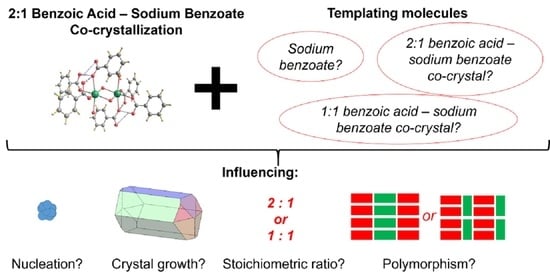
Co-Crystallization Kinetics of 2:1 Benzoic Acid–Sodium Benzoate Co-Crystal: The Effect of Templating Molecules in a Solution
Abstract
:1. Introduction
2. Theory
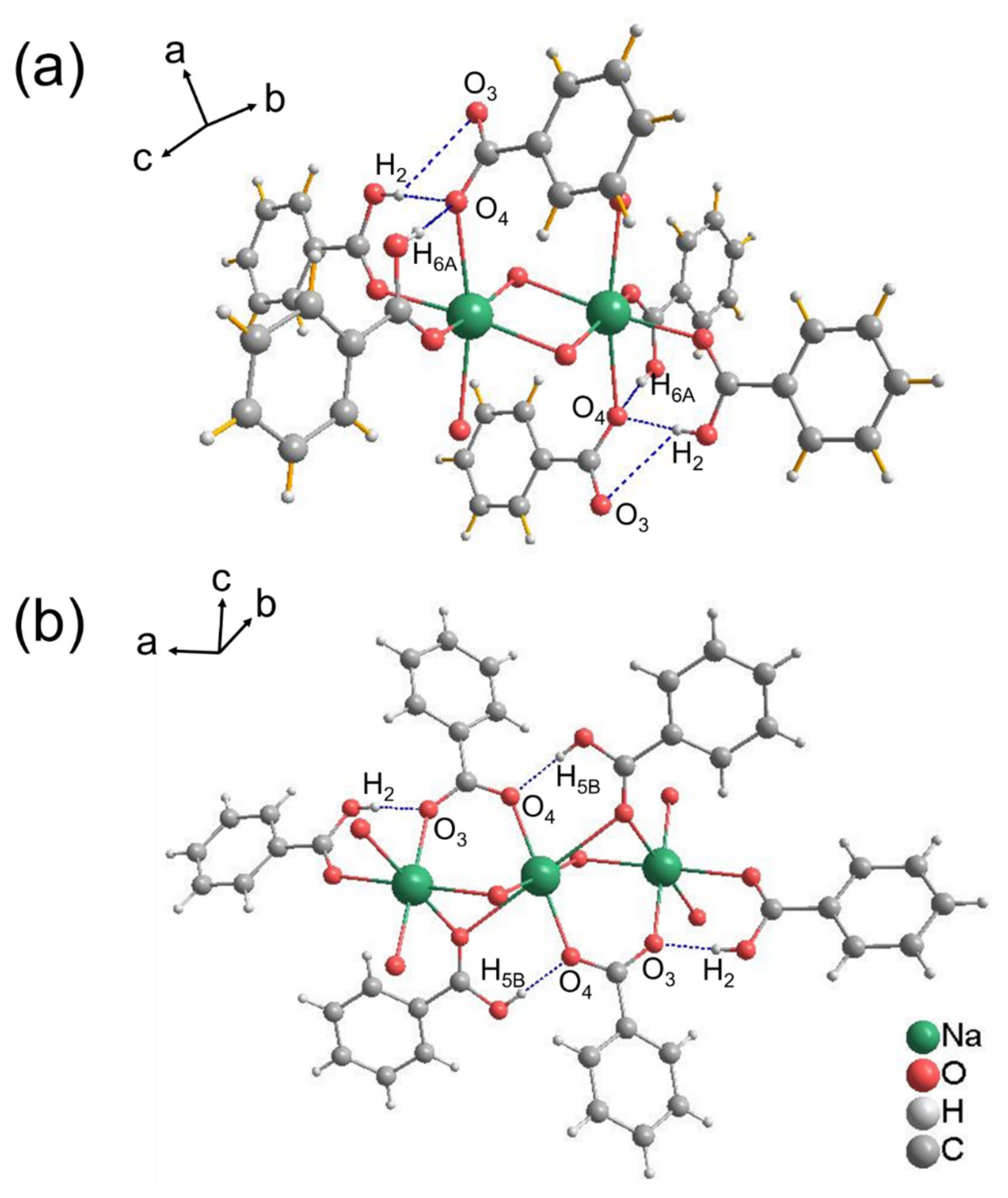
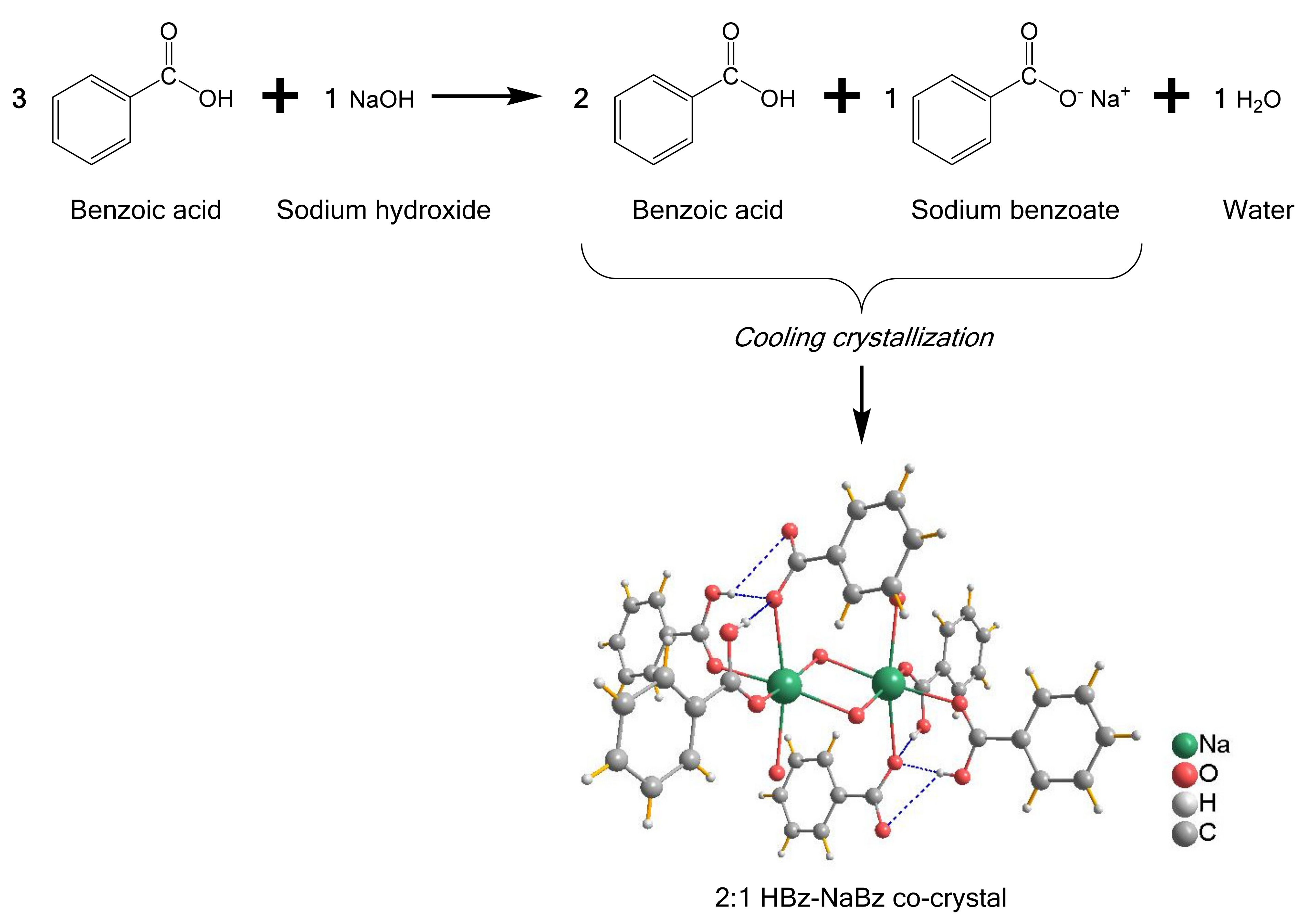
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Nomenclatures
| Symbol | Description | Unit |
| At | Overall crystal surface area at a given time | m2 |
| At,i | Surface area of an individual crystal at a given time | m2 |
| C0 | Initial concentration of 2:1 HBz–NaBz co-crystal | kg L−1 |
| Ct | Concentration of 2:1 HBz–NaBz co-crystal at a given time | kg L−1 |
| C* | Solubility value of 2:1 HBz–NaBz co-crystal | kg L−1 |
| dmt/dt | Crystal mass growth rate | kg s−1 |
| fN | Minimum detectable number of nuclei per unit volume | m−3 |
| g | Order of crystal growth kinetics | dimensionless |
| i* | Theoretical number of molecules in the critical nucleus | dimensionless |
| J | Rate of primary nucleation | s−1 m−3 |
| J0 | Pre-exponential factor of nucleation rate equation | s−1 m−3 |
| KG | Overall crystal growth rate coefficient | kg m−2 s−1 |
| KG′ | Modified overall crystal growth rate coefficient | kg1/3 s−1 |
| k | Boltzmann’s constant, 1.38065 × 10−23 | m2 kg s−2 K−1 |
| Lt,i | Characteristic length of a single crystal at a given time | m |
| mt | Overall crystal mass | kg |
| mt,i | Mass of an individual crystal at a given time | kg |
| r | Radius of a nucleus | m |
| rcrit | Critical nucleus size | m |
| S0 | Initial degree of supersaturation | dimensionless |
| T | Temperature | K |
| tg | Time required for nuclei to grow into a detectable size | s |
| tn | Time required for for the solutes to undergo primary nucleation | s |
| tr | Time required for a supersaturation to reach steady-state distribution of molecular clusters | s |
| Vsolution | Volume of solution | m3 |
| Vt,i | Volume of an individual crystal at a given time | m3 |
| υ | Molecular volume | m3 |
| α | Volumetric shape factor | dimensionless |
| β | Surface shape factor | dimensionless |
| γ | Interfacial energy between the surface and solution | J m−2 |
| ΔG | Overall free energy change in homogeneous nucleation | J |
| ∆Gcrit | Critical free energy required to form a critical nucleus size | J |
| ΔGS | Excess free energy between the surface and bulk solid of a particle | J |
| ΔGV | Excess free energy between bulk solid and solute in solution phase | J |
| ∆Gv | Free energy change in the transformation per unit volume | J m−3 |
| ρc | Density of crystal | kg m−3 |
| τ | Induction period | s |
References
- Berry, D.J.; Steed, J. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Mukaida, M.; Sato, H.; Sugano, K.; Terada, K. Stability Orders of Acetaminophen and Theophylline Co-crystals Determined by Co-crystal Former Exchange Reactions and Their Correlation with In Silico and Thermal Parameters. J. Pharm. Sci. 2017, 106, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefter, E.; Sackman, P. Structural Studies on Molecular Complexes V: Crystal Structures of Sulfathiazole—Sulfanilamide and Sulfathiazole—Theophylline Complexes. J. Pharm. Sci. 1971, 60, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.L.; Lee, T. Intensified Crystallization Processes for 1:1 Drug–Drug Cocrystals of Sulfathiazole–Theophylline, and Sulfathiazole–Sulfanilamide. Cryst. Growth Des. 2018, 18, 1339–1349. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Babu, N.J.; Nangia, A. Nitrofurantoin– p -aminobenzoic acid cocrystal: Hydration stability and dissolution rate studies. J. Pharm. Sci. 2011, 100, 3233–3244. [Google Scholar] [CrossRef]
- Sugandha, K.; Kaity, S.; Mukherjee, S.; Isaac, J.; Ghosh, A. Solubility Enhancement of Ezetimibe by a Cocrystal Engineering Technique. Cryst. Growth Des. 2014, 14, 4475–4486. [Google Scholar] [CrossRef]
- Butterhof, C.; Milius, W.; Breu, J. Co-crystallisation of benzoic acid with sodium benzoate: The significance of stoichiometry. CrystEngComm 2012, 14, 3945. [Google Scholar] [CrossRef]
- Butterhof, C.; Bärwinkel, K.; Senker, J.; Breu, J. Polymorphism in co-crystals: A metastable form of the ionic co-crystal 2 HBz·1 NaBz crystallised by flash evaporation. CrystEngComm 2012, 14, 6744. [Google Scholar] [CrossRef]
- Sandhu, B.; Sinha, A.S.; Desper, J.; Aakeröy, C.B. Modulating the physical properties of solid forms of urea using co-crystallization technology. Chem. Commun. 2018, 54, 4657–4660. [Google Scholar] [CrossRef]
- Lee, T.; Chen, J.W.; Lee, H.L.; Lin, T.Y.; Tsai, Y.C.; Cheng, S.-L.; Lee, S.-W.; Hu, J.-C.; Chen, L.-T. Stabilization and spheroidization of ammonium nitrate: Co-crystallization with crown ethers and spherical crystallization by solvent screening. Chem. Eng. J. 2013, 225, 809–817. [Google Scholar] [CrossRef]
- Li, Z.-H.; Yu, T.; Lee, T.; Kim, W.-S. Cocrystallization of Caffeine–Maleic Acid in a Batchelor Vortex Flow. Cryst. Growth Des. 2020, 20, 1618–1627. [Google Scholar] [CrossRef]
- Chen, T.-H.; Yeh, K.L.; Chen, C.W.; Lee, H.L.; Hsu, Y.C.; Lee, T. Mixing Effect on Stoichiometric Diversity in Benzoic Acid–Sodium Benzoate Cocrystals. Cryst. Growth Des. 2019, 19, 1576–1583. [Google Scholar] [CrossRef]
- United States Food and Drug Administration OTC Active Ingredient. Available online: https://www.fda.gov/media/75750/download (accessed on 11 June 2021).
- Butterhof, C.; Milius, W.; Breu, J. Influence of Cation Size on the Co-crystallisation of Benzoic Acid with Different Benzoates. Z. Anorg. Allg. Chem. 2013, 639, 308–311. [Google Scholar] [CrossRef]
- Brittain, H.G. Vibrational Spectroscopic Studies of Cocrystals and Salts. 3. Cocrystal Products Formed by Benzenecarboxylic Acids and Their Sodium Salts. Cryst. Growth Des. 2010, 10, 1990–2003. [Google Scholar] [CrossRef]
- Patel, M.A.; Nguyen, B.; Chadwick, K. Predicting the Nucleation Induction Time Based on Preferred Intermolecular Interactions. Cryst. Growth Des. 2017, 17, 4613–4621. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, S.; Liu, S.; Tang, W.; Fu, X.; Gong, J. Novel Strategy to Control Polymorph Nucleation of Gamma Pyrazinamide by Preferred Intermolecular Interactions during Heterogeneous Nucleation. Cryst. Growth Des. 2018, 18, 4874–4879. [Google Scholar] [CrossRef]
- Bora, P.; Saikia, B.; Sarma, B. Oriented Crystallization on Organic Monolayers to Control Concomitant Polymorphism. Chem. A Eur. J. 2020, 26, 699–710. [Google Scholar] [CrossRef]
- Agnew, L.R.; McGlone, T.; Wheatcroft, H.P.; Robertson, A.; Parsons, A.R.; Wilson, C.C. Continuous Crystallization of Paracetamol (Acetaminophen) Form II: Selective Access to a Metastable Solid Form. Cryst. Growth Des. 2017, 17, 2418–2427. [Google Scholar] [CrossRef]
- Yeh, K.L.; Lee, T. Selective formation of form II paracetamol through the assistance of paracetamol co-crystals as templates in a solution. CrystEngComm 2021, 23, 3940–3945. [Google Scholar] [CrossRef]
- O’Donnell, J.L.; Zuo, X.; Goshe, A.J.; Sarkisov, L.; Snurr, R.Q.; Hupp, J.T.; Tiede, D.M. Solution-Phase Structural Characterization of Supramolecular Assemblies by Molecular Diffraction. J. Am. Chem. Soc. 2007, 129, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, H.L. Direct Co-crystal Assembly from Synthesis to Co-crystallization. CrystEngComm 2015, 17, 9002–9006. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth-Heinemann: Woburn, MA, USA, 2001. [Google Scholar]
- Lee, T.; Chen, Y.H.; Wang, Y.W. Effects of Homochiral Molecules of (S)-(+)-Ibuprofen and (S)-(−)-Sodium Ibuprofen Dihydrate on the Crystallization Kinetics of Racemic (R,S)-(±)-Sodium Ibuprofen Dihydrate. Cryst. Growth Des. 2008, 8, 415–426. [Google Scholar] [CrossRef]
- Mersmann, A.; Bartosch, K. How to predict the metastable zone width. J. Cryst. Growth 1998, 183, 240–250. [Google Scholar] [CrossRef]
- Boomadevi, S.; Dhanasekaran, R.; Ramasamy, P. Investigations on nucleation and growth kinetics of urea crystals from methanol. Cryst. Res. Technol. 2002, 37, 159–168. [Google Scholar] [CrossRef]
- Mersmann, A.; Eble, A.; Heyer, C. Crystal Growth. In Crystallization Technology Handbook; Informa UK Limited: London, UK, 2001. [Google Scholar]
- Saleemi, A.; Onyemelukwe, I.I.; Nagy, Z. Effects of a structurally related substance on the crystallization of paracetamol. Front. Chem. Sci. Eng. 2013, 7, 79–87. [Google Scholar] [CrossRef]
- Shiau, L.-D.; Lu, T.-S. A model for determination of the interfacial energy from the induction time or metastable zone width data based on turbidity measurements. CrystEngComm 2014, 16, 9743–9752. [Google Scholar] [CrossRef]
- Shiau, L.-D.; Wang, H.-P. Simultaneous determination of interfacial energy and growth activation energy from induction time measurements. J. Cryst. Growth 2016, 442, 47–51. [Google Scholar] [CrossRef]
- Shiau, L.-D. Investigations into the Influence of Solvents on the Nucleation Kinetics for Isonicotinamide, Lovastatin, and Phenacetin. ACS Omega 2019, 4, 17352–17358. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Rasmuson, Å.C. Nucleation and growth of succinic acid in a batch cooling crystallizer. AIChE J. 1991, 37, 1293–1304. [Google Scholar] [CrossRef]
- Helt, J.E.; Larson, M.A. Effects of temperature on the crystallization of potassium nitrate by direct measurement of supersaturation. AIChE J. 1977, 23, 822–830. [Google Scholar] [CrossRef]
- Glade, H.; Ilyaskarov, A.; Ulrich, J. Determination of Crystal Growth Kinetics Using Ultrasonic Technique. Chem. Eng. Technol. 2004, 27, 736–740. [Google Scholar] [CrossRef]
- Lee, T.; Lin, Y.K. The Origin of Life and the Crystallization of Aspartic Acid in Water. Cryst. Growth Des. 2010, 10, 1652–1660. [Google Scholar] [CrossRef]
- Sangtani, E.; Sahu, S.K.; Thorat, S.H.; Gawade, R.L.; Jha, K.; Munshi, P.; Gonnade, R.G. Furosemide Cocrystals with Pyridines: An Interesting Case of Color Cocrystal Polymorphism. Cryst. Growth Des. 2015, 15, 5858–5872. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Zhang, Q.; Peng, B.; Zhu, B.; Wang, J.-R.; Liu, L.; Mei, X. Fine-Tuning the Colors of Natural Pigment Emodin with Superior Stability through Cocrystal Engineering. Cryst. Growth Des. 2018, 18, 6123–6132. [Google Scholar] [CrossRef]
- Butterhof, C.; Martin, T.; Milius, W.; Breu, J. Microphase Separation with Small Amphiphilic Molecules: Crystal Structure of Preservatives Sodium Benzoate (E 211) and Potassium Benzoate (E 212). Z. Anorg. Allg. Chem. 2013, 639, 2816–2821. [Google Scholar] [CrossRef]
- Martin, T.; Gorelik, T.; Greim, D.; Butterhof, C.; Kolb, U.; Senker, J.; Breu, J. Microphase Separation upon Crystallization of Small Amphiphilic Molecules: ‘Low’ Temperature Form II of Sodium Benzoate (E 211). CrystEngComm 2016, 18, 5811–5817. [Google Scholar] [CrossRef]






| S0 = C0/C* | τ (s) | γ × 105 (J·m−2) | J0 × 10−9 (Nucleus s−1·m−3) |
|---|---|---|---|
| Without templating molecules | |||
| 1.66 | 370 ± 14 | 191.92 ± 2.28 | 8.04 ± 0.30 |
| 1.54 | 1080 ± 27 | ||
| 1.48 | 1494 ± 49 | ||
| 1.43 | 1848 ± 180 | ||
| With NaBz templating molecules | |||
| 1.66 | 277 ± 18 | 202.38 ± 3.59 | 15.97 ± 2.79 |
| 1.54 | 714 ± 47 | ||
| 1.48 | 1086 ± 39 | ||
| 1.43 | 1816 ± 68 | ||
| With 2:1 HBz–NaBz co-crystal templating molecules | |||
| 1.66 | 205 ± 35 | 194.34 ± 3.37 | 17.05 ± 3.25 |
| 1.54 | 538 ± 38 | ||
| 1.48 | 717 ± 45 | ||
| 1.43 | 1097 ± 96 | ||
| With 1:1 HBz–NaBz co-crystal templating molecules | |||
| 1.66 | 187 ± 20 | 190.62 ± 1.96 | 18.62 ± 2.26 |
| 1.54 | 366 ± 11 | ||
| 1.48 | 533 ± 32 | ||
| 1.43 | 894 ± 47 | ||
| S0 = C0/C* | ∆Gv × 10−6 (J·m−3) | ∆Gcrit × 1022 (J) | J × 10−7 (Nucleus s−1·m−3) | rcrit × 1011 (m) | i* |
|---|---|---|---|---|---|
| Without templating molecules | |||||
| 1.66 | −4.35 | 62.61 ± 2.22 | 167.34 ± 3.25 | 88.25 ± 1.05 | 6.19 ± 0.22 |
| 1.54 | −3.70 | 86.26 ± 3.06 | 92.56 ± 3.68 | 103.59 ± 1.23 | 10.01 ± 0.36 |
| 1.48 | −3.36 | 104.63 ± 3.71 | 58.45 ± 3.27 | 114.09 ± 1.35 | 13.38 ± 0.48 |
| 1.43 | −3.07 | 125.70 ± 4.46 | 34.51 ± 2.58 | 125.05 ± 1.48 | 17.62 ± 0.63 |
| With NaBz templating molecules | |||||
| 1.66 | −4.35 | 73.44 ± 3.89 | 250.35 ± 20.29 | 93.06 ± 1.65 | 7.26 ± 0.38 |
| 1.54 | −3.70 | 101.18 ± 5.36 | 124.62 ± 5.59 | 109.23 ± 1.94 | 11.75 ± 0.62 |
| 1.48 | −3.36 | 122.74 ± 6.50 | 72.54 ± 1.20 | 120.30 ± 2.13 | 15.69 ± 0.83 |
| 1.43 | −3.07 | 147.46 ± 7.81 | 39.04 ± 0.64 | 131.86 ± 2.34 | 20.66 ± 1.09 |
| With 2:1 HBz–NaBz co-crystal templating molecules | |||||
| 1.66 | −4.35 | 65.03 ± 3.36 | 330.00 ± 40.45 | 89.36 ± 1.55 | 6.43 ± 0.33 |
| 1.54 | −3.70 | 89.60 ± 4.63 | 177.76 ± 17.48 | 104.89 ± 1.82 | 10.40 ± 0.54 |
| 1.48 | −3.36 | 108.68 ± 5.62 | 110.00 ± 8.96 | 115.52 ± 2.00 | 13.90 ± 0.72 |
| 1.43 | −3.07 | 130.57 ± 6.75 | 63.48 ± 4.21 | 126.62 ± 2.20 | 18.30 ± 0.95 |
| With 1:1 HBz–NaBz co-crystal templating molecules | |||||
| 1.66 | −4.35 | 61.33 ± 1.88 | 398.61 ± 32.16 | 87.65 ± 0.90 | 6.07 ± 0.19 |
| 1.54 | −3.70 | 84.50 ± 2.59 | 222.79 ± 14.68 | 102.88 ± 1.06 | 9.81 ± 0.30 |
| 1.48 | −3.36 | 102.50 ± 3.14 | 141.80 ± 7.81 | 113.31 ± 1.17 | 13.10 ± 0.40 |
| 1.43 | −3.07 | 123.14 ± 3.78 | 84.48 ± 3.73 | 124.20 ± 1.28 | 17.26 ± 0.53 |
| S0 = C0/C* | g | KG′ × 103 (kg1/3 s−1) |
|---|---|---|
| Without templating molecules | ||
| 1.66 | 1.1 | 9.9 |
| 1.54 | 1.6 | 38.0 |
| 1.48 | 1.0 | 3.6 |
| 1.43 | 1.1 | 7.0 |
| With NaBz templating molecules | ||
| 1.66 | 1.9 | 83.0 |
| 1.54 | 1.9 | 97.0 |
| 1.48 | 1.8 | 66.8 |
| 1.43 | 1.9 | 80.7 |
| With 2:1 HBz–NaBz co-crystal templating molecules | ||
| 1.66 | 0.8 | 4.1 |
| 1.54 | 0.9 | 4.3 |
| 1.48 | 1.0 | 8.9 |
| 1.43 | 1.4 | 19.7 |
| With 1:1 HBz–NaBz co-crystal templating molecules | ||
| 1.66 | 1.1 | 7.6 |
| 1.54 | 0.8 | 3.9 |
| 1.48 | 1.1 | 10.0 |
| 1.43 | 0.9 | 3.2 |
| 2:1 HBz–NaBz Co-Crystals | Average Aspect Ratio |
|---|---|
| Without templating molecules | 6.0 ± 1.7 |
| With NaBz templating molecules | 9.6 ± 3.7 |
| With 2:1 HBz–NaBz co-crystal templating molecules | 5.3 ± 1.4 |
| With 1:1 HBz–NaBz co-crystal templating molecules | 4.3 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lini, F.Z.; Pratama, D.E.; Lee, T. Co-Crystallization Kinetics of 2:1 Benzoic Acid–Sodium Benzoate Co-Crystal: The Effect of Templating Molecules in a Solution. Crystals 2021, 11, 812. https://doi.org/10.3390/cryst11070812
Lini FZ, Pratama DE, Lee T. Co-Crystallization Kinetics of 2:1 Benzoic Acid–Sodium Benzoate Co-Crystal: The Effect of Templating Molecules in a Solution. Crystals. 2021; 11(7):812. https://doi.org/10.3390/cryst11070812
Chicago/Turabian StyleLini, Freshsya Zata, Dhanang Edy Pratama, and Tu Lee. 2021. "Co-Crystallization Kinetics of 2:1 Benzoic Acid–Sodium Benzoate Co-Crystal: The Effect of Templating Molecules in a Solution" Crystals 11, no. 7: 812. https://doi.org/10.3390/cryst11070812