeacetto, F. Design and characterization of novel glass-ceramic sealants for solid oxide electrolysis cell (SOEC) applications. Int. J. Appl. Ceram. Technol. 2018, 15, 999–1010. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Rodríguez-López, S.; Pascual, M.J. Processing technologies for sealing glasses and glass-ceramics. Int. J. Appl. Glass. Sci. 2020, 11, 552–568. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-López, S.; Wei, J.; Laurenti, K.C.; Mathias, I.; Justo, V.M.; Serbena, F.C.; Baudín, C.; Malzbender, J.; Pascual, M.J. Mechanical properties of solid oxide fuel cell glass-ceramic sealants in the system BaO/SrO-MgO-B2O3-SiO2. J. Eur. Ceram. Soc. 2017, 37, 3579–3594. [Google Scholar] [CrossRef]
- Rodríguez-López, S.; Malzbender, J.; Justo, V.M.; Serbena, F.C.; Groß-Barsnick, S.M.; Pascual, M.J. Thermo-Mechanical Stability and Gas-Tightness of Glass-Ceramics Joints for SOFC in the System MgO-BaO/SrO-B2O3-SiO2. Front. Mater. 2020, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-López, S.; Haanappel, V.A.C.; Durán, A.; Muñoz, F.; Mather, G.C.; Pascual, M.J.; Gross-Barsnick, S.M. Glass-ceramic seals in the system MgO-BaO-B2O3-SiO2 operating under simulated SOFC conditions. Int. J. Hydrog. Energy 2016, 41, 15335–15345. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Avramov, I.; Avramova, K.; Rüssel, C. Useful method to analyze data on overall transformation kinetics. J. Non. Cryst. Solids 2010, 356, 1201–1203. [Google Scholar] [CrossRef]
- Avramov, I.; Avramova, K.; Rüssel, C. New method to analyze data on overall crystallization kinetics. J. Cryst. Growth. 2005, 285, 394–399. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Marseglia, E.A. Kinetic theory of crystallization of amorphous materials. J. Non. Cryst. Solids 1980, 41, 31–36. [Google Scholar] [CrossRef]
- Matusita, K.; Sakka, S. Kinetic study of crystallization of glass by differential thermal analysis—criterion on application of Kissinger plot. J. Non. Cryst. Solids 1980, 38–39, 741–746. [Google Scholar] [CrossRef]
- Geasse, P. Development of Crystallizing Glass Sealants for High Temperature Planar Solid Oxide Fuel Cells. Ph.D. Thesis, RWT Aachen, Aachen, Germany, 2003. [Google Scholar]
- Pascual, M.J.; Prado, M.O.; Durán, A. A new method for determining fixed viscosity points of glasses. Phys. Chem. Glas. 2005, 46, 512–520. [Google Scholar]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Influence of particle size on the crystallization kinetics of glasses produced from waste materials. J. Non-Cryst. Solids 2011, 357, 211–219. [Google Scholar] [CrossRef]
- Karamanov, A.; Avramov, I.; Arrizza, L.; Pascova, R.; Gutzow, I. Variation of Avrami parameter during non-isothermal surface crystallization of glass powders with different sizes. J. Non-Cryst. Solids 2012, 358, 1486–1490. [Google Scholar] [CrossRef]
- Donald, I.W. Crystallization kinetics of a lithium zinc silicate glass studied by DTA and DSC. J. Non. Cryst. Solids. 2004, 345–346, 120–126. [Google Scholar] [CrossRef]
- Pascual, M.J.; Lara, C.; Durán, A. Non-isothermal crystallization kinetics of devitriying RO-BaO-SiO2 (R = Mg, Zn) glasses. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 2006, 47, 572–581. [Google Scholar]
- Skarmoutsos, D.; Tietz, F.; Nikolopoulos, P. Structure-Property Relationships of Ni/YSZ and Ni/(YSZ+TiO2)Cermets. Fuel Cells 2001, 1, 243–248. [Google Scholar] [CrossRef]
- Kerstan, M.; Rüssel, C. Barium silicates as high thermal expansion seals for solid oxide fuel cells studied by high-temperature X-ray diffraction (HT-XRD). J. Power Sources 2011, 196, 7578–7584. [Google Scholar] [CrossRef]
- Kerstan, M.; Müller, M.; Rüssel, C. Thermal expansion of Ba2ZnSi2O7, BaZnSiO4 and the solid solution series BaZn2−xMgxSi2O7 (0 ≤ x ≤ 2) studied by high-temperature X-ray diffraction and dilatometry. J. Solid State Chem. 2012, 188, 84–91. [Google Scholar] [CrossRef]
- Thieme, C.; Rüssel, C. Thermal expansion behavior of SrSiO3 and Sr2SiO4 determined by high-temperature X-ray diffraction and dilatometry. J. Mater. Sci. 2015, 50, 5533–5539. [Google Scholar] [CrossRef]









| Glass | 7.5B(Ba) | 10B(Sr) | ||||||
|---|---|---|---|---|---|---|---|---|
| Component * | Theoretical | Analized | Theoretical | Analized | ||||
| mol%. | wt%. | mol%. | wt%. | mol%. | wt%. | mol%. | wt%. | |
| SiO2 (± 0.3) | 47.5 | 34.6 | 48.95 | 35.40 | 45 | 39.0 | 45.77 | 39.50 |
| MgO (± 0.05) | 18 | 8.8 | 15.67 | 7.60 | 18 | 10.5 | 17.03 | 9.86 |
| BaO (± 0.3 **) | 27 | 50.2 | 26.93 | 49.70 | - | - | 0.60 | 0.27 |
| SrO (± 0.3 **) | - | - | 0.09 | 0.11 | 27 | 40.4 | 26.41 | 39.3 |
| B2O3 (± 0.05) | 7.5 | 6.3 | 7.52 | 6.30 | 10 | 10.1 | 10.00 | 10.00 |
| K2O (± 0.03) | - | - | 0.19 | 0.21 | - | - | 0.16 | 0.22 |
| Na2O (± 0.05) | - | - | 0.66 | 0.49 | - | - | 0.20 | 0.18 |
| CaO (± 0.02) | - | - | - | - | - | - | 0.15 | 0.12 |
| Composition | Designation | D(v,0.1) | D(v,0.5) | D(v,0.9) |
|---|---|---|---|---|
| 7.5B(Ba) | 80–20 μm | 8.1 | 44.5 | 100.6 |
| Fine | 5.3 | 13.0 | 38.7 | |
| 10B(Sr) | <63 μm | 3.1 | 22.2 | 54.2 |
| Fine | 5.3 | 13.0 | 56.4 |
| Glass | Fraction | Heating Rate (°C/min) | TFS ± 10 | TMS ± 10 | TS ± 10 | THB ± 3 | TF ± 3 |
|---|---|---|---|---|---|---|---|
| (°C) | |||||||
| 7.5B(Ba) | Fine | 2 | 673 | 723 | 778 | 974 | 1040 |
| 5 | 694 | 740 | 790 | 990 | 1049 | ||
| 10 | 703 | 752 | 807 | 1025 | 1060 | ||
| 80–20 µm | 2 | 673 | 729 | 970 | 988 | 1028 | |
| 5 | 704 | 761 | 995 | 1003 | 1090 | ||
| 10 | 704 | 765 | 1000 | 1007 | 1102 | ||
| 10B(Sr) | Fine | 2 | 717 | 754 | 800 | 867 | 1030 |
| 5 | 719 | 755 | 810 | 906 | 1045 | ||
| 10 | 734 | 771 | 826 | 921 | 1086 | ||
| <63 µm | 2 | 722 | 756 | 803 | 952 | 1030 | |
| 5 | 723 | 769 | 811 | 974 | 1041 | ||
| 10 | 735 | 776 | 850 | 1010 | 1078 | ||
| Glass | Particle Size | Heating Rate (°C/min) | Tg ± 7 | Tx± 9 | Tp1 ± 9 | Tp2 ± 9 | Tg − Tx |
|---|---|---|---|---|---|---|---|
| °C | |||||||
| 7.5B(Ba) | Fine | 2 | 630 | 707 | 745 | 786 | 77 |
| 3 | 632 | 710 | 751 | 803 | 78 | ||
| 5 | 634 | 725 | 763 | 807 | 91 | ||
| 10 | 640 | 736 | 779 | 831 | 96 | ||
| 15 | 645 | 746 | 788 | 843 | 101 | ||
| 20 | 645 | 754 | 794 | 850 | 109 | ||
| 30 | 647 | 762 | 809 | 864 | 115 | ||
| 40 | 649 | 766 | 817 | 873 | 117 | ||
| 80–20 µm | 2 | 635 | 711 | 749 | 796 | 76 | |
| 3 | 635 | 721 | 753 | 807 | 86 | ||
| 5 | 635 | 729 | 769 | 822 | 94 | ||
| 10 | 638 | 738 | 782 | 839 | 100 | ||
| 15 | 640 | 748 | 792 | 845 | 108 | ||
| 20 | 642 | 757 | 798 | 858 | 115 | ||
| 30 | 648 | 765 | 812 | 871 | 117 | ||
| 40 | 650 | 772 | 821 | 878 | 122 | ||
| 10B(Sr) | Fine | 2 | 636 | 725 | 774 | 842 | 88 |
| 3 | 642 | 730 | 779 | 856 | 88 | ||
| 5 | 651 | 746 | 789 | 877 | 95 | ||
| 10 | 653 | 748 | 802 | 893 | 95 | ||
| 15 | 657 | 758 | 808 | 910 | 100 | ||
| <63 μm | 2 | 640 | 734 | 776 | 845 | 94 | |
| 3 | 647 | 743 | 782 | 860 | 97 | ||
| 5 | 653 | 750 | 795 | 881 | 98 | ||
| 10 | 654 | 755 | 807 | 906 | 101 | ||
| 15 | 662 | 763 | 818 | 915 | 102 | ||
| Activation Energy (KJ/mol) | Particle Size | |||
|---|---|---|---|---|
| Fine | 80–20 µm | |||
| Peak 1, Ea1 | Peak 2, Ea2 | Peak 1, Ea1 | Peak 2, Ea2 | |
| Kissinger | 364 ± 12 | 335 ± 13 | 365 ± 14 | 358 ± 11 |
| Takhor | 381 ± 12 | 353 ± 13 | 383 ± 14 | 377 ± 11 |
| Augis-Bennett | 372 ± 12 | 344 ± 13 | 374 ± 14 | 367 ± 11 |
| Marseglia | 372 ± 12 | 344 ± 13 | 374 ± 14 | 367 ± 11 |
| Matusita (m) | 0.98 | 0.97 | 0.98 | 0.97 |
| Activation Energy (KJ/mol) | Particle Size | |||
|---|---|---|---|---|
| Fine | <63 µm | |||
| Peak 1, Ea1 | Peak 2, Ea2 | Peak 1, Ea1 | Peak 2, Ea2 | |
| Kissinger | 523 ± 20 | 312 ± 20 | 439 ± 22 | 290 ± 16 |
| Takhor | 541 ± 20 | 331 ± 20 | 456 ± 22 | 309 ± 16 |
| Augis-Bennett | 532 ± 20 | 322 ± 20 | 447 ± 22 | 299 ± 16 |
| Marseglia | 532 ± 20 | 322 ± 20 | 447 ± 22 | 299 ± 16 |
| Matusita (m) | 0.98 | 0.97 | 0.98 | 0.97 |
| TEC 200–500 °C ± 0.5 (10−6 K−1) | 7.5B(Ba) | 10B(Sr) |
|---|---|---|
| Glass | 9.7 | 9.2 |
| 24 h | 12.4 | 9.2 |
| 100 h | 11.0 | 9.0 |
| 300 h | 9.7 | 9.0 |
| 800 h | 10.4 | 9.1 |
| 1500 h | 10.1 | 9.5 |
| Crofer22APU | 11.2 | |
| Crofer22H | 11.2 | |
| 8YSZ [22] | 10.5 (25–1000 °C) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-López, S.; Pascual, M.J. Sintering/Crystallization and Viscosity of Sealing Glass-Ceramics. Crystals 2021, 11, 737. https://doi.org/10.3390/cryst11070737
Rodríguez-López S, Pascual MJ. Sintering/Crystallization and Viscosity of Sealing Glass-Ceramics. Crystals. 2021; 11(7):737. https://doi.org/10.3390/cryst11070737
Chicago/Turabian StyleRodríguez-López, Sonia, and Maria J. Pascual. 2021. "Sintering/Crystallization and Viscosity of Sealing Glass-Ceramics" Crystals 11, no. 7: 737. https://doi.org/10.3390/cryst11070737
APA StyleRodríguez-López, S., & Pascual, M. J. (2021). Sintering/Crystallization and Viscosity of Sealing Glass-Ceramics. Crystals, 11(7), 737. https://doi.org/10.3390/cryst11070737






