Effect of Internal Radiation on Process Parameters in the Global Simulation of Growing Large-Size Bulk β-Ga2O3 Single Crystals with the Czochralski Method
Abstract
:1. Introduction
2. Simulation
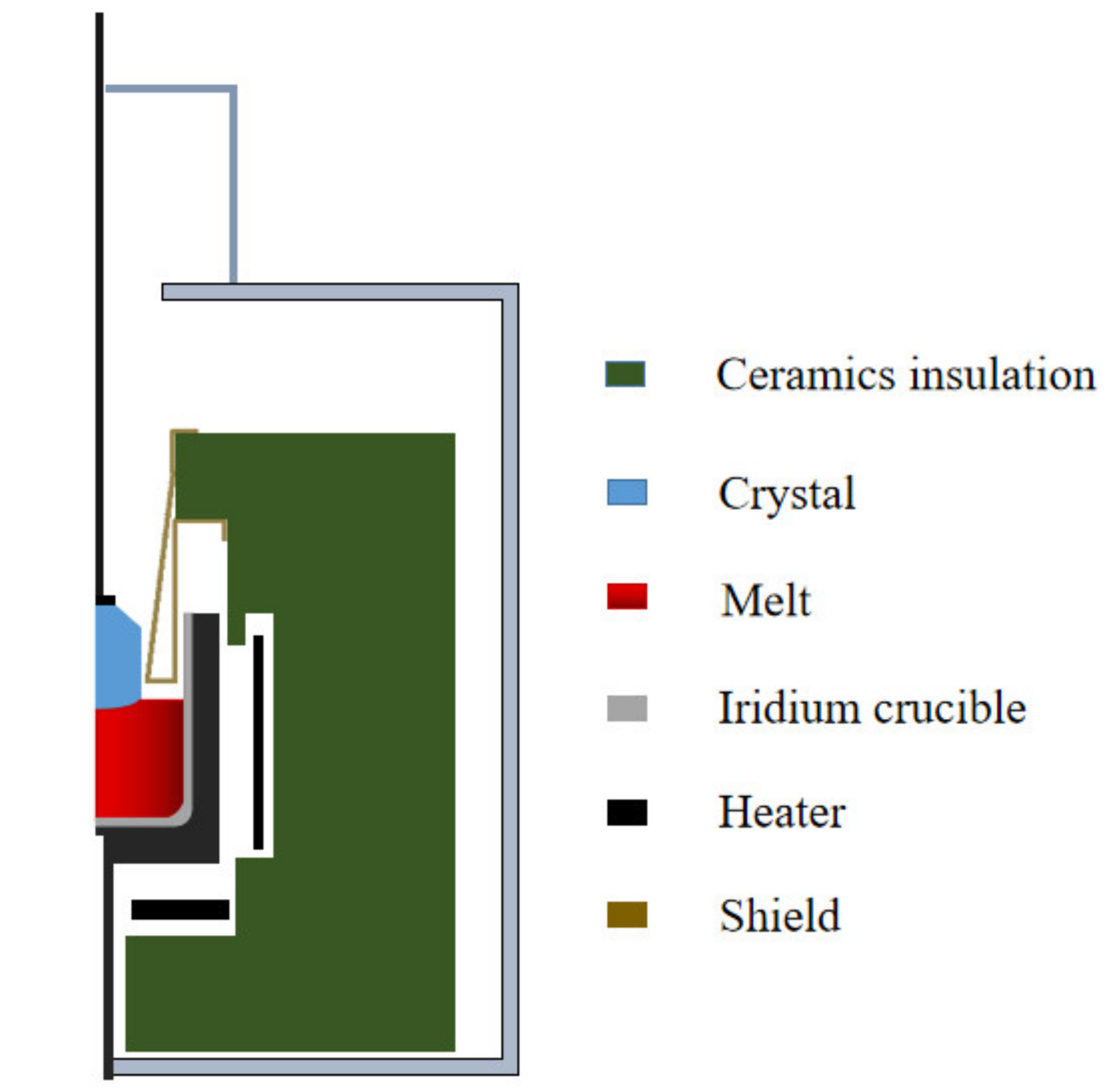
2.1. Geometric Model
2.2. Mathematical Model
- (1)
- The crystal is fully cylindrical and isotropic
- (2)
- The melt is considered as an incompressible liquid. The temperature field in the crucible is axially symmetric.
- (3)
- Due to the relatively low crystallization rates (2 mm/h), the growing process can be seen as quasi-static.
- (4)
- The phase interface shape has little effect on the overall temperature distribution and heater power. It is convex over the entire growth process with slight changes slightly in thickness. Therefore, at this stage, we have neglected the changes in the phase interface.
2.2.1. Thermophysical Properties
2.2.2. The Actual Governing Equations of the Model
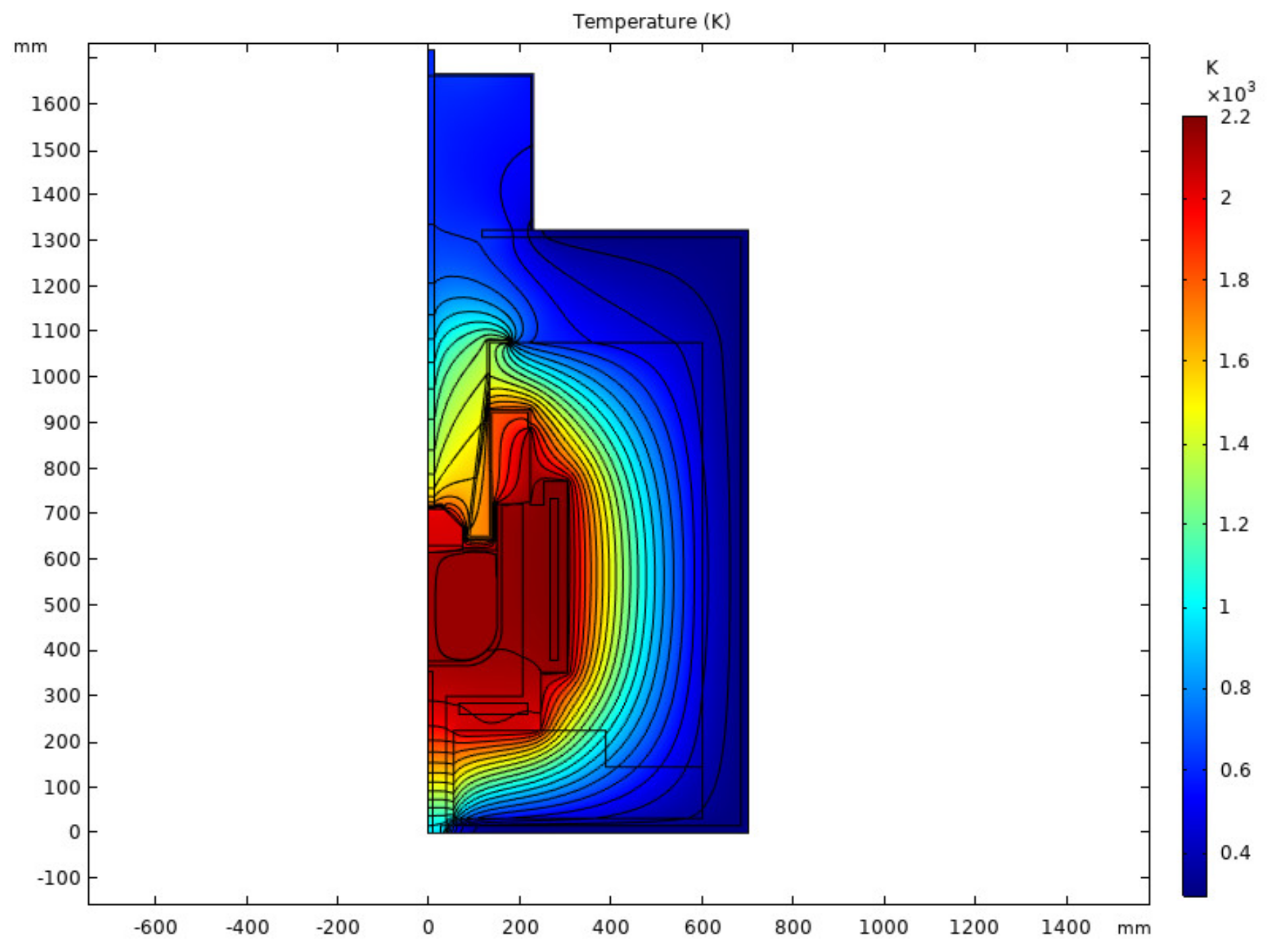
2.2.3. Mesh Model and Boundaries
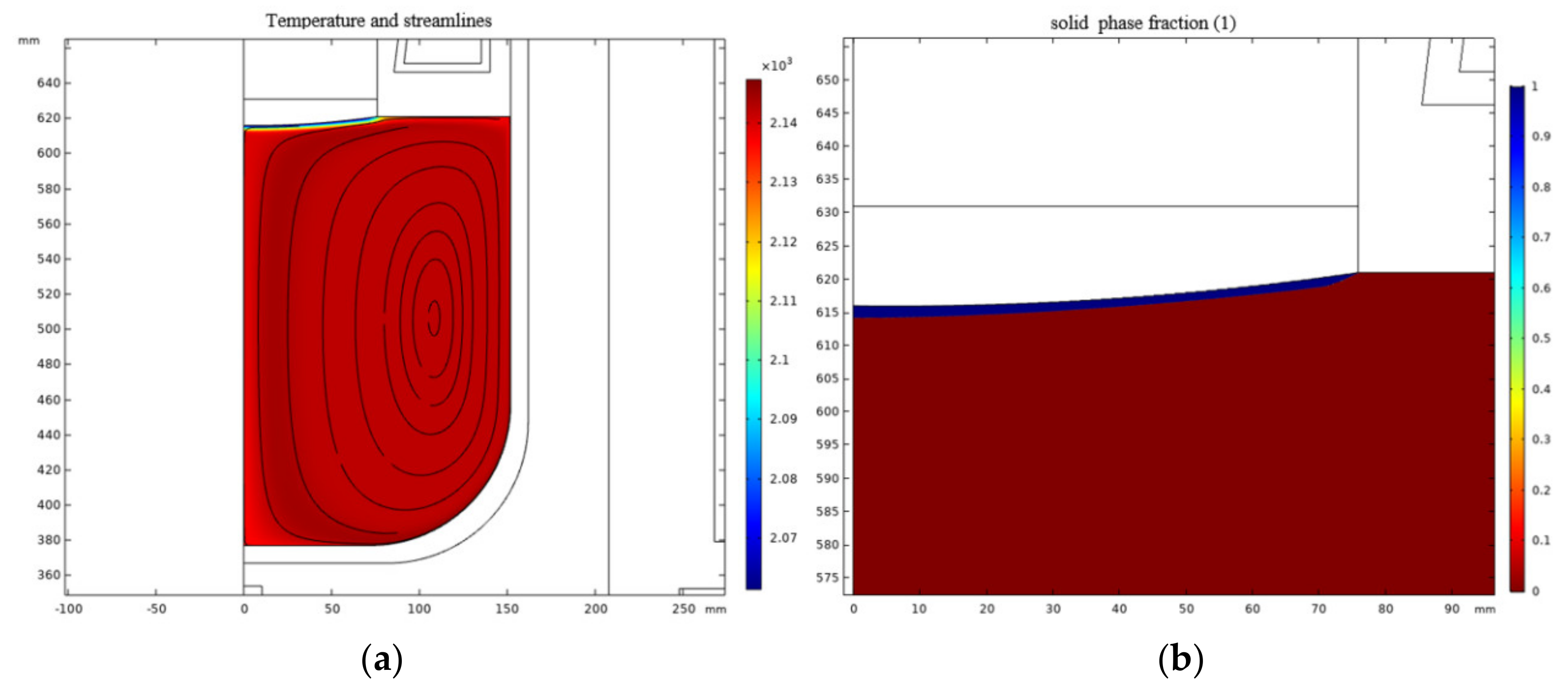
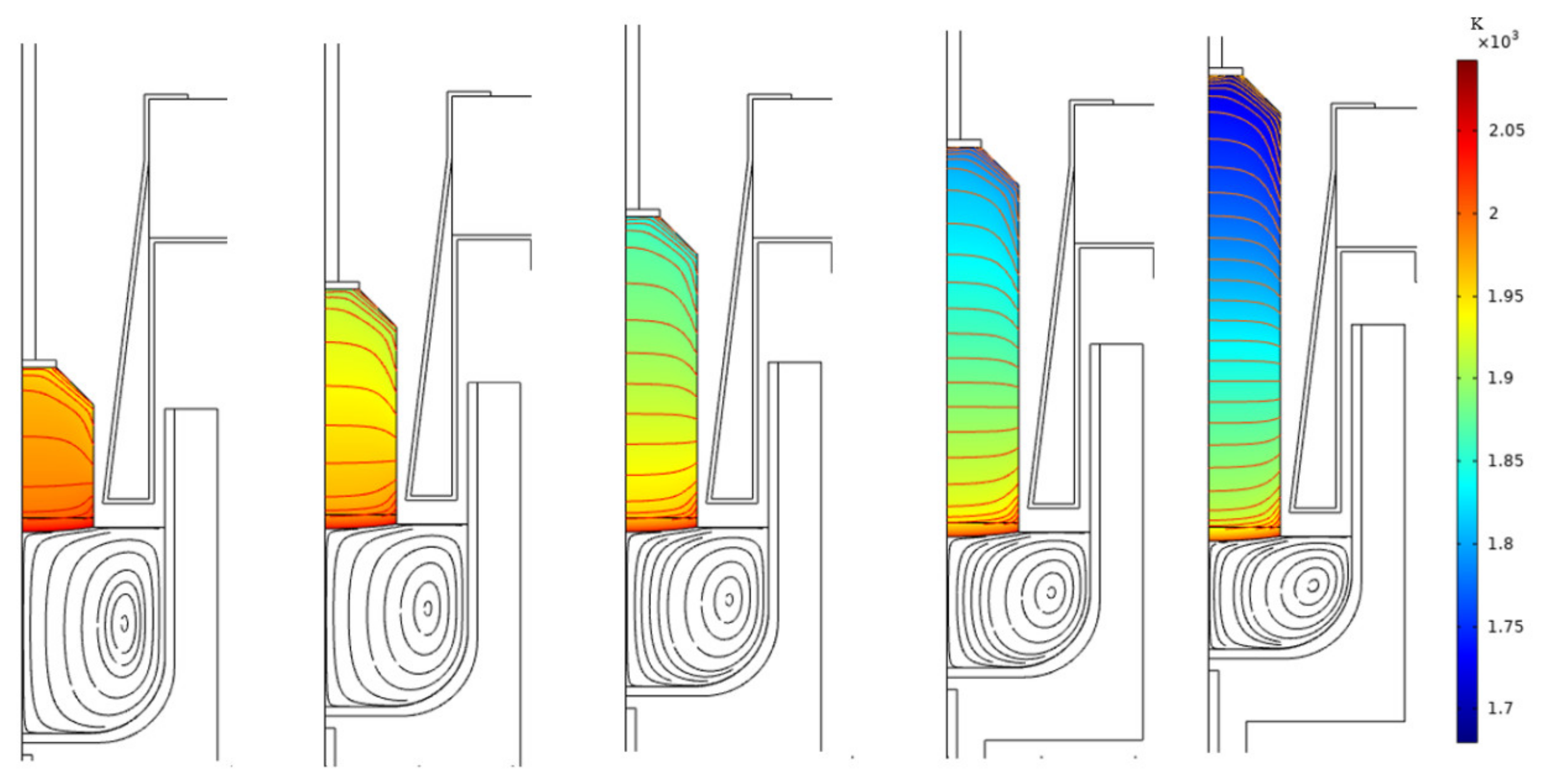
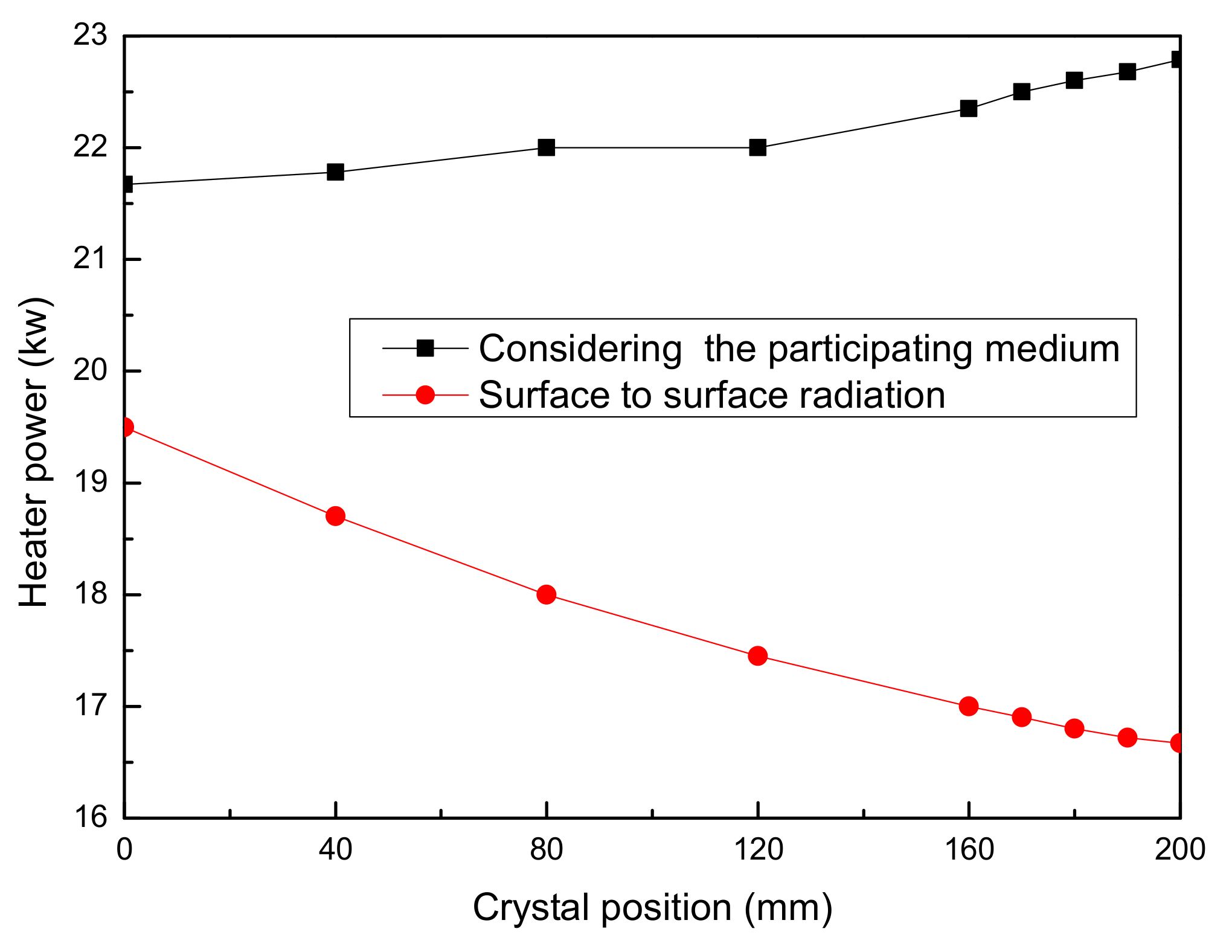
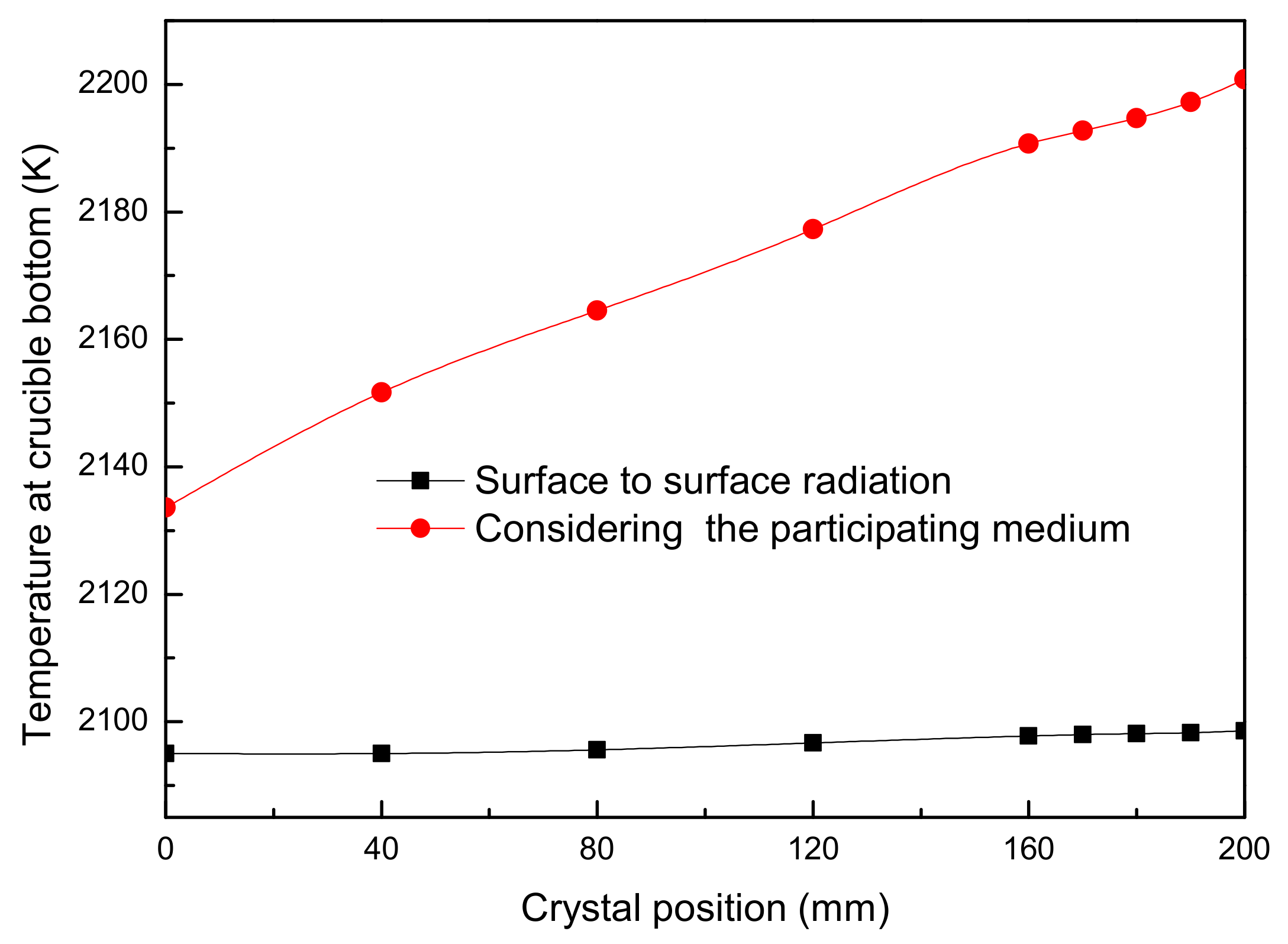
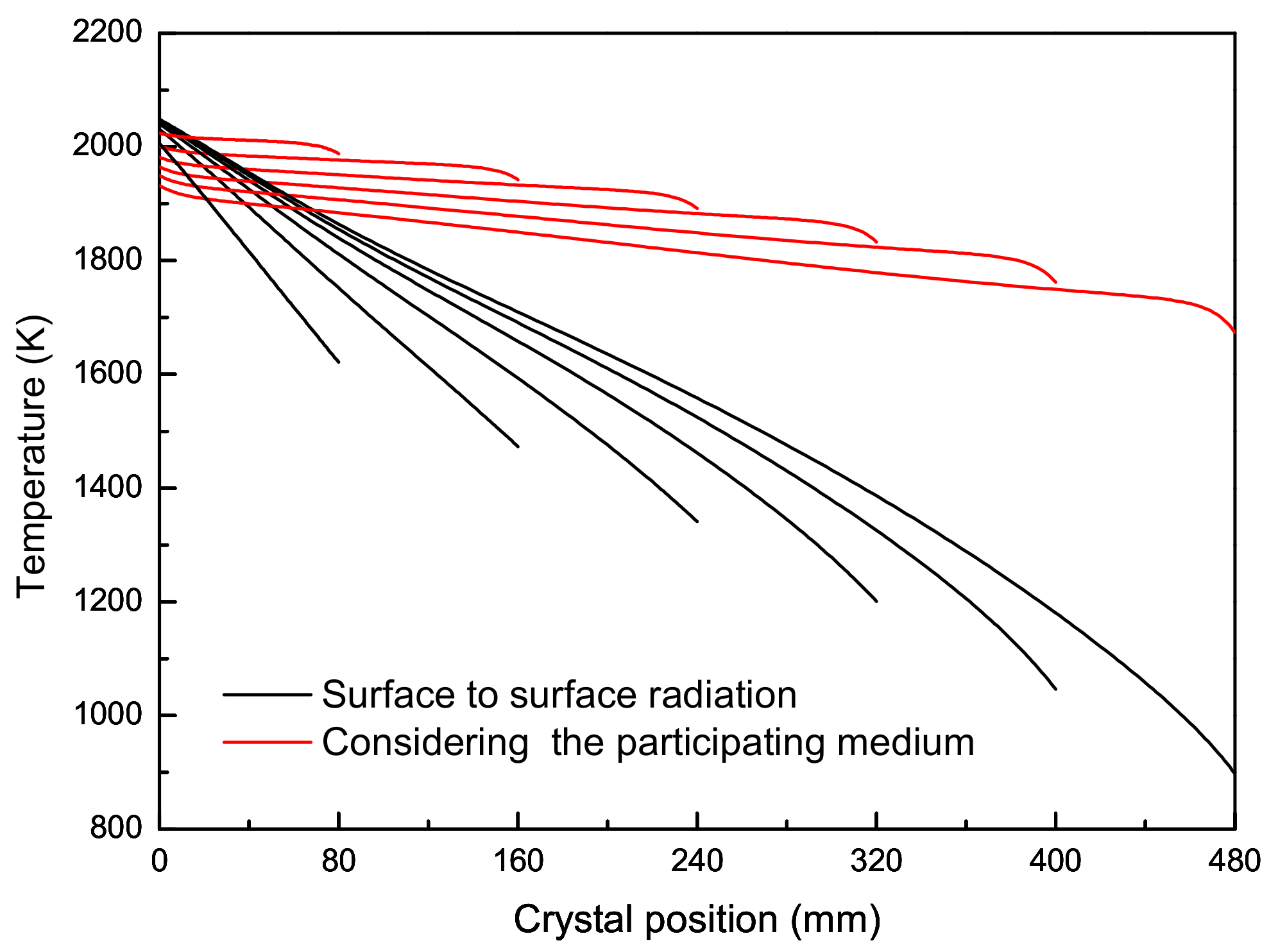
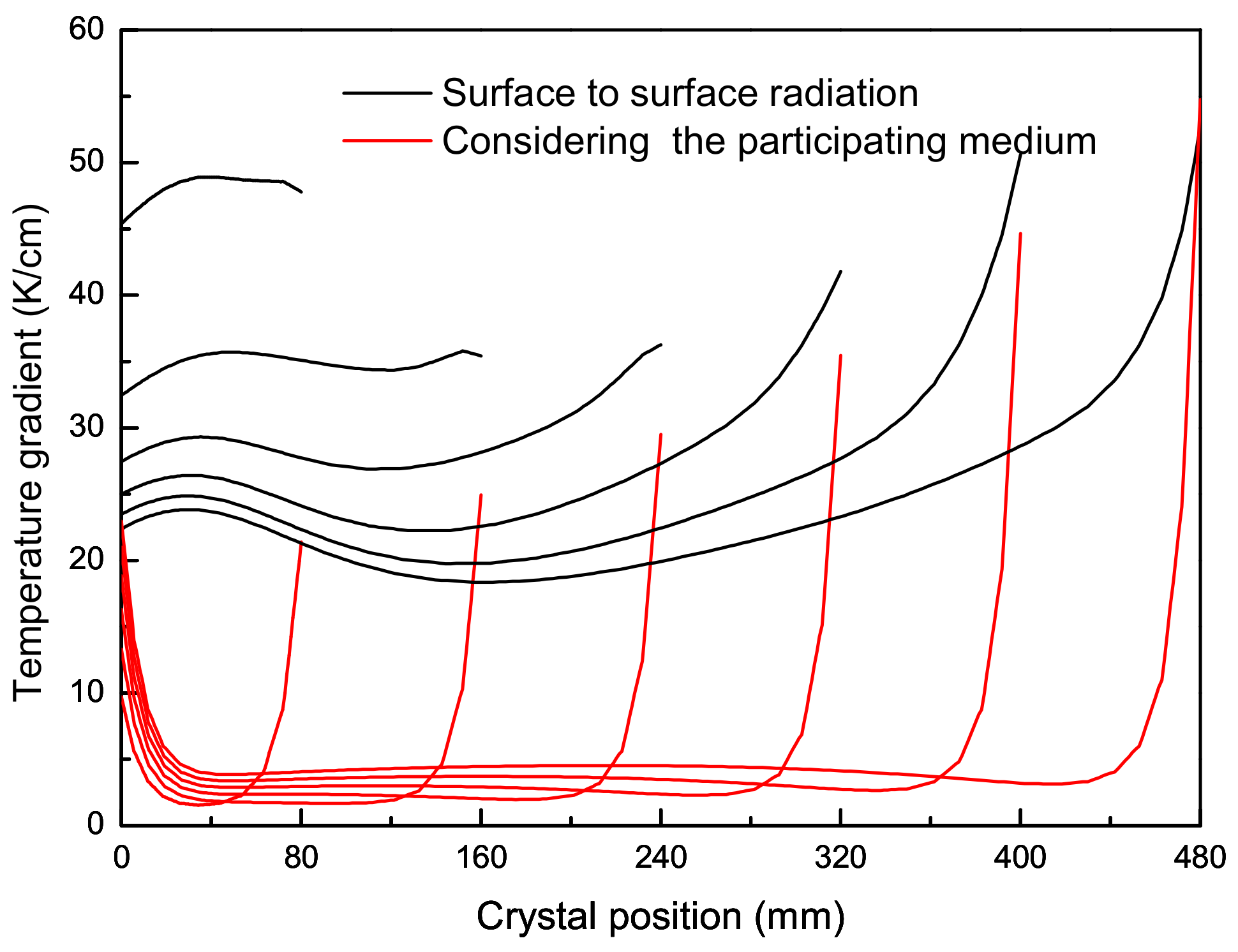
2.3. Results and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cp | the Specific Heat Capacity |
| Fr | force in r direction |
| force in the circumferential direction | |
| force in z-direction | |
| G | irradiation |
| J | radiosity |
| k | thermal conductivity |
| p | pressure |
| Q | external heating source |
| r | radial coordinate of the melt/crystal |
| T | temperature |
| u | fluid velocity |
| ε | emissivity |
| υ | rotational velocity |
| ω | axial angular velocity |
| ρ | density |
| µ | viscosity |
| σ | blackbody radiation constant |
References
- Fornari, R.; Pavesi, M.; Montedoro, V.; Klimm, D.; Mezzadri, F.; Cora, I.; Pécz, B.; Boschi, F.; Parisinia, A.; Baraldi, A.; et al. Thermal stability of ε-Ga2O3 polymorph. Acta Mater. 2017, 140, 411–416. [Google Scholar] [CrossRef]
- Lee, S.D.; Akaiwa, K.; Fujita, S. Thermal stability of single crystalline alpha gallium oxide films on sapphire substrates. Phys. Status Solidi (c) 2013, 10, 1592–1595. [Google Scholar] [CrossRef]
- Kim, M.; Seo, J.-H.; Singisetti, U.; Ma, Z. Recent advances in free-standing single crystalline wide band-gap semiconductors and their applications: GaN, SiC, ZnO, β-Ga2O3, and diamond. J. Mater. Chem. C 2017, 5, 8338–8354. [Google Scholar] [CrossRef]
- Mastro, M.A.; Kuramata, A.; Calkins, J.; Kim, J.; Ren, F.; Pearton, S. Perspective—opportunities and future directions for Ga2O3. ECS J. Solid State Sci. Technol. 2017, 6, P356–P359. [Google Scholar] [CrossRef]
- Sasaki, K.; Higashiwaki, M.; Kuramata, A.; Masui, T.; Yamakoshi, S. MBE grown Ga2O3 and its power device applications. J. Cryst. Growth 2013, 378, 591–595. [Google Scholar] [CrossRef]
- β-Ga2O3 Substrates. Available online: http://www.tamura-ss.co.jp/en/products/gao/index.html (accessed on 1 July 2020).
- Hoshikawa, K.; Ohba, E.; Kobayashi, T.; Yanagisawa, J.; Miyagawa, C.; Nakamura, Y. Growth of beta-Ga2O3 single crystals using vertical bridgman method in ambient air. J. Cryst. Growth 2016, 447, 36–41. [Google Scholar] [CrossRef]
- Galazka, Z.; Uecker, R.; Irmscher, K.; Albrecht, M.; Klimm, D.; Pietsch, M.; Brützam, M.; Bertram, R.; Ganschow, S.; Fornari, R. Czochralski growth and characterization of β-Ga2O3 single crystals. Cryst. Res. Technol. 2010, 45, 1229–1236. [Google Scholar] [CrossRef]
- Banerjee, J.; Muralidhar, K. Role of internal radiation during czochralski growth of YAG and Nd: YAG crystals. Int. J. Therm. Sci. 2006, 45, 151–167. [Google Scholar] [CrossRef]
- Galazka, Z.; Irmscher, K.; Uecker, R.; Bertram, R.; Pietsch, M.; Kwasniewski, A.; Naumann, M.; Schulz, T.; Schewski, R.; Klimm, D.; et al. On the bulk β-Ga2O3 single crystals grown by the czochralski method. J. Cryst. Growth 2014, 404, 184–191. [Google Scholar] [CrossRef]
- Galazka, Z.; Uecker, R.; Klimm, D.; Irmscher, K.; Naumann, M.; Pietsch, M.; Kwasniewski, A.; Bertram, R.; Ganschow, S.; Bickermann, M. Scaling-up of bulk β-Ga2O3 single crystals by the czochralski method. ECS J. Solid State Sci. Technol. 2016, 6, Q3007. [Google Scholar] [CrossRef]
- Galazka, Z.; Ganschow, S.; Fiedler, A.; Bertram, R.; Klimm, D.; Irmscher, K.; Schewski, R.; Pietsch, M.; Albrecht, M.; Bickermann, M. Doping of czochralski-grown bulk β-Ga2O3 single crystals with Cr, Ce and Al. J. Cryst. Growth 2018, 486, 82–90. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Mihelčić, M.; Wingerath, K.; Pirron, C. Three-dimensional simulations of the czochralski bulk flow. J. Cryst. Growth 1984, 69, 473–488. [Google Scholar] [CrossRef]
- Kimura, H.; Numazawa, T.; Sato, M.; Maeda, H. Single crystal growth and characterization of (Dy1−xGdx)3Ga5O12 Garnets. J. Cryst. Growth 1988, 87, 523–528. [Google Scholar] [CrossRef]
- Uecker, R.; Wilke, H.; Schlom, D.; Velickov, B.; Reiche, P.; Polity, A.; Bernhagen, M.; Rossberg, M. Spiral formation during czochralski growth of rare-earth scandates. J. Cryst. Growth 2006, 295, 84–91. [Google Scholar] [CrossRef]
- Fei, Y.; Chou, M.M.; Chai, B.H. Crystal growth and morphology of substituted gadolinium gallium garnet. J. Cryst. Growth 2002, 240, 185–189. [Google Scholar] [CrossRef]
- Brandle, C.; Fratello, V.; Valentino, A.; Stokowski, S. Effects of impurities and atmosphere on the growth of Cr-doped gadolinium scandium gallium garnet. I. J. Cryst. Growth 1987, 85, 223–228. [Google Scholar] [CrossRef]
- Fratello, V.; Brandle, C.; Valentino, A.; Stokowski, S. Effects of impurities and atmosphere on the growth of Cr-doped gadolinium scandium gallium garnet. II. J. Cryst. Growth 1987, 85, 229–233. [Google Scholar] [CrossRef]
- Okano, Y.; Ikeya, T.; Hoshikawa, K.; Fukuda, T. Behavior of RAlO3 (R=Ho, Er and Dy) during crystal growth by the Czochralski method. J. Cryst. Growth 1993, 131, 616–619. [Google Scholar] [CrossRef]
- Kochurikhin, V.; Shimamura, K.; Fukuda, T. Investigation of spiral bending of straight Czochralski grown dysprosium gallium aluminium garnet crystals. J. Cryst. Growth 1996, 160, 181–183. [Google Scholar] [CrossRef]
- Kochurikhin, V.; Shimamura, K.; Fukuda, T. The influence of dopants on the interface stability during Dy3Ga5O12 single crystal growth. J. Cryst. Growth 1994, 143, 232–236. [Google Scholar] [CrossRef]
- Schwabe, D.; Uecker, R.; Bernhagen, M.; Galazka, Z. An analysis of and a model for spiral growth of czochralski-grown oxide crystals with high melting point. J. Cryst. Growth 2011, 335, 138–147. [Google Scholar] [CrossRef]
- Czochralski, J. Ein neues verfahren zur messung der kristallisationsgeschwindigkeit der metalle. Z. Phys. Chem. 1918, 92, 219–221. [Google Scholar] [CrossRef]
- Teal, G.K.; Little, J.B. Growth of germanium single crystals. One physics ellipse, college PK, MD 20740-3844 USA: American Physical Soc. Phys. Rev. 1950, 78, 647. [Google Scholar]
- Nassau, K.; van Uitert, L.G. Preparation of large calcium-tungstate crystals containing paramagnetic ions for maser applications. J. Appl. Phys. 1960, 31, 1508. [Google Scholar] [CrossRef]
- Bardsley, W.; Green, G.W.; Holliday, C.H.; Hurle, D.T.J. Automatic control of czochralski crystal growth. J. Cryst. Growth 1972, 16, 277–279. [Google Scholar] [CrossRef]
- Kyle, T.R.; Zydzik, G. Automated crystal puller. Mater. Res. Bull. 1973, 8, 443–450. [Google Scholar] [CrossRef]
- Miller, W.; Böttcher, K.; Galazka, Z.; Schreuer, J. Numerical modelling of the czochralski growth of β-Ga2O3. Crystals 2017, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Liu, B.; Yu, Y.; Liu, S.; Gao, B. Numerical analysis of difficulties of growing large-size bulk β-Ga2O3 single crystals with the Czochralski Method. Crystals 2021, 11, 25. [Google Scholar] [CrossRef]
- Galazka, Z. Czochralski method. In Gallium Oxide; Higashiwaki, M., Fujita, S., Eds.; Springer Series in Materials Science; Springer: Cham, Switzerland, 2020; Volume 293. [Google Scholar] [CrossRef]
- Jackson, A.J.; Walsh, A. Oxidation of GaN: An ab initio thermodynamic approach. Phys. Rev. B 2013, 88. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Niu, S.; Ho, S.L.; Fu, W.N.; Zhu, J. A parameterized mesh generation and refinement method for finite element parameter sweeping analysis of electromagnetic devices. IEEE Trans. Magn. 2012, 48, 239–242. [Google Scholar] [CrossRef]
- Nikolaev, V.I.; Stepanov, S.I.; Romanov, A.E.; Bougrov, V.E. Single crystals of electronic materials growth and properties. In Electronic and Optical Materials; Woodhead Publishing: Sawston, Britain, 2019; pp. 487–521. [Google Scholar]
- Srivastava, R.D.; Farber, M. Thermodynamic properties of group 3 oxides. Chem. Rev. 1978, 78, 627–638. [Google Scholar] [CrossRef]
- Derby, J.J.; Xiao, Q. Some effects of crystal rotation on large-scale czochralski oxide growth: Analysis via a hydrodynamic thermal-capillary model. J. Cryst. Growth 1991, 113, 575–586. [Google Scholar] [CrossRef]
- Dingwell, D.B. Density of Ga2O3 liquid. J. Am. Ceram. Soc. 1992, 75, 1656–1657. [Google Scholar] [CrossRef] [Green Version]
- Santia, M.D.; Tandon, N.; Albrecht, J.D. Lattice thermal conductivity in β−Ga2O3 from first principles. Appl. Phys. Lett. 2015, 107. [Google Scholar] [CrossRef]
- Slomski, M.; Blumenschein, N.; Paskov, P.P.; Muth, J.F.; Paskova, T. Anisotropic thermal conductivity of β-Ga2O3 at elevated temperatures: Effect of Sn and Fe dopants. J. Appl. Phys. 2017, 121. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Yang, X.; Ma, W.; Lv, G.; Wei, K.; Luo, T.; Chen, D. A modified vacuum directional solidification system of multicrystalline silicon based on optimizing for heat transfer. J. Cryst. Growth 2014, 400, 7–14. [Google Scholar] [CrossRef]
- Gresho, P.M.; Sani, R.L. Incompressible Flow and the FInite Element Method. Volume 1: Advection-Diffusion and Isothermal Laminar Flow; John Wiley & Sons: Hoboken, NJ, USA, 1998; Volume 2, p. 469. [Google Scholar]
| Property | Symbol | Melt | Solid |
|---|---|---|---|
| Density | ρ (kg/m3) | 4800 | 5945 |
| Specific heat | Cp (J/(kg·K)) | 720 | 560 |
| Thermal conductivity | λ (W/(m·K)) | 5 | 21 at 300 K 7.83 at 2110 K |
| Viscosity | µ (Ns/m2) | 0.1 | - |
| Reference temperature | Tm | 1820 ± 10 °C | |
| Thermal expansion coefficient | β (K−1) | ||
| Emissivity | ε | 0.3 | |
| Enthalpy | ΔH kJ/mol | −1089 | |
| Absorption coefficient | 4–100 (1/cm) | ||
| Refractive index | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Liu, B.; Yu, Y.; Song, B.; Han, P.; Liu, S.; Gao, B. Effect of Internal Radiation on Process Parameters in the Global Simulation of Growing Large-Size Bulk β-Ga2O3 Single Crystals with the Czochralski Method. Crystals 2021, 11, 763. https://doi.org/10.3390/cryst11070763
Tang X, Liu B, Yu Y, Song B, Han P, Liu S, Gao B. Effect of Internal Radiation on Process Parameters in the Global Simulation of Growing Large-Size Bulk β-Ga2O3 Single Crystals with the Czochralski Method. Crystals. 2021; 11(7):763. https://doi.org/10.3390/cryst11070763
Chicago/Turabian StyleTang, Xia, Botao Liu, Yue Yu, Botao Song, Pengfei Han, Sheng Liu, and Bing Gao. 2021. "Effect of Internal Radiation on Process Parameters in the Global Simulation of Growing Large-Size Bulk β-Ga2O3 Single Crystals with the Czochralski Method" Crystals 11, no. 7: 763. https://doi.org/10.3390/cryst11070763







