Have Covalent Organic Framework Films Revealed Their Full Potential?
Abstract
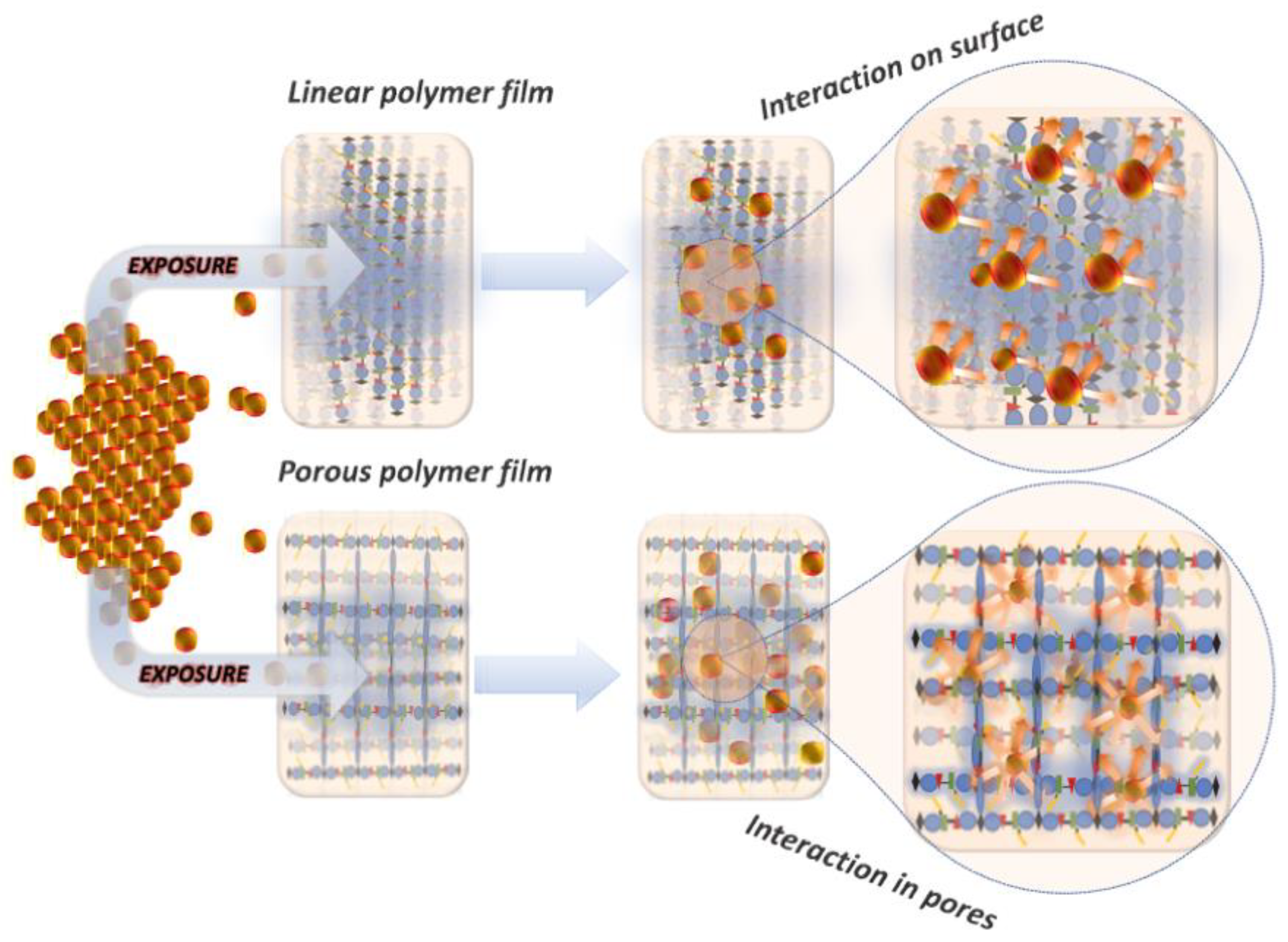
:1. Introduction
2. Techniques for Covalent Organic Framework Film Formation
3. Outlook and Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, K.V.; Preuss, K.; Titirici, M.M.; Rodríguez-Reinoso, F. Nanoporous Materials for the Onboard Storage of Natural Gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, T.D.; Coudert, F.X.; James, S.L.; Cooper, A.I. The changing state of porous materials. Nat. Mater. 2021, 60, 5890. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Standl, S.; Hinrichsen, O. Kinetic Modeling of Catalytic Olefin Cracking and Methanol-to-Olefins (MTO) over Zeolites: A Review. Catalysts 2018, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, P.; Anderson, R.; Wang, X.; Zhang, X.; Robison, L.; Redfern, L.R.; Moribe, S.; Islamoglu, T.; Gómez-Gualdrón, D.A.; et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303. [Google Scholar] [CrossRef]
- Jaros, S.W.; Smoleński, P.; Guedes da Silva, M.F.C.; Florek, M.; Król, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New silver BioMOFs driven by 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTA = S): Synthesis, topological analysis and antimicrobial activity. Cryst. Eng. Comm. 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, C.H. Metal-organic framework thin films: Fabrication, modification, and patterning. Processes 2020, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Freund, R.; Canossa, S.; Cohen, S.M.; Yan, W.; Deng, H.; Guillerm, V.; Eddaoudi, M.; Madden, D.G.; Fairen-Jimenez, D.; Lyu, H.; et al. 25 years of Reticular Chemistry. Angew. Chemie Int. Ed. 2021. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Liu, X.; Pan, L.; Shi, C.; Zhang, X.; Zou, J.J. Heterogeneous Photocatalytic Organic Transformation Reactions Using Conjugated Polymers-Based Materials. ACS Catal. 2020, 10, 12256–12283. [Google Scholar] [CrossRef]
- Thomas, A. Much ado about nothing—A decade of porous materials research. Nat. Commun. 2020, 11, 4985. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chemie Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Díaz de Greñu, B.; Torres, J.; García-González, J.; Muñoz-Pina, S.; de los Reyes, R.; Costero, A.M.; Amorós, P.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. ChemSusChem 2021, 14, 208–233. [Google Scholar] [CrossRef]
- Matsumoto, M.; Dasari, R.R.; Ji, W.; Feriante, C.H.; Parker, T.C.; Marder, S.R.; Dichtel, W.R. Rapid, Low Temperature Formation of Imine-Linked Covalent Organic Frameworks Catalyzed by Metal Triflates. J. Am. Chem. Soc. 2017, 139, 4999–5002. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Z.; Alemany, L.B.; Li, Y.; Nnorom, N.; Barnes, M.; Khalil, S.; Rahman, M.M.; Ajayan, P.M.; Verduzco, R. Rapid, Ambient Temperature Synthesis of Imine Covalent Organic Frameworks Catalyzed by Transition-Metal Nitrates. Chem. Mater. 2021, 33, 3394–3400. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, M.; Zhang, M.; Chen, J.; Zhang, S.; Zhou, X.; He, L.; Sheridan, M.V.; Yuan, M.; Zhang, M.; et al. Electron Beam Irradiation as a General Approach for the Rapid Synthesis of Covalent Organic Frameworks under Ambient Conditions. J. Am. Chem. Soc. 2020, 142, 9169–9174. [Google Scholar] [CrossRef]
- Hu, J.; Gupta, S.K.; Ozdemir, J.; Beyzavi, M.H. Applications of Dynamic Covalent Chemistry Concept toward Tailored Covalent Organic Framework Nanomaterials: A Review. ACS Appl. Nano Mater. 2020, 3, 6239–6269. [Google Scholar] [CrossRef]
- Liu, R.; Tan, K.T.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent organic frameworks: An ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 2021, 50, 120–242. [Google Scholar] [CrossRef]
- Lyle, S.J.; Waller, P.J.; Yaghi, O.M. Covalent Organic Frameworks: Organic Chemistry Extended into Two and Three Dimensions. Trends Chem. 2019, 1, 172–184. [Google Scholar] [CrossRef]
- Chaoui, N.; Trunk, M.; Dawson, R.; Schmidt, J.; Thomas, A. Trends and challenges for microporous polymers. Chem. Soc. Rev. 2017, 46, 3302–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Chemistry: Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Diercks, C.S.; Zhu, C.; Yaghi, O.M. Porous Crystalline Olefin-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 6848–6852. [Google Scholar] [CrossRef]
- Acharjya, A.; Pachfule, P.; Roeser, J.; Schmitt, F.J.; Thomas, A. Vinylene-Linked Covalent Organic Frameworks by Base-Catalyzed Aldol Condensation. Angew. Chem. Int. Ed. 2019, 58, 14865–14870. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klöck, C.; O’Keeffe, M.; Yaghi, O.M. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhao, W.; Zhang, F.; Cao, Y.; Liu, F.; Bi, S.; Feng, X. A two-dimensional conjugated polymer framework with fully sp2-bonded carbon skeleton. Polym. Chem. 2016, 7, 4176–4181. [Google Scholar] [CrossRef] [Green Version]
- Jin, E.; Asada, M.; Xu, Q.; Dalapati, S.; Addicoat, M.A.; Brady, M.A.; Xu, H.; Nakamura, T.; Heine, T.; Chen, Q.; et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 2017, 357, 673–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwijnenburg, M.A.; Cheng, G.; McDonald, T.O.; Jelfs, K.E.; Jiang, J.X.; Ren, S.; Hasell, T.; Blanc, F.; Cooper, A.I.; Adams, D.J. Shedding light on structure-property relationships for conjugated microporous polymers: The importance of rings and strain. Macromolecules 2013, 46, 7696–7704. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Jiang, D. Conjugated microporous polymers as molecular sensing devices: Microporous architecture enables rapid response and enhances sensitivity in fluorescence-on and fluorescence-off sensing. J. Am. Chem. Soc. 2012, 134, 8738–8741. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Bildirir, H.; Dawson, R.; Thomas, A.; Yagci, Y. Microporous thioxanthone polymers as heterogeneous photoinitiators for visible light induced free radical and cationic polymerizations. Macromolecules 2014, 47, 4607–4614. [Google Scholar] [CrossRef]
- Bildirir, H.; Osken, I.; Ozturk, T.; Thomas, A. Reversible Doping of a Dithienothiophene-Based Conjugated Microporous Polymer. Chem. A Eur. J. 2015, 21, 9306–9311. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Noda, Y.; Kumar Barange, D.; Kochergin, Y.S.; Lyu, P.; Balcarova, B.; Nachtigall, P.; Bojdys, M.J. Real-time optical and electronic sensing with a β-amino enone linked, triazine-containing 2D covalent organic framework. Nat. Commun. 2019, 10, 3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Acharjya, A.; Ye, M.-Y.; Rabeah, J.; Li, S.; Kochovski, Z.; Youk, S.; Roeser, J.; Grüneberg, J.; Schwarze, M.; et al. Protonated imine-linked covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2021, n/a. [Google Scholar] [CrossRef]
- Edhborg, F.; Bildirir, H.; Bharmoria, P.; Moth-Poulsen, K.; Albinsson, B. Intramolecular Triplet–Triplet Annihilation Photon Upconversion in Diffusionally Restricted Anthracene Polymer. J. Phys. Chem. B 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Qiu, T.; Guo, W.; Liang, Z.; Tabassum, H.; Xia, D.; Zou, R. Covalent organic framework-based materials for energy applications. Energy Environ. Sci. 2021, 14, 688–728. [Google Scholar] [CrossRef]
- Skabara, P.J.; Arlin, J.B.; Geerts, Y.H. Close encounters of the 3D kind—Exploiting high dimensionality in molecular semiconductors. Adv. Mater. 2013, 25, 1948–1954. [Google Scholar] [CrossRef]
- Bildirir, H.; Gregoriou, V.G.; Avgeropoulos, A.; Scherf, U.; Chochos, C.L. Porous organic polymers as emerging new materials for organic photovoltaic applications: Current status and future challenges. Mater. Horizons 2017, 4, 546–556. [Google Scholar] [CrossRef]
- Fritz, P.W.; Coskun, A. The Prospect of Dimensionality in Porous Semiconductors. Chem. A Eur. J. 2021, 27, 7489–7501. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abadía, M.; Strutyński, K.; Lerma-Berlanga, B.; Stoppiello, C.T.; Khlobystov, A.N.; Martí-Gastaldo, C.; Saeki, A.; Melle-Franco, M.; Mateo-Alonso, A. π-Interpenetrated 3D Covalent Organic Frameworks from Distorted Polycyclic Aromatic Hydrocarbons. Angew. Chem. Int. Ed. 2021, 60, 9941–9946. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y. Responsive Guest Encapsulation of Dynamic Conjugated Microporous Polymers. Sci. Rep. 2016, 6, 28784. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.M.; Mileson, B.; Sadagopan, A.; Lopez, S.A. Molecular Recognition and Band Alignment in 3D Covalent Organic Frameworks for Cocrystalline Organic Photovoltaics. J. Phys. Chem. C 2020, 124, 9126–9133. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Dichtel, W.R. Direct detection of RDX vapor using a conjugated polymer network. J. Am. Chem. Soc. 2013, 135, 8357–8362. [Google Scholar] [CrossRef] [PubMed]
- Namgung, H.; Lee, J.J.; Gwon, Y.J.; Lee, T.S. Synthesis of tetraphenylethylene-based conjugated microporous polymers for detection of nitroaromatic explosive compounds. RSC Adv. 2018, 8, 34291–34296. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.-W.; Xu, S.-Q.; Wang, X.-Z.; Chen, Y.; Dai, L.; Zhao, X. The construction of fluorescent heteropore covalent organic frameworks and their applications in spectroscopic and visual detection of trinitrophenol with high selectivity and sensitivity. Chem. Commun. 2018, 54, 2308–2311. [Google Scholar] [CrossRef]
- Chakravarty, C.; Mandal, B.; Sarkar, P. Multifunctionalities of an Azine-Linked Covalent Organic Framework: From Nanoelectronics to Nitroexplosive Detection and Conductance Switching. J. Phys. Chem. C 2018, 122, 3245–3255. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Dong, R.; Feng, X.; Kaskel, S.; Matoga, D.; Stavila, V. Electronic Devices Using Open Framework Materials. Chem. Rev. 2020, 120, 8581–8640. [Google Scholar] [CrossRef]
- Bai, B.; Wang, D.; Wan, L.J. Synthesis of covalent organic framework films at interfaces. Bull. Chem. Soc. Jpn. 2021, 94, 1090–1098. [Google Scholar] [CrossRef]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 2011, 332, 228–231. [Google Scholar] [CrossRef]
- Dogru, M.; Handloser, M.; Auras, F.; Kunz, T.; Medina, D.; Hartschuh, A.; Knochel, P.; Bein, T. A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew. Chem. Int. Ed. 2013, 52, 2920–2924. [Google Scholar] [CrossRef]
- Medina, D.D.; Rotter, J.M.; Hu, Y.; Dogru, M.; Werner, V.; Auras, F.; Markiewicz, J.T.; Knochel, P.; Bein, T. Room temperature synthesis of covalent-organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 2015, 137, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, Y.; Zhang, G.; Peng, Z.; Ye, L.; Chen, Y.; Zhang, T.; Xing, G.; Chen, L. An In-situ Film-to-Film Transformation Approach toward Highly Crystalline Covalent Organic Framework Films. CCS Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Bisbey, R.P.; DeBlase, C.R.; Smith, B.J.; Dichtel, W.R. Two-dimensional Covalent Organic Framework Thin Films Grown in Flow. J. Am. Chem. Soc. 2016, 138, 11433–11436. [Google Scholar] [CrossRef]
- Ratsch, M.; Ye, C.; Yang, Y.; Zhang, A.; Evans, A.M.; Börjesson, K. All-Carbon-Linked Continuous Three-Dimensional Porous Aromatic Framework Films with Nanometer-Precise Controllable Thickness. J. Am. Chem. Soc. 2020, 142, 6548–6553. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.; Heidary, N.; Horch, M.; Gernert, U.; Zebger, I.; Schmidt, J.; Fischer, A.; Thomas, A. Microporous polymer network films covalently bound to gold electrodes. Chem. Commun. 2015, 51, 4283–4286. [Google Scholar] [CrossRef] [Green Version]
- Feldblyum, J.I.; McCreery, C.H.; Andrews, S.C.; Kurosawa, T.; Santos, E.J.G.; Duong, V.; Fang, L.; Ayzner, A.L.; Bao, Z. Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Commun. 2015, 51, 13894–13897. [Google Scholar] [CrossRef]
- Dai, W.; Shao, F.; Szczerbiński, J.; McCaffrey, R.; Zenobi, R.; Jin, Y.; Schlüter, A.D.; Zhang, W. Synthesis of a Two-Dimensional Covalent Organic Monolayer through Dynamic Imine Chemistry at the Air/Water Interface. Angew. Chem. Int. Ed. 2016, 55, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sahabudeen, H.; Qi, H.; Glatz, B.A.; Tranca, D.; Dong, R.; Hou, Y.; Zhang, T.; Kuttner, C.; Lehnert, T.; Seifert, G.; et al. Wafer-sized multifunctional polyimine-based two-dimensional conjugated polymers with high mechanical stiffness. Nat. Commun. 2016, 7, 13461. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Valentino, L.; Stiehl, G.M.; Balch, H.B.; Corcos, A.R.; Wang, F.; Ralph, D.C.; Mariñas, B.J.; Dichtel, W.R. Lewis-Acid-Catalyzed Interfacial Polymerization of Covalent Organic Framework Films. Chem 2018, 4, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Song, X.; Li, Y.; Li, Y.; Peng, Z.; Ye, L.; Chen, L. 2D covalent organic framework thin films: Via interfacial self-polycondensation of an A2B2 type monomer. Chem. Commun. 2020, 56, 3253–3256. [Google Scholar] [CrossRef]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, S.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef]
- Sasmal, H.S.; Halder, A.; Kunjattu, S.H.; Dey, K.; Nadol, A.; Ajithkumar, T.G.; Ravindra Bedadur, P.; Banerjee, R. Covalent Self-Assembly in Two Dimensions: Connecting Covalent Organic Framework Nanospheres into Crystalline and Porous Thin Films. J. Am. Chem. Soc. 2019, 141, 20371–20379. [Google Scholar] [CrossRef]
- Ying, Y.; Yang, Z.; Shi, D.; Peh, S.B.; Wang, Y.; Yu, X.; Yang, H.; Chai, K.; Zhao, D. Ultrathin covalent organic framework film as membrane gutter layer for high-permeance CO2 capture. J. Memb. Sci. 2021, 632, 119384. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 2013, 4, 2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.G.; Lee, C.C.; EL-Mahdy, A.F.M.; Lüder, J.; Yu, M.H.; Li, Z.; Zhu, Z.; Chueh, C.C.; Kuo, S.W. Exploitation of two-dimensional conjugated covalent organic frameworks based on tetraphenylethylene with bicarbazole and pyrene units and applications in perovskite solar cells. J. Mater. Chem. A 2020, 8, 11448–11459. [Google Scholar] [CrossRef]
- Haldar, S.; Chakraborty, D.; Roy, B.; Banappanavar, G.; Rinku, K.; Mullangi, D.; Hazra, P.; Kabra, D.; Vaidhyanathan, R. Anthracene-Resorcinol Derived Covalent Organic Framework as Flexible White Light Emitter. J. Am. Chem. Soc. 2018, 140, 13367–13374. [Google Scholar] [CrossRef]
- Smith, B.J.; Parent, L.R.; Overholts, A.C.; Beaucage, P.A.; Bisbey, R.P.; Chavez, A.D.; Hwang, N.; Park, C.; Evans, A.M.; Gianneschi, N.C.; et al. Colloidal Covalent Organic Frameworks. ACS Cent. Sci. 2017, 3, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Bradshaw, N.P.; Litchfield, B.; Strauss, M.J.; Seckman, B.; Ryder, M.R.; Castano, I.; Gilmore, C.; Gianneschi, N.C.; Mulzer, C.R.; et al. High-Sensitivity Acoustic Molecular Sensors Based on Large-Area, Spray-Coated 2D Covalent Organic Frameworks. Adv. Mater. 2020, 32, 2004205. [Google Scholar] [CrossRef]
- Rotter, J.M.; Weinberger, S.; Kampmann, J.; Sick, T.; Shalom, M.; Bein, T.; Medina, D.D. Covalent Organic Framework Films through Electrophoretic Deposition—Creating Efficient Morphologies for Catalysis. Chem. Mater. 2019, 31, 10008–10016. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrochemically Generated Thin Films of Microporous Polymer Networks: Synthesis, Properties, and Applications. Macromol. Chem. Phys. 2016, 217, 827–841. [Google Scholar] [CrossRef]
- Gu, C.; Chen, Y.; Zhang, Z.; Xue, S.; Sun, S.; Zhang, K.; Zhong, C.; Zhang, H.; Pan, Y.; Lv, Y.; et al. Electrochemical route to fabricate film-like conjugated microporous polymers and application for organic electronics. Adv. Mater. 2013, 25, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-Based Microporous Polymer Networks via Chemical or Electrochemical Oxidative Coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef] [Green Version]
- Räupke, A.; Palma-Cando, A.; Shkura, E.; Teckhausen, P.; Polywka, A.; Görrn, P.; Scherf, U.; Riedl, T. Highly sensitive gas-phase explosive detection by luminescent microporous polymer networks. Sci. Rep. 2016, 6, 29118. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Huang, N.; Chen, Y.; Qin, L.; Xu, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. π-Conjugated Microporous Polymer Films: Designed Synthesis, Conducting Properties, and Photoenergy Conversions. Angew. Chem. Int. Ed. 2015, 54, 13594–13598. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Huang, N.; Chen, Y.; Zhang, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. Porous Organic Polymer Films with Tunable Work Functions and Selective Hole and Electron Flows for Energy Conversions. Angew. Chem. Int. Ed. 2016, 55, 3049–3053. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Zhang, X.; Zheng, Z.; Bi, S.; Zhang, Y.; Zhou, H. A conjugated microporous polymer film fabricated by in situ electro-chemical deposition as a hole transporting layer in organic photovoltaics. J. Mater. Chem. C 2018, 6, 9044–9048. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wu, J.; Liu, K.; Li, D.; Meng, Q.; Zhu, G. Electropolymerization Porous Aromatic Framework Film As a Hole-Transport Layer for Inverted Perovskite Solar Cells with Superior Stability. ACS Appl. Mater. Interfaces 2017, 9, 43688–43695. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Zhang, X.; Lu, Z.; Xiao, H.; Li, C.; Li, G.; Xu, X.; Chen, X.; Bo, Z. Hyperbranched polymer as an acceptor for polymer solar cells. Chem. Commun. 2017, 53, 537–540. [Google Scholar] [CrossRef]
- Bildirir, H.; Di Carlo Rasi, D.; Wienk, M.M.; Janssen, R.A.J.; Avgeropoulos, A.; Gregoriou, V.G.; Allard, S.; Scherf, U.; Chochos, C.L. New n-Type Solution Processable All Conjugated Polymer Network: Synthesis, Optoelectronic Characterization, and Application in Organic Solar Cells. Macromol. Rapid Commun. 2018, 39, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Xu, Z.; Tang, X.; Liu, T.; Dong, X.; Zhang, X.; Zheng, N.; Xie, Z.; Liu, Y. Multifunctional Two-Dimensional Conjugated Materials for Dopant-Free Perovskite Solar Cells with Efficiency Exceeding 22%. ACS Energy Lett. 2021, 6, 1521–1532. [Google Scholar] [CrossRef]
- Castano, I.; Evans, A.M.; Dos Reis, R.; Dravid, V.P.; Gianneschi, N.C.; Dichtel, W.R. Mapping Grains, Boundaries, and Defects in 2D Covalent Organic Framework Thin Films. Chem. Mater. 2021, 33, 1341–1352. [Google Scholar] [CrossRef]
- Fenton, J.L.; Burke, D.W.; Qian, D.; La Cruz, M.O.D.; Dichtel, W.R. Polycrystalline Covalent Organic Framework Films Act as Adsorbents, Not Membranes. J. Am. Chem. Soc. 2021, 143, 1466–1473. [Google Scholar] [CrossRef]
- Dey, K.; Bhunia, S.; Sasmal, H.S.; Reddy, C.M.; Banerjee, R. Self-Assembly-Driven Nanomechanics in Porous Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2021, 143, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-X.; Li, Z.-J.; Wei, L.; Ding, S.-Y.; Zhang, Y.-B.; Wang, W. A Dynamic Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 4995–4998. [Google Scholar] [CrossRef]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500. [Google Scholar] [CrossRef]
- Mähringer, A.; Medina, D.D. Taking stock of stacking. Nat. Chem. 2020, 12, 985–987. [Google Scholar] [CrossRef]
- Souto, M.; Perepichka, D.F. Electrically conductive covalent organic frameworks: Bridging the fields of organic metals and 2D materials. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Kang, J.; Huang, S.; Jiang, K.; Lu, C.; Chen, Z.; Zhu, J.; Yang, C.; Ciesielski, A.; Qiu, F.; Zhuang, X. 2D Porous Polymers with sp2-Carbon Connections and Sole sp2-Carbon Skeletons. Adv. Funct. Mater. 2020, 30, 2000857. [Google Scholar] [CrossRef]
- Byun, Y.; Xie, L.S.; Fritz, P.; Ashirov, T.; Dincă, M.; Coskun, A. A Three-Dimensional Porous Organic Semiconductor Based on Fully sp2-Hybridized Graphitic Polymer. Angew. Chem. Int. Ed. 2020, 59, 15166–15170. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Liu, H.; Duan, C.; Pan, Q.; Zhu, J.; Hu, F.; Ma, X.; Jiu, T.; Li, Z.; et al. Highly Conjugated Three-Dimensional Covalent Organic Frameworks Based on Spirobifluorene for Perovskite Solar Cell Enhancement. J. Am. Chem. Soc. 2018, 140, 10016–10024. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, B.; Liu, F.; Stevens, C.; Brady, M.A.; Cai, S.; Wang, C.; Russell, T.P.; Tan, T.W.; Liu, Y. Orientation transitions during the growth of imine covalent organic framework thin films. J. Mater. Chem. C 2017, 5, 5090–5095. [Google Scholar] [CrossRef] [Green Version]
- Phelan, N.F.; Orchin, M. Cross conjugation. J. Chem. Educ. 1968, 45, 633. [Google Scholar] [CrossRef]
- Van Pruissen, G.W.P.; Brebels, J.; Hendriks, K.H.; Wienk, M.M.; Janssen, R.A.J. Effects of cross-conjugation on the optical absorption and frontier orbital levels of donor-acceptor polymers. Macromolecules 2015, 48, 2435–2443. [Google Scholar] [CrossRef]
- Gross, Y.M.; Trefz, D.; Tkachov, R.; Untilova, V.; Brinkmann, M.; Schulz, G.L.; Ludwigs, S. Tuning Aggregation by Regioregularity for High-Performance n-Type P(NDI2OD-T2) Donor-Acceptor Copolymers. Macromolecules 2017, 50, 5353–5366. [Google Scholar] [CrossRef]
- Fall, S.; Biniek, L.; Odarchenko, Y.; Anokhin, D.V.; De Tournadre, G.; Lévêque, P.; Leclerc, N.; Ivanov, D.A.; Simonetti, O.; Giraudet, L.; et al. Tailoring the microstructure and charge transport in conjugated polymers by alkyl side-chain engineering. J. Mater. Chem. C 2016, 4, 286–294. [Google Scholar] [CrossRef]
- Jin, S.; Ding, X.; Feng, X.; Supur, M.; Furukawa, K.; Takahashi, S.; Addicoat, M.; El-Khouly, M.E.; Nakamura, T.; Irle, S.; et al. Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. 2013, 52, 2017–2021. [Google Scholar] [CrossRef]
- Jin, S.; Supur, M.; Addicoat, M.; Furukawa, K.; Chen, L.; Nakamura, T.; Fukuzumi, S.; Irle, S.; Jiang, D. Creation of Superheterojunction Polymers via Direct Polycondensation: Segregated and Bicontinuous Donor-Acceptor π-Columnar Arrays in Covalent Organic Frameworks for Long-Lived Charge Separation. J. Am. Chem. Soc. 2015, 137, 7817–7827. [Google Scholar] [CrossRef]
- Haase, F.; Gottschling, K.; Stegbauer, L.; Germann, L.S.; Gutzler, R.; Duppel, V.; Vyas, V.S.; Kern, K.; Dinnebier, R.E.; Lotsch, B. V Tuning the stacking behaviour of a 2D covalent organic framework through non-covalent interactions. Mater. Chem. Front. 2017, 1, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Pütz, A.M.; Terban, M.W.; Bette, S.; Haase, F.; Dinnebier, R.E.; Lotsch, B. V Total scattering reveals the hidden stacking disorder in a 2D covalent organic framework. Chem. Sci. 2020, 11, 12647–12654. [Google Scholar] [CrossRef]
- Castet, F.; D’Avino, G.; Muccioli, L.; Cornil, J.; Beljonne, D. Charge separation energetics at organic heterojunctions: On the role of structural and electrostatic disorder. Phys. Chem. Chem. Phys. 2014, 16, 20279–20290. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shao, P.; Chen, S.; Li, G.; Feng, X.; Chen, X.; Zhang, H.-J.; Lin, J.; Jiang, Y.-B. Supramolecular Alternating Donor–Acceptor Assembly toward Intercalated Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 3712–3717. [Google Scholar] [CrossRef]
- Chen, X.; Addicoat, M.; Irle, S.; Nagai, A.; Jiang, D. Control of Crystallinity and Porosity of Covalent Organic Frameworks by Managing Interlayer Interactions Based on Self-Complementary π-Electronic Force. J. Am. Chem. Soc. 2013, 135, 546–549. [Google Scholar] [CrossRef]
- Qian, C.; Qi, Q.Y.; Jiang, G.F.; Cui, F.Z.; Tian, Y.; Zhao, X. Toward Covalent Organic Frameworks Bearing Three Different Kinds of Pores: The Strategy for Construction and COF-to-COF Transformation via Heterogeneous Linker Exchange. J. Am. Chem. Soc. 2017, 139, 6736–6743. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Coupry, D.E.; Addicoat, M.A.; Okushita, K.; Nishimura, K.; Heine, T.; Jiang, D. Multiple-component covalent organic frameworks. Nat. Commun. 2016, 7, 12325. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, T.; Haase, F.; Trenker, S.; Biswal, B.P.; Savasci, G.; Duppel, V.; Moudrakovski, I.; Ochsenfeld, C.; Lotsch, B.V. Sub-stoichiometric 2D covalent organic frameworks from tri- and tetratopic linkers. Nat. Commun. 2019, 10, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.L.; Yang, A.; Flanders, N.C.; Yeung, M.T.; Sheppard, D.T.; Dichtel, W.R. Two-Dimensional Covalent Organic Framework Solid Solutions. J. Am. Chem. Soc. 2021, 143, 7081–7087. [Google Scholar] [CrossRef]
- Stassin, T.; Verbeke, R.; Cruz, A.J.; Rodríguez-Hermida, S.; Stassen, I.; Marreiros, J.; Krishtab, M.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J.; et al. Porosimetry for Thin Films of Metal–Organic Frameworks: A Comparison of Positron Annihilation Lifetime Spectroscopy and Adsorption-Based Methods. Adv. Mater. 2021, 33, 2006993. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Kamencek, T.; Zojer, E. Understanding the origin of serrated stacking motifs in planar two-dimensional covalent organic frameworks. Nanoscale 2021, 13, 9339–9353. [Google Scholar] [CrossRef] [PubMed]
- Osterrieth, J.; Rampersad, J.; Madden, D.G.; Rampal, N.; Skoric, L.; Connolly, B.; Allendorf, M.; Stavila, V.; SNIDER, J.; Ameloot, R. How Reproducible Are Surface Areas Calculated from the BET Equation? ChemRxiv 2021. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bildirir, H. Have Covalent Organic Framework Films Revealed Their Full Potential? Crystals 2021, 11, 762. https://doi.org/10.3390/cryst11070762
Bildirir H. Have Covalent Organic Framework Films Revealed Their Full Potential? Crystals. 2021; 11(7):762. https://doi.org/10.3390/cryst11070762
Chicago/Turabian StyleBildirir, Hakan. 2021. "Have Covalent Organic Framework Films Revealed Their Full Potential?" Crystals 11, no. 7: 762. https://doi.org/10.3390/cryst11070762
APA StyleBildirir, H. (2021). Have Covalent Organic Framework Films Revealed Their Full Potential? Crystals, 11(7), 762. https://doi.org/10.3390/cryst11070762






