1. Introduction
Currently, white light emitting diodes (WLEDs) are displacing traditional light sources due to their high luminous efficiency, energy saving ability, long lifetime, and environmental friendliness [
1]. At present, a conventional WLED in the so-called volume casting approach (VCA) is based on the blue LED chip and yellow emitting Y
3Al
5O
12:Ce (YAG:Ce) powder phosphor embedded in the epoxy resin [
2]. However, YAG:Ce ceramics or crystal plates also accessible for light conversion, producing high power WLEDs in the planar casting approach (PCA) [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12]. Ceramic phosphors based on the different kinds of Ce
3+-doped Ln
3Al
5O
12 garnets have also been developed for PCA application [
4,
5,
6,
7,
8,
9,
10,
11,
12].
The {Y}
3[Al]
2(Al)
3O
12 garnet structure is characterized by a great flexibility, which allows for the relatively simple replacing of cations in the dodecahedral { }, octahedral [ ], and tetrahedral ( ) sites. For this reason, it is possible to modify the content of this garnet for optimization of the Ce
3+ spectroscopic properties for the demands of applications in WLEDs. Recently, a new class of garnet phosphors based on Ce
3+-doped A
3B
2C
3O
12 (A = Ca, R = Y, Lu; B = Mg, Sc, Al, Ga; C = Ga, Al, Si) silicate garnets has been proposed for creation of high-power WLEDs [
13,
14,
15,
16,
17,
18,
19,
20]. The ceramics of Ce
3+-doped {Ca
2R}[B
2-x C
x](Si
3-yC
y)O
12:Ce (R = Y, Lu; B = Sc, Ga; C = Ga, Al; x, y = 0–1) and {Ca
2Y}[Sc]
2(Si)
3O
12:Ce garnets were crystallized for use as LED converters and their luminescent properties were investigated as well [
21,
22]. The {Ca
3}[Sc]
2(Si)
3O
12:Ce and {Ca
2Y}[Sc]
2(Si)
3O
12:Ce garnets were also obtained in the form of single crystalline films (SCFs) using the liquid phase epitaxy (LPE) growth method for application as blue LED converters and laser media [
23,
24,
25]. Furthermore, these two types of garnets and other garnet compounds of this family are currently considered as prospective materials for the creation of new advanced SCF scintillators, cathodoluminescent screens, and solar cells [
23,
24].
However, many questions in studying the luminescent properties of mixed silicate garnets are still open today due to the lack of single crystal samples grown by traditional methods, such as Czochralski, Bridgman, and micro-pulling-down techniques, for basic investigations and practical applications. First, it is interesting to investigate the influence of the Ca2+ -Si4+ pair in the YAG host with regards to the creation of different kinds of Ce3+-based centers due to the different local disorder induced by doping with +2 and +4 charged cations. Another important task of these investigations is connected with the estimation of the real potential of luminescent materials based on different kinds of crystals and ceramics of Ca2+-Si4+-based mixed garnets for different optoelectronic applications, including photovoltaic devices, LED convertors, and scintillators.
In this work, we present the results on the crystallization and investigation of the structural and optical properties of phosphors based on the SCFs of Ce3+-doped Y3−xCaxAl5−ySiyO12 garnet, where x = 0.13–0.52 and y = 0.065–0.5. We hope that the results of this pilot research will be useful for the development of luminescent materials for white LED converters, scintillators, cathodoluminescent screens, and other optoelectronic devices based on the epitaxial structures of Ca2+-Si4+-containing garnets, grown using the LPE method on doped or undoped substrates of garnet compounds.
2. Growth of Y3−xCaxAl5−ySiyO12 SCF by LPE Method
Five sets of optically perfect Y
3−xCa
xAl
5−ySi
yO
12:Ce SCF samples were crystallized using the LPE method on YAG substrates with an orientation close to (110) from the super cooling melt solution containing nominal equimolar (x = y) Ca and Si content in the 0.5–2 range (
Figure 1). The melt of PbO-B
2O
3 (12:1) oxides was used as a flux in the LPE growth procedure. The PbO, B
2O
3, Y
2O
3, Al
2O
3, CaO, SiO
2, and CeO
2 raw materials were of 4N purity.
The real composition of SCF samples was determined using a JEOL JSM-820 electronic microscope (Tokyo, Japan), equipped with IXRF 500 and LN2 Eumex EDX detectors, and is presented in
Table 1. The measurements were performed in the 5–10 points of the SCF sample with subsequent averaging of results for improving the accuracy of content determination to the 0.001–0.003 at.% level depending on the cation type.
From the microanalysis of the real content of the SCF samples (
Table 1), we also found the Ca
2+ and Si
4+ segregation coefficients in Y
3−xCa
xAl
5−ySi
yO
12:Ce SCF samples at nominal Ca (x) and Si (y) content in the melt solution in the 0.5–2 range (
Figure 2). As can be seen from this figure, the variation in the ratio between the Y/Ca–Si/Al content in the melt solution and the SCF growth temperature T
g leads to a noticeable change of their segregation coefficients. Namely, the Ca
2+ and Si
4+ segregation coefficients are nonlinearly varied in the 0.17–0.27 and 0.065–0.25 ranges, respectively, when the nominal Ca (x) and Si (y) content in the melt solution was changed from 0.5 to 2.0 and the respective growth temperature T
g was changed within the 960–1020 °C range. The segregation coefficient of Ce
3+ ions in the mentioned mixed garnet hosts was very low and equal to around 0.025–0.0325.
Figure 1.
Photo of YAG substrate (
a), as-grown Y
2.95Ce
0.05Al
5O
12:Ce (
b), and Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 (
c) SCFs (see also
Table 1).
Figure 1.
Photo of YAG substrate (
a), as-grown Y
2.95Ce
0.05Al
5O
12:Ce (
b), and Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 (
c) SCFs (see also
Table 1).
Table 1.
Nominal (in melt solution) and real (in film) content of Y3−xCaxAl5−ySiyO12 and YAG: Ce SCFs, LPE grown on YAG substrates by using PbO-B2O3 flux.
Table 1.
Nominal (in melt solution) and real (in film) content of Y3−xCaxAl5−ySiyO12 and YAG: Ce SCFs, LPE grown on YAG substrates by using PbO-B2O3 flux.
| No | Nominal SCF Content | Real SCF Content |
|---|
| R | Y3Al5O12:Ce | Y2.95Ce0.05Al5O12:Ce |
| 1 | Y2.5Ca0.5Al4.5Si0.5O12:Ce | Y2.825Ca0.13Ce0.065Al4.935Si0.065O12 |
| 2 | Y2.25Ca0.75Al4.25Si0.75O12:Ce | Y2.765Ca0.18Ce0.055Al4.875Si0.125O12 |
| 3 | Y2CaAl4SiO12:Ce | Y2.77Ca0.185Ce0.045Al4.845Si0.155O12 |
| 4 | Y1.5Ca1.5Al3.5Si1.5O12:Ce | Y2.685Ca0.26Ce0.055Al4.785Si0.195O12 |
| 5 | YCa2Al3Si2O12:Ce | Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 |
It is important to note here that the real Ca and Si content in SCFs is not equal at the equimolar concentration of these ions in the melt solution, especially at a low (x = 0.13–0.18 and y = 0.065–0.12) content of these ions (
Table 1). As can be seen from this table, the Ca
2+ concentration was systematically larger than that of the Si
4+. This means that for local charge compensation, the other 4+ ion states can be created—for instance, Ce
4+ ions or Pb
4+ flux related dopants—or local charge compensation can occur by means of formation of the oxygen vacancies. We can predict here both types of charge compensation of Ca
2+ excess in the Y
3−xCa
xAl
5−ySi
yO
12 SCFs: the predominant formation of Ce
4+ and Pb
4+ states at the mentioned low Ca-Si content and the preferable formation of oxygen vacancies at a large Ca-Si concentration (
Figure 2).
The XRD measurements were used for the characterization of the structural quality of Y
3−xCa
xAl
5−y Si
yO
12 SCFs with different content x of Ca and Si cations, grown onto the YAG substrate with the (110) orientation and a lattice constant of 12.0069 Ȧ (
Figure 3). From the respective XRD patterns of the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF sample, the difference between the lattice constants of the YAG substrate and film Δa = (a
SCF–a
sub)/a
sub × 100% was estimated, which was equal to 0.53% (
Figure 3). Additionally, for this garnet composition, the lattice constant was calculated, which equals 12.0705 Ȧ (
Figure 3).
3. Optical Properties of Y3−xCaxAl5−ySiyO12 SCF
For the characterization of the optical properties of the Ce
3+-doped Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs, the absorption spectra (
Figure 4), cathodoluminescence (CL) spectra (
Figure 5), photoluminescence (PL) spectra (
Figure 6), PL excitation spectra (
Figure 7), and PL decay kinetics (
Figure 8 and
Table 2) were used. We also performed the measurement of the scintillation decay kinetics and photoelectron light yield for these SCF samples under excitation by α–particles (
Table 3 and
Figure 9). The absorption, luminescent, and scintillation properties of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs with different Ca/Si content were compared with the properties of the reference YAG:Ce SCF sample (
Table 2 and
Table 3). The absorption spectra of the SCFs were measured using a Jasco V730 spectrophotometer (Oklahoma, OK, USA). The PL emission and excitation spectra as well as the PL decay kinetics of the SCFs were measured using an Edinburg Instrument FS5 spectrofluorometer (Livingston, UK).
The CL spectra were recorded at room temperature (RT) with a scanning electron microscope JEOL JSM-820, which was also equipped with a spectrometer Ocean Electronics and a TE-cooled CCD detector that worked in the 200–925 nm range. The scintillation LY with a shaping time of 12 s and decay kinetics under irradiation by α-particles of Pu239 (5.15 MeV) source were measured using a setup based on the Hamamatsu H6521 PMP (Hamamatsu, Japan), multichannel analyzer, and digital TDS3052 oscilloscope (Colby, UK). All optical measurements were performed at room temperature.
3.1. Absorption Spectra
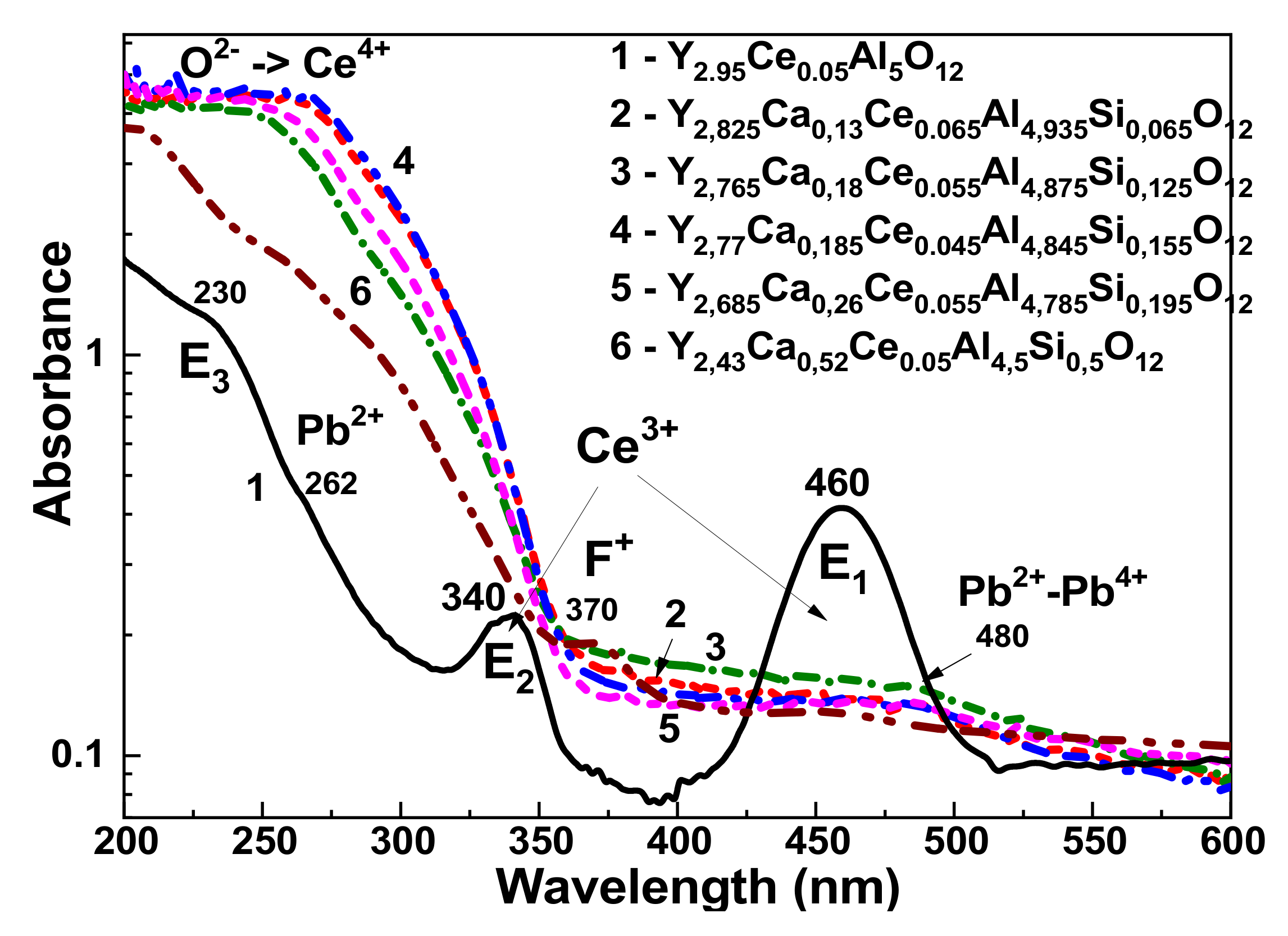
The absorption spectra of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs with different Ca and Si content in the x = 0.13–0.52 and y = 0.065–0.5 ranges, respectively, are shown in
Figure 4 in comparison to the YAG:Ce counterpart’s spectra. In the YAG:Ce garnet, the measured absorption bands E
1 and E
2 peaked at 460 and 340 nm, respectively, and correspond to the Ce
3+ ion’s 4f
1(
2F
5/2)→5d (
2E) transitions in the garnet host (
Figure 4, curve 1). The Ca
2+ and Si
4+ alloying in YAG SCFs in the mentioned concentration ranges leads to the strong decrease in the intensity of the Ce
3+ absorption bands. Furthermore, in the Y
3−xCa
xAl
5−ySi
yO
12:Ce SCF samples, these bands are almost dissipated (
Figure 4). On the other hand, the wide absorption bands that peaked at approximately 250 nm dominate in the spectra of these SCFs (
Figure 4, curves 2–3). The nature of these bands is related to the O
2−→Ce
4+ charge transfer transitions (CTT) [
23]. These CTT bands at similar positions are also observed in Mg
2+ and Ca
2+-doped Lu
3Al
5O
12:Ce and Gd
3Ga
3Al
2O
12:Ce garnets [
26,
27]. This means that in the as-grown Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs, the main charge state of cerium ions is the Ce
4+ valence states. It is worth noting here that the onset of O
2−→Ce
4+ CTT in these SCFs can even be shifted up to 400 nm, leading to a significant overlap with the E
2 absorption bands of Ce
3+ ions.
Apart from the Ce
3+ and O
2−→Ce
4+ CTT-related bands, the bump around 260 nm is also observed in the absorption spectra of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs grown from the flux based on PbO. This band is related to the
1S
0→
3P
1 transitions of Pb
2+ ions as the main flux contamination in the SCFs [
28]. The similar band is observed at 262 nm in the YAG:Ce SCF (
Figure 3, curve 1). Furthermore, the Ca-Si alloying also leads in the SCFs to the formation of a wide complex absorption band peaking in the 400–500 nm range. Within the frame of assumptions concerning Pb
4+ ion formation for the charge compensation of Ca
2+ excess in the SCF samples, we can attribute this complex band to the O
2− →Pb
4+ and Pb
2+ →Pb
4+ CTTs. Such types of transitions are observed by Scott and Page in the absorption spectra of the YGG:Pb garnet [
29].
Figure 4.
RT absorption spectra of Y3 − xCaxAl5 − ySiyO12:Ce SCFs in (log scale) with different Ca and Si content (2–6) in comparison with absorption spectra of YAG:Ce SCF (1).
Figure 4.
RT absorption spectra of Y3 − xCaxAl5 − ySiyO12:Ce SCFs in (log scale) with different Ca and Si content (2–6) in comparison with absorption spectra of YAG:Ce SCF (1).
The band peaking at 370 nm in the absorption spectra of the Y
2.43Ca
0.52Ce
0.05 Al
4.5Si
0.5O
12 SCF is most probably related to the intrinsic 1A→1B transitions of the F
+ center [
30]. This band also coincides with excitation band of the F
+ luminescence in Y
3 − xCa
xAl
5 − ySi
yO
12:Ce SCFs (
Figure 6b). The evidence of this characteristic absorption/excitation band confirms the presence of oxygen vacancy formation in Ca
2+-Si
4+-based SCFs even at the conditions of their low-temperature (below 1000 °C) growth in an oxygen-containing (air) atmosphere [
23]. This also means that the charge compensation of Ca
2+ excess in the CFs under study can also occur due to the formation of the charged oxygen vacancies.
3.2. Cathodoluminescence Spectra
The normalized RT CL spectra of the Y
3 − xCa
xAl
5 − ySi
yO
12:Ce SCFs with different Ca and Si content in the x = 0.18–0.52 and 0.125–0.5 ranges, respectively, are displayed in
Figure 4 in comparison to the YAG:Ce SCF counterpart. The 5d
1→ 4f(
2F
5/2;7/2) transitions of Ce
3+ ion in these garnets correlate with the main luminescence band peaking at 533 nm in the YAG:Ce SCF (
Figure 5, curves 1). Meanwhile, in comparison with the YAG:Ce, the position of these bands is strongly redshifted to 545–547 nm in Y
3 − xCa
xAl
5 − ySi
yO
12:Ce SCFs (
Figure 5, curves 3 and 4). It is important to note here that the Ce
3+ emission bands are notably broadened in these SCFs with respect to spectra of YAG:Ce SCFs. Particularly, the respective FWHM values of Ce
3+ emission bands are equal to 0.465 eV in Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF and only 0.396 eV in YAG:Ce (
Figure 5, curve 1). Such broadening of the Ce
3+ emission band in Ca-Si-doped garnets can be related to increasing the electron–phonon interaction or/and with the formation of the Ce
3+ multicenter in the dodecahedral position of these garnet hosts.
Figure 5.
Normalized CL spectra at RT of Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content (2–4) in comparison with CL spectra of YAG:Ce SCF (1).
Figure 5.
Normalized CL spectra at RT of Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content (2–4) in comparison with CL spectra of YAG:Ce SCF (1).
It is also worth noting that in all the SCFs under study, the luminescence of the Y
Al antisite defects [
31] is not observed due to the low temperatures of their crystallization below 1000 °C. Only in the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF sample is the very low intensity band peaking at 400 nm observed (not shown in
Figure 5). This band can be associated with the luminescence of F
+ centers in this garnet. The investigation of the PL emission/ excitation spectra, as well as the PL decay kinetics of F
+ centers, provided additional validation of this conclusion (
Figure 6b,
Figure 7b,
Figure 8b).
3.3. Photoluminescence Spectra
Under excitation at the E
1 Ce
3+ absorption band at 340 nm, the PL spectra of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs exhibit luminescence in double wide bands, which is related to the 5d
1→ 4f(
2F
5/2,7/2) transitions of Ce
3+ ions (
Figure 6a). Namely, these bands are peaked at 520 and 553 nm for Y
2.765Ca
0.18Ce
0.05 5Al
4.875Si
0.125O
12 SCFs and at 537 and 570 nm for the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF. The low intensity of this luminescence is caused by a very low concentration of Ce
3+ ions in the as-grown SCFs due to the preferable formation of Ce
4+ ions in them (
Figure 4). Furthermore, as opposed to the CL spectra, the position of PL emission bands and their FHWM in Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs under excitation at 340 nm shows more complicated dependence on the x, y values of Ca
2+-Si
4+ content. Namely, the PL spectra of the Y
2.765Ca
0.18 Ce
0.055Al
4.875Si
0.125O
12 SCF are significantly (11 nm) redshifted and notably broadened (FWHM = 0.479 eV) with respect to the spectra of the YAG:Ce SCF (FWHM = 0.457 eV), when the PL spectrum of Ce
3+ in the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF shows a small (5 nm) blueshift and practically the same FWHM = 0.458 eV as that in the YAG:Ce SCF (
Figure 6a). Such phenomena of the PL spectra of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs under a fixed excitation wavelength also indicate the complicated character of Ce
3+ center formation in these garnets and influence at least of two factors in this process.
The luminescence of F
+ centers in the band peaking at 397 nm is also observed in the Y
2.43Ca
0.52 Ce
0.05Al
4.5Si
0.5O
12 SCF sample under excitation at 370 nm in the respective absorption band of this center (
Figure 6b). Another low intensity band peaking at 605 nm can be related to the dimmer or more complicated centers, based on the charged oxygen vacancies in the YAG host (
Figure 6b) (see [
32] for details).
The excitation spectra of the Ce
3+ luminescence in Y
3−xCa
xAl
5−y Si
yO
12:Ce SCFs exhibit two bands peaking in the 454–457.5 nm and 340–344 nm ranges, respectively, associated with the 4f(
2F
5/2)→5d
1,2 transitions of Ce
3+ ions and corresponding also to the E
1 and E
2 absorption bands in these garnets (
Figure 7a). The difference in the position of these bands ΔE = E
2 – E
1, proportional to the crystal field strength in the dodecahedral position of garnet, is equal to 0.872, 0.894, and 0.905 eV for samples 2, 3, and 4 of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs, respectively (see
Figure 7). These ΔE values deviate somewhat from the values of ΔE = 0.93 eV in YAG:Ce SCFs. Meanwhile, the Stokes shift, the difference in the position of emission and low-energy excitation bands, is much lower in sample 2 (66 nm; 0.342 eV) and sample 5 (81 nm; 0.41 eV) of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs compared to the YAG:Ce SCF (84.5 nm; 0.422 eV).
Figure 6.
(a)—RT PL spectra of Y3−xCaxAl5−ySiyO12:Ce SCF with different Ca and Si content x/y = 0.18/0.125 (2) and y = 0.52/0.5 (3) in comparison with PL spectra of YAG:Ce SCF (1) under excitation in the vicinity of Ce3+ absorption band at 340 nm. (b)—RT PL spectra of F+ center in Y2.43Ca0.52 Ce0.05Al4.5Si0.5O12 SCF under excitation at 370 nm in the respective absorption band of this center.
Figure 6.
(a)—RT PL spectra of Y3−xCaxAl5−ySiyO12:Ce SCF with different Ca and Si content x/y = 0.18/0.125 (2) and y = 0.52/0.5 (3) in comparison with PL spectra of YAG:Ce SCF (1) under excitation in the vicinity of Ce3+ absorption band at 340 nm. (b)—RT PL spectra of F+ center in Y2.43Ca0.52 Ce0.05Al4.5Si0.5O12 SCF under excitation at 370 nm in the respective absorption band of this center.
Figure 7.
(a)—RT excitation spectra of Ce3+ luminescence at 530 nm in Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content (2–4) in comparison with respective excitation spectra in YAG:Ce SCF (1). (b)—RT excitation spectra of F+ center luminescence in Y2.43Ca0.52Ce0.05Al4.5 Si0.5O12 SCF at registration of emission at 400 nm.
Figure 7.
(a)—RT excitation spectra of Ce3+ luminescence at 530 nm in Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content (2–4) in comparison with respective excitation spectra in YAG:Ce SCF (1). (b)—RT excitation spectra of F+ center luminescence in Y2.43Ca0.52Ce0.05Al4.5 Si0.5O12 SCF at registration of emission at 400 nm.
The band peaking at 372 nm in the excitation spectra of the Ce
3+ luminescence in Y
3−xCa
xAl
5−y Si
yO
12:Ce SCFs at x values in the 0.26–0.52 range is connected to the intrinsic 1A→1B transitions of the F
+ center [
30]. The association of this band with F
+ centers proves the availability of the characteristic excitation bands of these centers at 370 nm and 274 nm in the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF at a registration of emission at 410 nm (
Figure 7b). As a result, the Ce
3+ centers in these SCFs can be excited by the luminescence of F
+ centers in the 397 nm band, which strikingly overlapped with the E
2 absorption bands of Ce
3+ ions (
Figure 4).
3.4. Decay Kinetics of Photoluminescence
In comparison to the YAG:Ce SCF counterpart, the decay kinetics of the Ce
3+ luminescence in the Y
3−xCa
x Al
5−ySi
yO
12:Ce SCFs with different Ca and Si content in the x = 0.13–0.52 and y = 0.065–0.5 ranges, respectively, under excitation at 340 nm in the vicinity of E
2 Ce
3+ absorption bands are shown in
Figure 8a. Similar to other Ca
2+-Si
4+-based garnets [
13,
24,
31] and contrary to the YAG:Ce SCFs (
Figure 8a, curves 1), the decay kinetics of the Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs (
Figure 8a, curves 2–5) are strongly nonexponential, and the decay curves become faster and nonexponential when increasing the x and y values. For this reason, these decay curves can be presented by the two or even more components with the characteristic decay time values
t at 1/e: 0.1 and 0.001 intensity decay levels (
Figure 8a). The respective decay times τ
1/e, τ
1/10, and τ
1/100 are presented in
Table 2.
The key reasons for the nonexponential decay kinetics of the Ce
3+ luminescence in the as-grown Y
3−xCa
xAl
5−y Si
yO
12:Ce SCFs are the presence of Ce
4+ valence states and the formation of Ce1 and Ce2 multicenters due to the substitution of Ca
2+ and Y
3+ cations by Ce
3+ ions, accordingly. The effect of the acceleration of the Ce
3+ decay in the case of Ce
4+ presence can also potentially be related to the direct intervalence charge transfer (IVCT) transitions that induce fast nonradiative decay channels [
33,
34,
35]. This effect has recently been described for Eu
2+/Eu
3+ pairs in fluorides by L. Seijo et al. [
33] and also predicted for Ce
3+/Ce
4+ pairs in SrS [
34] and garnet compounds [
35].
Figure 8.
(a)—RT decay kinetics of Ce3+ luminescence at 530 nm in Y3−xCaxAl5-xSiyO12:Ce SCFs with different Ca and Si content (2–5) under excitation of PL at 340 nm and registration of PL at 530 nm in comparison with respective decay kinetics of Ce3+ emission in YAG:Ce SCFs (1). (b)—RT decay kinetics of F+ luminescence at 415 nm in Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCFs.
Figure 8.
(a)—RT decay kinetics of Ce3+ luminescence at 530 nm in Y3−xCaxAl5-xSiyO12:Ce SCFs with different Ca and Si content (2–5) under excitation of PL at 340 nm and registration of PL at 530 nm in comparison with respective decay kinetics of Ce3+ emission in YAG:Ce SCFs (1). (b)—RT decay kinetics of F+ luminescence at 415 nm in Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCFs.
Table 2.
Decay times of PL at RT in Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content under excitation at 340 nm and registration of PL at 530 nm.
Table 2.
Decay times of PL at RT in Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content under excitation at 340 nm and registration of PL at 530 nm.
| No | Real SCF Content | t1/e,ns | t1/10,ns | t1/100,ns |
|---|
| R | Y2.95Ce0.05Al5O12:Ce | 62.6 | 141 | 294 |
| 1 | Y2.825Ca0.13Ce0.065Al4.935Si0.065O12 | 2.9 | 8.5 | 86.2 |
| 2 | Y2.765Ca0.18Ce0.055Al4.875Si0.125O12 | 2.95 | 6.6 | 39 |
| 3 | Y2.77Ca0.185Ce0.045Al4.845Si0.155O12 | 2.0 | 5.7 | 21.8 |
| 4 | Y2.685Ca0.26Ce0.055Al4.785Si0.195O12 | 1.95 | 4.8 | 18.2 |
| 5 | Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 | 1.6 | 7.5 | 29.5 |
The Ce
4+ ions, which act as very effective electron trapping centers, can significantly accelerate the decay kinetics of Ce
3+ luminescence when excited with energies higher than the band gap of garnet or close to the energies of the O
2−→Ce
4+ CTT [
36,
37]. Due to the extended long-wavelength wings of these CTT bands in the garnets under study, the presence of O
2−→Ce
4+ transitions is also feasible under 340 nm excitation in the area of the E
2 absorption band of Ce
3+ ions [
36,
37]. Therefore, even under 340 nm excitation, we can observe the luminescence of Ce
3+ ions due to the charge transformation of Ce
4+ ions: Ce
4+ + hv
(340 nm)→(Ce
3+) * +
p→ Ce
3+(530 nm) +
p→Ce
4+ [
22,
23].
Under this supposition, the Ce
4+ centers can be responsible for the presence of the fast components of cerium luminescence with a lifetime of t
1/e = 3.5–5.85 ns in Y
2.43Ca
0.52Ce
0.05Al
4.5 Si
0.5O
12 SCF when excited at 340 nm. In the meantime, the slower decay components of luminescence in these garnets, with decay times of t
1/20 = 49.4–62.9 ns and 98–121 ns, are mostly attributable to Ce
3+ ion radiative transitions. The decay time constants of the Ce
3+ luminescence in YAG:Ce SCF are t
1/e = 60.5 ns and t
1/20 = 183 ns, correspondingly (
Figure 8a, curve 1).
It is worth noting that the presence of the fast component of cerium luminescence in the ns range in silicate garnet compounds and the nonexponential form of the decay curves are also related to the formation of Ce
3+ multicenters [
13,
14,
21,
22,
23,
24,
25]. In particular, such decay curves can imply the possibility of energy transfer between low-energy and high-energy emitting Ce
3+-based centers [
25]. Nonetheless, in the as-grown Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs, the contribution of such an energy transfer mechanism to the nonexponential kinetics of PL is strongly masked by the presence of Ce
4+ centers. Therefore, the study of the influence of the energy transfer processes between Ce
3+ multicenters on the nonexponential kinetics of the Ce
3+ luminescence in these garnets can be performed only after removing the Ce
4+ centers, for instance, by using the thermal annealing of SCFs in the reducing atmosphere [
33].
3.5. Scintillation Properties of Ce3+ Doped Y3−xCaxAl5−ySiyO12 SCFs
Under α-particle excitation by a
239Pu (5.15 MeV) source, the as-grown Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs show significantly lower scintillation LY in comparison with the YAG: Ce SCF counterpart with an LY of 2.6 photons per keV (
Table 3). The low scintillation efficiency of Ca-Si-based SCFs is due to the recharging of the majority of Ce
3+ ions to the Ce
4+ state in as-grown samples. The scintillation behavior of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs corresponds to the properties of Ca
3ScSi
3O
12:Ce and Ca
2YMgScSi
3O
12:Ce SCFs [
23,
24,
25], as well as (Lu, Y)
2SiO
5: Ce SCFs [
38,
39], where the low scintillation response is caused by the main Ce
4+ valence state of cerium ions, which formed during the LPE growth of these SCFs from the flux based on PbO oxide.
The scintillation decay kinetics of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs are presented in
Figure 9. As can be seen from this figure, the scintillation response of these SCFs becomes faster with increasing the Ca-Si concentrations. The respective decay times are equal to t
1/e = 50 ns, 43.5, and 30 ns and t
1/20 = 152 ns, 148 ns, and 79 ns for SCF samples with Ca/Si content x/y = 0.18/0.125, 0.26/0.195, and 0.52/0.5, respectively, in comparison with t
1/e = 50 ns and t
1/20 = 152 ns for YAG: Ce SCF. This effect also correlates with the significant decrease in the LY of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCF samples when increasing the Ca/Si concentration (
Table 3).
Table 3.
Scintillation LY and t1/e, t1/20 decay times of Y3−xCaxAl5−y SiyO12:Ce SCFs with different Ca and Si content under excitation by particles of 239Pu (5.15 MeV) in comparison with YAG: Ce standard sample with an LY of 2.6 photons/keV. *—not measured.
Table 3.
Scintillation LY and t1/e, t1/20 decay times of Y3−xCaxAl5−y SiyO12:Ce SCFs with different Ca and Si content under excitation by particles of 239Pu (5.15 MeV) in comparison with YAG: Ce standard sample with an LY of 2.6 photons/keV. *—not measured.
| No | SCF Content | LY, % | t1/e, ns | t1/e, ns |
|---|
| R | Y2.95Ce0.05Al5O12:Ce | 100 | 141 | 294 |
| 1 | Y2.825Ca0.13Ce0.065Al4.935Si0.065O12 | 9.5 | 8.5 | 86.2 |
| 2 | Y2.765Ca0.18Ce0.055Al4.875Si0.125O12 | 8.5 | 6.6 | 39 |
| 3 | Y2.77Ca0.185Ce0.045Al4.845Si0.155O12 | 7.0 | 5.7 | 21.8 |
| 4 | Y2.685Ca0.26Ce0.055Al4.785Si0.195O12 | 6.6 | 4.8 | 18.2 |
| 5 | Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 | 5.9 | 7.5 | 29.5 |
| 5a | Y2.43Ca0.52Ce0.05Al4.5Si0.5O12; TT = 1000 °C | 10.9 | * | * |
| 5b | Y2.43Ca0.52Ce0.05Al4.5Si0.5O12; TT = 1300 °C | 38 | 44.3 | 152 |
Figure 9.
Scintillation decay kinetics of Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content under excitation by α–particles of 239Pu (5.15 MeV) source in comparison with respective scintillation decay kinetics of YAG: Ce SCF (1).
Figure 9.
Scintillation decay kinetics of Y3−xCaxAl5−ySiyO12:Ce SCFs with different Ca and Si content under excitation by α–particles of 239Pu (5.15 MeV) source in comparison with respective scintillation decay kinetics of YAG: Ce SCF (1).
4. Optical Properties of Y3−xCaxAl5−ySiyO12 SCFs, Annealing in the Reducing Atmosphere
The impact of thermal treatment (TT) at the 1000–1300 °C range in the 95% N
2–5% H
2 reducing atmosphere on the optical properties of Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs was investigated as well (
Figure 10,
Figure 11,
Figure 12) with the example of the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF sample. The mentioned TT results in the change of the relative concentration of Ce
4+ and Ce
3+ centers in the SCFs under study due to the reaction O
2− + 2Ce
4+ → V
O + 2Ce
3+, where V
O is the oxygen vacancy.
The different absorption spectra of the untreated and annealed samples at 1000 °C and 1300 °C support this result (
Figure 10, curves 4 and 5, respectively).
As shown in
Figure 10, the intensity of the O
2−→Ce
4+ CTT absorption band in the UV region, peaking at 250 nm, falls significantly in annealed samples. This decrease in absorption of Ce
4+ centers is accompanied by an increase in absorption in the bands peaking at 446 and about 340 nm, which correspond to the E
1 and E
2 absorption bands of Ce
3+ ions. Additionally, the rate of increase in the Ce
3+ absorption is proportionate to the annealing temperature (
Figure 10, curves 4 and 5).
Figure 10.
Influence of the TT in N2 95% + H2 5% atmosphere at 1000 °C (2) and 1300 °C (3) temperatures on the absorption spectra of Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCF. Curve 4 is the difference spectra of untreated and annealed samples at temperature of 1300 °C.
Figure 10.
Influence of the TT in N2 95% + H2 5% atmosphere at 1000 °C (2) and 1300 °C (3) temperatures on the absorption spectra of Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCF. Curve 4 is the difference spectra of untreated and annealed samples at temperature of 1300 °C.
The annealing of Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF in a reducing environment causes a significant change in the structure emission and excitation bands, which is connected with Ce
3+ centers. Particularly, in the as-grown sample, the maximum of the Ce
3+ emission band is located at 540 nm in the as-grown sample, excited in the major bands peaking at 458 nm, and bumped at 342 nm (
Figure 11, curve 1). We assumed that these bands are linked to the Ce1 center. The difference in the locations of the E
1 and E
2 excitation bands for such a Ce1 center is 0.917 eV. For this center, the difference in the locations of the emission and low-energy excitation bands (Stokes shift) is 82 nm (0.41 eV).
The excitation band peaking at the 380–385 nm range is related to the excitation of F+ center emission and follows the excitation of the Ce3+ luminescence via the emission of these centers.
Figure 11.
Influence of the thermal treatment in N2 95% + H2 5% atmosphere at 1000 °C (2) and 1300 °C (3) temperatures on the excitation spectra (1–3) and emission spectra (1′–3′) of Ce3+ luminescence in Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCF sample.
Figure 11.
Influence of the thermal treatment in N2 95% + H2 5% atmosphere at 1000 °C (2) and 1300 °C (3) temperatures on the excitation spectra (1–3) and emission spectra (1′–3′) of Ce3+ luminescence in Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCF sample.
Meanwhile, the TT at a temperature of 1000 °C and 1300 °C results in: (i) a significant shift in the maximum of the Ce3+ emission spectrum to 531 and 537 nm; (ii) a shift of the main excitation bands to 450 and 449 nm, respectively; and (iii) a strong decrease of the intensity of the F+ center excitation band peaking at 380 nm and the appearance of an excitation band peaking at 336 nm. After TT, such changes in the emission and excitation spectra of Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCFs can be attributed to an increase in the relative concentrations of Ce2 centers. For such a Ce2 center, the difference in the positions of E1 and E2 excitation bands is equal to 0.928 eV. Therefore, the Ce2 centers are characterized by a slightly larger crystal field strength than that of the Ce1 centers, and for this reason, the position of emission bands of this center is redshifted with respect to the Ce1 center. The Stokes shift of the Ce2 center is equal to 88 nm (0.452 eV).
Figure 12a displays the effect of the TT in the N
2 95% + H
2 5% atmosphere at temperatures of 1000 °C and 1300 °C on the decay kinetics of the Ce
3+ luminescence in Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF. Annealing in a reducing environment reduces the concentration of Ce
4+ centers while increasing the content of Ce
3+ centers, resulting in variations in the decay kinetics of the SCF samples. Particularly, the treatment in the 1000–1300 °C range provides more flat-shaped decay curves of the Ce
3+ luminescence. This suggests that the intrinsic transitions of Ce
3+ ions have a dominant contribution to the PL decay kinetics of Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 SCF after TT, specifically in the sample annealed at 1300 °C (
Figure 12, curve 3). Taking into account the dominant contribution of the Ce2 centers to the PL of this sample, the decay kinetics of SCFs, annealed at 1300 °C, are mostly connected to the luminescence of this center. The Ce
4+→Ce
3+ recharge in SCF samples after TT allows investigating the possibility of energy transfer across various Ce
3+ multicenters in the Y
2.43Ca
0.52Ce
0.05Al
4.5Si
0.5O
12 garnet. Specifically, the slightly nonexponential form of Ce
3+ luminescence in the SCF sample treated at 1300 °C (
Figure 12a, curve 3) can be generated by energy transfer between Ce1 and Ce2 centers.
Figure 12.
(a)—RT decay kinetics of Ce3+ luminescence at 530 nm in Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCFs under excitation of PL at 340 nm and registration of PL at 530 nm before (1) and after TT in the reducing N2 + H2 (95 + 5%) atmosphere at 1000 °C (2) and 1300 °C (3). (b)—Scintillation decay kinetics of this SCF after TT at 1300 °C (2) in comparison with as-grown sample (1).
Figure 12.
(a)—RT decay kinetics of Ce3+ luminescence at 530 nm in Y2.43Ca0.52Ce0.05Al4.5Si0.5O12 SCFs under excitation of PL at 340 nm and registration of PL at 530 nm before (1) and after TT in the reducing N2 + H2 (95 + 5%) atmosphere at 1000 °C (2) and 1300 °C (3). (b)—Scintillation decay kinetics of this SCF after TT at 1300 °C (2) in comparison with as-grown sample (1).
The abovementioned conclusion is confirmed by the measurements of the scintillation LY of the Y
2.43Ca
0.52 Ce
0.05Al
4.5Si
0.5O
12 SCF after TT at 1000 °C and 1300 °C in the reducing (N
2 + H
2) atmosphere. Specifically, a significant (up to 1.4 times) rise in LY is seen after TT of this sample even at 1000 °C due to the recharging of certain part of Ce
4+ ions to the Ce
3+ form (
Table 2). Simultaneously, the TT of the SCF at 1300 °C resulted in a greater (up to 6.5 times) increase in LY when compared to the untreated sample. Furthermore, the scintillation decay kinetics of this SCF sample also become slower and close to YAG:Ce SCF (
Figure 12b) due to the recharging of the significant part of the Ce
4+ ions to the Ce
3+ state. However, the significant part of the LY in the annealed sample is probably lost due to the formation of the large concentration of oxygen vacancies and the strong continuous absorption of this sample in the range of the Ce
3+ luminescence (see
Figure 10, curve 5).
5. Regularities of Ce3+⇿Ce4+ Recharge in Y3−xCaxAl5−ySiyO12:Ce SCFs
The comparison of the absorption spectra of Y
3−xCa
xAl
5−ySi
yO
12:Ce and YAG:Ce SCFs (
Figure 3) as well as the absorption spectra of the initial and annealed in the reducing atmosphere of Y
2.43Ca
0.52Ce
0.05Al
4.5 Si
0.5O
12 SCF sample (
Figure 10) clearly confirms
the formation of the main part of cerium ions in the Ce4+ state for the compensation of the access of Ca2+ ions in the as-grown SCFs of these garnets. Meanwhile, in contradiction with the dominant Ce
4+ state in the as-grown SCFs, the CL spectra, and the scintillation decay kinetics of these samples confirm that the energy transfer from garnet host to the emission centers is realized in the final stage via
the Ce3+ luminescence. This means that the excitation of the Ce
3+ center emission in the Y
3−xCa
x Al
5−ySi
yO
12:Ce SCFs under high energy irradiation (e-beam, α-particles) occurs via the initial recharging of Ce
4+ ions instead of the direct excitation of the Ce
3+ luminescence in the YAG:Ce SCF counterpart.
The excitation processes of the Ce
3+ luminescence in the Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs with preferable Ce
4+ state under high-energy excitation can be presented as follows (
Figure 13) [
40,
41,
42]. At the initial stage of the CL/scintillation process, the ionizing radiation produces electron-hole pairs. The trapping of electrons from the conductive band by the Ce
4+ ions leads to the formation of Ce
3+ centers in the excited state (step1). Their radiative decay results in the appearance of the Ce
3+ emission and formation of the temporary Ce
3+ ions in the ground state (step 2), which trap mobile holes from the valence band (step 3), and, as a consequence, Ce
4+ centers are recreated.
The preferable excitation of the Ce
3+ luminescence via the recharging of Ce
4+ ions in Y
3−xCa
xAl
5−ySi
yO
12:Ce SCFs can result also in the strong acceleration of their scintillation decay kinetics (
Figure 9). Indeed, the processes of the excitation of the Ce
3+ luminescence in these SCFs can decrease the delay in the migration stage of the energy transfer from the excited garnet hosts to the Ce
3+ ions due to the exclusion of the trapping of the charge carriers at the matrix defects [
41,
42] and unwanted impurities (e.g., Pb
2+ flux related dopant). Such slowing of energy transfer is typically realized under high-energy excitation in the Ce
3+-doped SCFs grown from PbO-B
2O
3 based fluxes [
43].
The respective changes in the absorption and PL emission/excitation spectra and decay kinetics in the Y
2.43Ca
0.52Ce
0.05 Al
4.5Si
0.5O
12 SCFs were observed. They are related to the change in concentration of the Ce
4+ and Ce
3+ centers after TT in the 1000–1300 °C range, and they confirm the assumption regarding the formation of Ce
3+ multicenters in Y
3−xCa
xAl
5−ySi
yO
12:Ce Ce SCFs and the nature of Ce1 and Ce2 centers in these garnets. The Ce1 centers are formed by Ce
3+ ions at the Y
3+ sites, whereas Ce2 centers form when Ce
3+ ions replace the Ca
2+ cations. However, due to the main Ce
4+ valence state of Ce2 centers in as-grown samples, these centers are barely visible in the PL emission and excitation spectra, as well as the decay kinetics of the Ce
3+ luminescence (
Figure 6,
Figure 7,
Figure 8). Ad interim, treatment in the reducing environment causes a part of the Ce
4+ ions to be recharged to the Ce
3+ states, allowing the detection of the Ce2 centers in the PL spectra and the PL decay kinetics of the Ce
3+ emission (
Figure 10 and
Figure 11).
6. Conclusions
The growth of the single crystalline films (SCFs) of Y3−xCaxAl5−ySiyO12:Ce garnets at Ca and Si concentration in the x = 0.13–0.52 and y = 0.065–0.5 ranges, respectively, was performed using the LPE method from PbO-B2O3 based flux onto Y3Al5O12 (YAG) substrates. Due to Ca2+ and Si4+ alloying in the mentioned compounds, the misfit between SCF-substrate lattice constants changed from 0 to 0.53%. The segregation coefficients of Ca and Si ions in the SCFs under study are nonlinearly changed in the 0.17–0.28 and y = 0.065–0.25 ranges, respectively, when changing the nominal Ca and Si content in the melt solution in the x = 0.5–2 range. The segregation coefficient of Ce3+ ions in the Y3−xCaxAl5−ySiyO12:Ce SCFs was equal to 0.007–0.0095.
We have also found that the real Ca and Si content in Y3−xCaxAl5−ySiyO12:Ce SCFs is not equal at the equimolar concentration of these ions in the melt solution: the Ca2+ content was systematically larger than that of the Si4+. Both types of charge compensation of the Ca2+ excess are expected in these SCFs: the predominant formation of Ce4+ and Pb4+ states at relatively low Ca-Si content and the preferable formation of oxygen vacancies at a large Ca-Si concentration.
The absorption, luminescent, and scintillation properties of Y3−xCaxAl5−ySiyO12:Ce SCFs were investigated and compared with those for the reference YAG:Ce SCF counterpart. The cathodoluminescence spectra of Ce3+ ions in Y3−xCaxAl5−ySiyO12:Ce SCFs are significantly extended in the red range compared to YAG:Ce SCF due to the formation of Ce3+ multicenters in the dodecahedral positions of the garnet lattices, additionally stimulated by the Ca2+ and Si4+ pair co-doping.
We have confirmed the formation of two types of Ce3+ centers in the Y3−xCaxAl5−ySiyO12:Ce garnets in the photoluminescence emission and excitation spectra SCFs of these compounds. These two centers (Ce1 and Ce2) possess various local surroundings due to the substitution by the Ce3+ ions of different dodecahedral cation positions (correspondingly Y3+ and Ca2+) and are characterized by differing spectral behaviors.
The fast F+ center luminescence band peaking at 372 nm and with a decay time of 5.7 ns is observed in Y3−xCaxAl5−ySiyO12:Ce SCFs at Ca (x) and Si (y) content approximately above 0.2. Due to the overlapping of the emission band of F+ centers with the absorption band of Ce3+ ions, Ce3+ luminescence in these SCFs can be partly excited via emission of F+ centers.
The as-grown Y3−xCaxAl5−ySiyO12:Ce SCFs show poor scintillation properties. Under α–particles excitation by 239Pu (5.15 MeV) source, these SCFs possess a faster scintillation response with decay times in the t1/e = 50–30 ns and t1/20 = 152–70 ns ranges but significantly low light yield (LY) of 6–8% in comparison with the reference YAG:Ce SCF (LY = 100%; t1/e = 66 ns; t1/20 = 194 ns). At the same time, the LY of as-grown Y3−xCaxAl5−ySiyO12:Ce SCFs can significantly increase (up to 1.4–6.5 times) after their thermal treatment (TT) in the reducing atmosphere (95% N2 + 5% H2) at temperatures in the range of 1000–1300 °C.
We have also observed the formation of Ce4+ valence states in the optical properties of Y3−xCaxAl5−y SiyO12:Ce SCFs. The presence of Ce4+ ions in the as-grown SCFs is confirmed by the observation of the O2+-Ce4+ charge transfer transitions in the absorption spectra of these SCFs. The Ce4+ centers are also responsible for acceleration of the initial stage of the PL decay kinetics of cerium and the presence of fast components with a lifetime in the few ns range in these SCFs. The Ce4+→Ce3+ recharge in these SCFs is achieved by the TT in the reducing atmosphere at temperatures above 1000 °C. Such TT also leads to more exponential-like decay kinetics of the Ce3+ luminescence in Y3−xCaxAl5−ySiyO12:Ce SCFs and enables studying the energy transfer processes between the different Ce3+ multicenters in these garnets.