Migration and Enrichment Behaviors of Ca and Mg Elements during Cooling and Crystallization of Boron-Bearing Titanium Slag Melt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experiment Producer
2.3. Characterization Method
3. Results and Discussion
3.1. The Micromorphology of the Modified Titanium Slag with Different Amounts of Additive (B2O3)
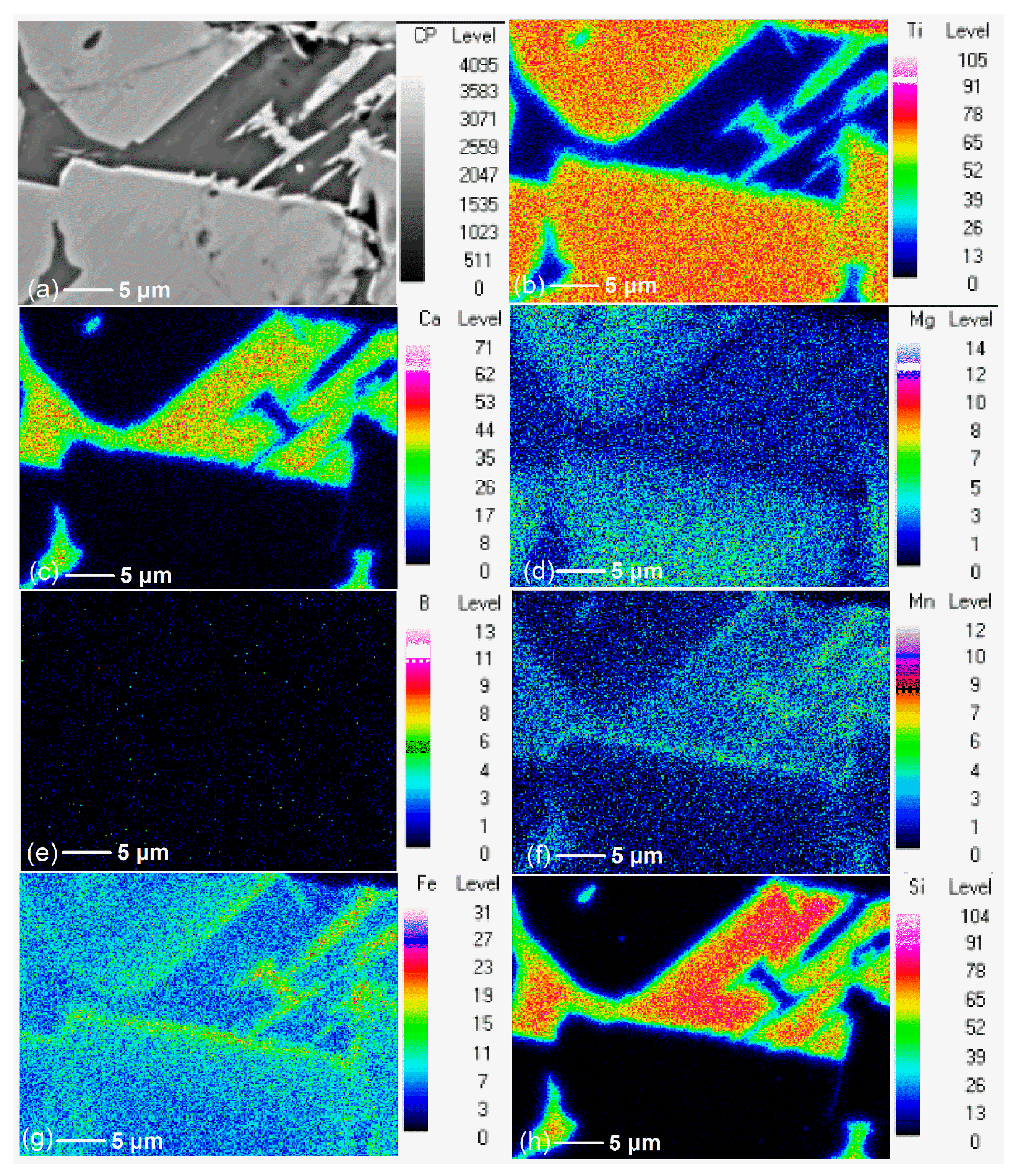
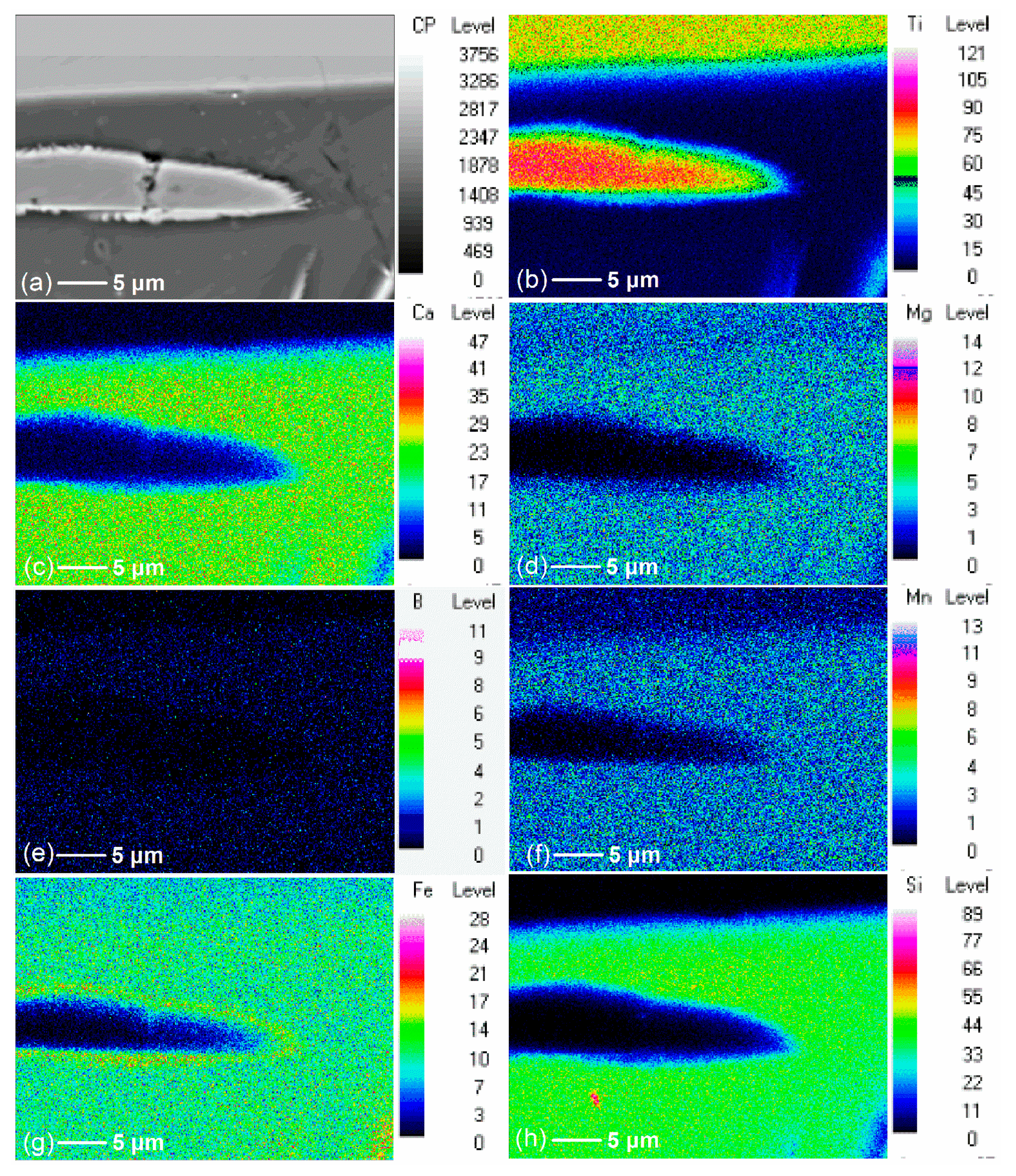
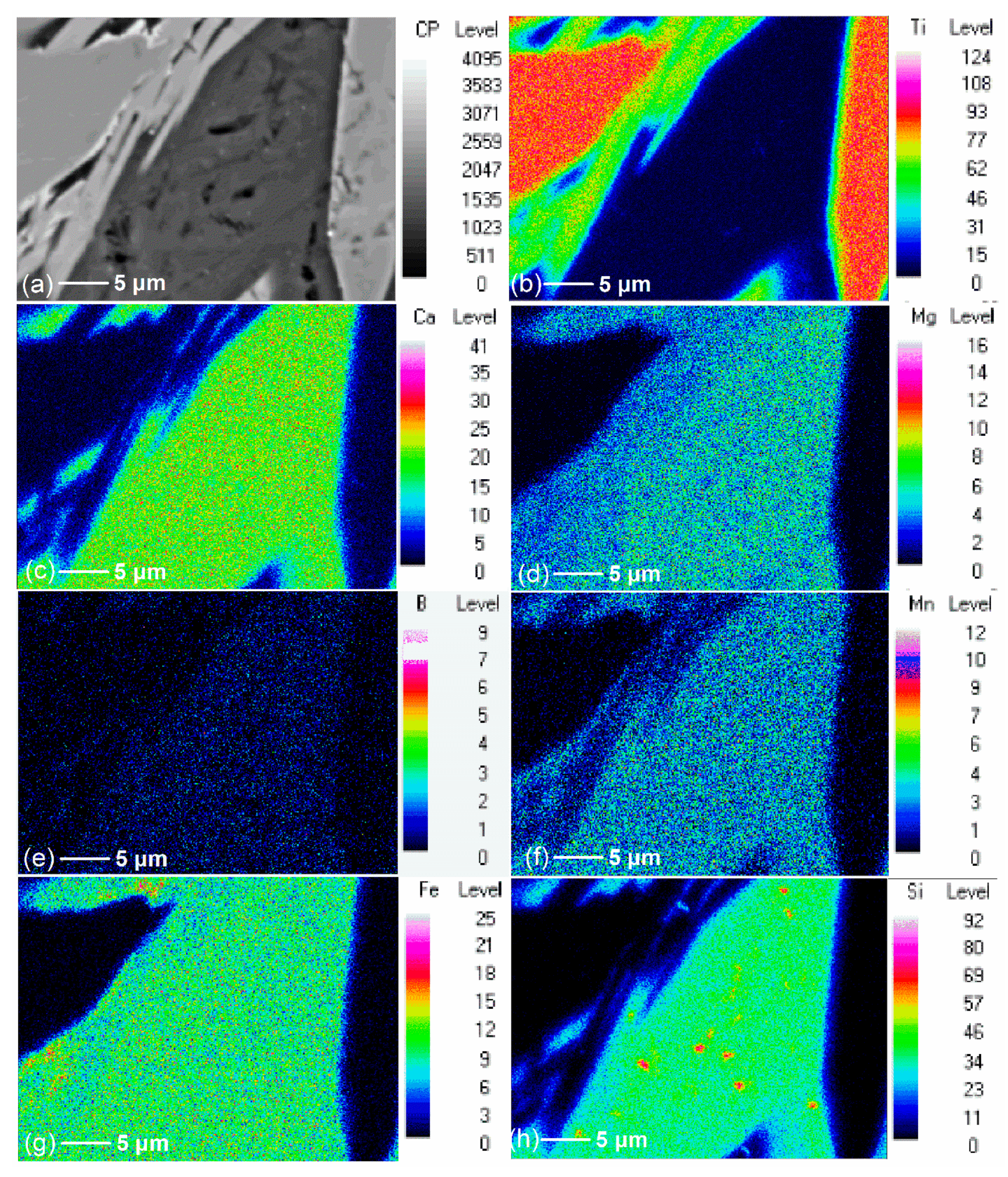
3.2. Effect of Additive (B2O3) Amount on the Element Distribution of the Modified Titanium Slag
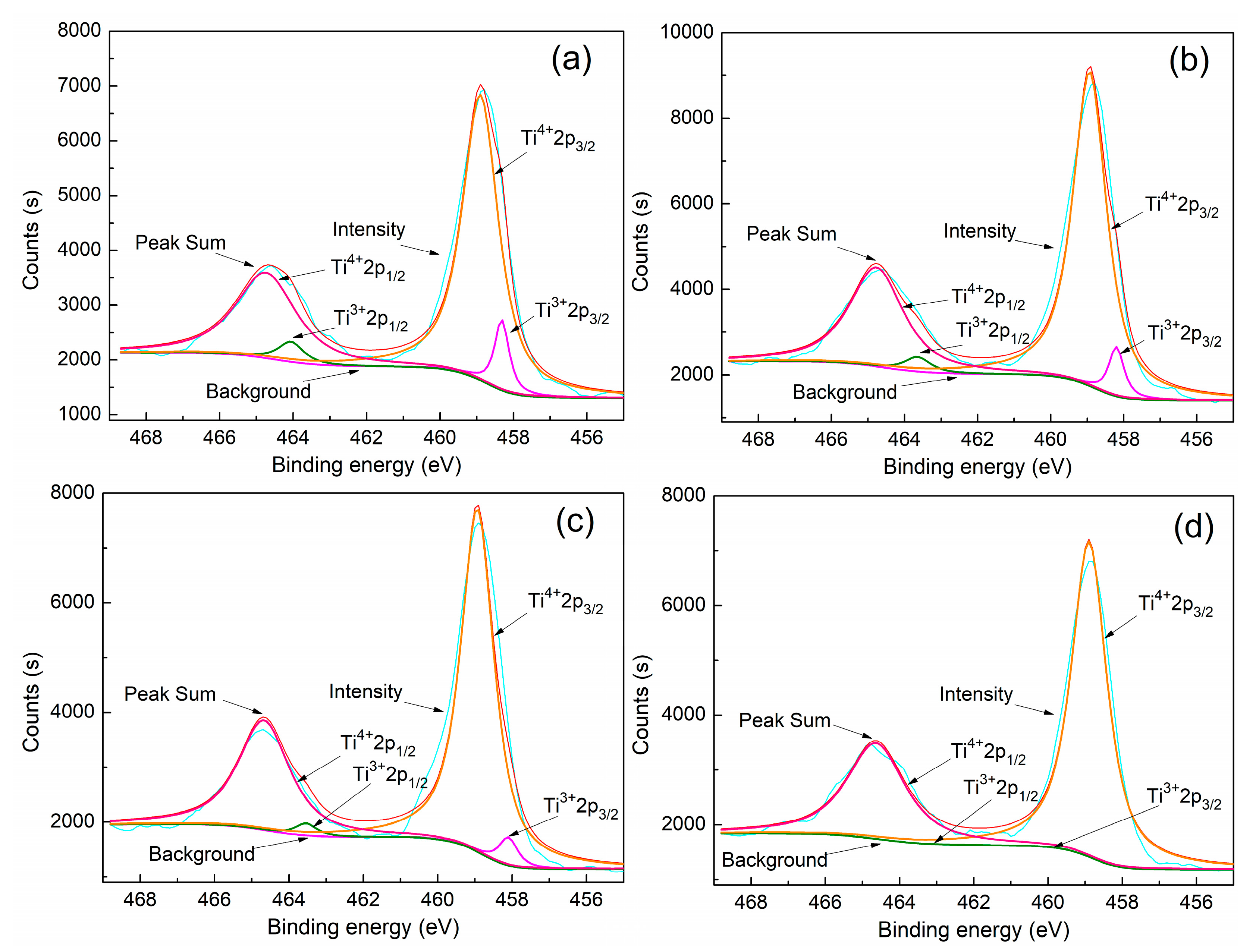
3.3. Effect of Additive (B2O3) Amount on the Valence State of Titanium in the Modified Titanium Slag
4. Conclusions
- (1)
- Ti was concentrated with the rutile form in the flake and bright areas, and B, Mg, and Ca elements were concentrated with the borate form in the long strip and dark areas during the cooling and crystallization of boron-bearing titanium slag melt.
- (2)
- When additive (B2O3) is added, Ti migrated and enriched in one area to form the rutile phase, while Ca, Mg, and B migrated and enriched in another area to form the borate phase. When the additive (B2O3) amount increased, Ca and Mg migration was complete and more thorough.
- (3)
- Additive (B2O3) has the effect of promoting the formation of the rutile phase and at the same time inhibiting the formation of the solid solution of anosovite during the cooling and crystallization of titanium slag melt.
- (4)
- With the additive (B2O3) amount increasing from 0% to 6%, the proportion of Ti3+ in the modified titanium slag reduces from 9.15% to 0%, and the proportion of Ti4+ increases from 90.85% to 100% under the same cooling and crystallization condition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fan, H.; Wang, R.; Duan, H.; Chen, D.; Xu, Z. Structural and transport properties of TiO2-SiO2-MgO-CaO system through molecular dynamics simulations. J. Mol. Liq. 2021, 325, 115226. [Google Scholar] [CrossRef]
- Chen, G.; Ling, Y.; Li, Q.; Zheng, H.; Jiang, Q.; Li, K.; Gao, L.; Omran, M.; Chen, J. Highly efficient oxidation of Panzhihua titanium slag for manufacturing welding grade rutile titanium dioxide. J. Mater. Res. Technol. 2020, 9, 7079–7086. [Google Scholar] [CrossRef]
- Sofronov, D.; Rucki, M.; Demidov, O.; Doroshenko, A.; Sofronova, E.; Shaposhnyk, A.; Kapustnik, O.; Mateychenko, P.; Kucharczyk, W. Formation of TiO2 particles during thermal decomposition of Ti(NO3)4, TiOF2 and TiOSO4. J. Mater. Res. Technol. 2020, 9, 12201–12212. [Google Scholar] [CrossRef]
- Fan, H.; Chen, D.; Liu, P.; Duan, H.; Huang, Y.; Long, M.; Liu, T. Structural and transport properties of FeO-TiO2 system through molecular dynamics simulations. J. Non Cryst. Solids 2018, 493, 57–64. [Google Scholar] [CrossRef]
- Sui, Y.; Guo, Y.; Jiang, T.; Qiu, G.-Z. Separation and recovery of iron and titanium from oxidized vanadium titano-magnetite by gas-based reduction roasting and magnetic separation. J. Mater. Res. Technol. 2019, 8, 3036–3043. [Google Scholar] [CrossRef]
- Xuefeng, X.; Tayyeb, A.; Lin, W.; Huanwu, C.; Zhe, Z.; Zixuan, N.; Xiaopin, L.; Anjin, L.; Binbin, Z.; Xingwang, C. Research on dynamic compression properties and deformation mechanism of Ti6321 titanium alloy. J. Mater. Res. Technol. 2020, 9, 11509–11516. [Google Scholar] [CrossRef]
- Ran, J.; Jiang, F.; Sun, X.; Chen, Z.; Tian, C.; Zhao, H. Microstructure and mechanical properties of Ti-6Al-4V fabricated by electron beam melting. Crystals 2020, 10, 972. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Ma, Y.; Wang, M.; Wang, Y.; Wang, X. A novel technology for the refinement of low-grade ilmenite concentrate. Hydrometallurgy 2018, 180, 36–40. [Google Scholar] [CrossRef]
- Zheng, F.; Guo, Y.; Duan, W.; Liu, S.; Qiu, G.; Chen, F.; Jiang, T.; Wang, S. Transformation of Ti-bearing mineral in Panzhihua electric furnace titanium slag during oxidation roasting process. J. Therm. Anal. Calorim. 2018, 131, 1767–1776. [Google Scholar] [CrossRef]
- Niu, L.-P.; Ni, P.-Y.; Zhang, T.-A.; Lv, G.-Z.; Zhou, A.-P.; Liang, X.-B.; Meng, D.-L. Mechanism of fluidized chlorination reaction of Kenya natural rutile ore. Rare Met. 2014, 33, 485–492. [Google Scholar] [CrossRef]
- Wu, K.-H.; Zhang, G.-H.; Chou, K.-C. Preparation technology of Ti-rich material from ilmenite via method of vacuum carbothermal reduction. Can. Metall. Q. 2019, 58, 196–203. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Liao, Z.; Chen, Q.; Tan, J. Hydrochloric acid leaching behavior of different treated Panxi ilmenite concentrations. Hydrometallurgy 2011, 107, 40–47. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries; Government Printing Office: Washington, DC, USA, 2021.
- Nie, W.; Wen, S.; Feng, Q.; Liu, D.; Zhou, Y. Mechanism and kinetics study of sulfuric acid leaching of titanium from titanium-bearing electric furnace slag. J. Mater. Res. Technol. 2020, 9, 1750–1758. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Duan, H. A new process of extracting titanium from vanadium—Titanium magnetite. Crystals 2021, 11, 327. [Google Scholar] [CrossRef]
- Lv, X.; Xin, Y.; Lv, X.; Lv, W.; Dang, J. High-titanium slag preparation process by carbothermic reduction of ilmenite and wet-magnetic separation. Metall. Mater. Trans. B 2021, 52, 351–362. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Mahmud, M.P.; Lang, C. Towards sustainable TiO2 production: An investigation of environmental impacts of ilmenite and rutile processing routes in Australia. J. Clean. Prod. 2018, 196, 1016–1025. [Google Scholar] [CrossRef]
- Jia, L.; Liang, B.; Lü, L.; Yuan, S.; Zheng, L.; Wang, X.; Li, C. Beneficiation of titania by sulfuric acid pressure leaching of Panzhihua ilmenite. Hydrometallurgy 2014, 150, 92–98. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Xie, Z.; Li, H. Influence of redox pretreatment on the pulverization of panzhihua ilmenite during hydrochloric acid leaching. Hydrometallurgy 2015, 157, 226–233. [Google Scholar] [CrossRef]
- Fan, H.; Chen, D.; Liu, T.; Duan, H.; Huang, Y.; Long, M.; He, W. Crystallization behaviors of anosovite and silicate crystals in high CaO and MgO titanium slag. Metals 2018, 8, 754. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Liu, S.; Jiang, T.; Qiu, G.; Chen, F. A process for producing synthetic rutile from Panzhihua titanium slag. Hydrometallurgy 2014, 147, 134–141. [Google Scholar] [CrossRef]
- Gueguin, M. Method of Preparing a Synthetic Rutile from a Titaniferous Slag Containing Magnesium Values. U.S. Patent 5,063,032, 5 November 1991. [Google Scholar]
- Borowiec, K.; Grau, A.E.; Gueguin, M.; Turgeon, J.-F. Method to Upgrade Titania Slag and Resulting Product. U.S. Patent 5,830,420, 3 November 1998. [Google Scholar]
- Elger, G.W.; Stadler, R.A.; Sanker, P.E. Process for Purifying a Titanium-Bearing Material and Upgrading Ilmenite to Synthetic Rutile with Sulfur Trioxide. U.S. Patent 4,120,694, 17 October 1978. [Google Scholar]
- Dong, H.; Jiang, T.; Guo, Y.; Chen, J.; Fan, X. Upgrading a Ti-slag by a roast-leach process. Hydrometallurgy 2012, 113, 119–121. [Google Scholar] [CrossRef]
- Zheng, F.-Q.; Guo, Y.-F.; Liu, S.-S.; Qiu, G.-Z.; Feng, C.; Jiang, T.; Shuai, W. Removal of magnesium and calcium from electric furnace titanium slag by H3PO4 oxidation roasting–leaching process. Trans. Nonferrous Met. Soc. China 2018, 28, 356–366. [Google Scholar] [CrossRef]
- Liu, S.-s.; Guo, Y.-f.; Qiu, G.-z.; Jiang, T. Preparation of Ti-rich material from titanium slag by activation roasting followed by acid leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 1174–1178. [Google Scholar] [CrossRef]
- Fan, H.; Duan, H.; Tan, K.; Li, Y.; Chen, D.; Long, M.; Liu, T. Production of synthetic rutile from molten titanium slag with the addition of B2O3. JOM 2017, 69, 1914–1919. [Google Scholar] [CrossRef]
- Carley, A.F.; Chalker, P.R.; Riviere, J.C.; Roberts, M.W. The identification and characterisation of mixed oxidation states at oxidised titanium surfaces by analysis of X-ray photoelectron spectra. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 351–370. [Google Scholar] [CrossRef]
- Siemensmeyer, B.; Schultze, J. XPS and UPS studies of gas-phase oxidation, electrochemistry and corrosion behaviour of Ti and Ti5Ta. Surf. Interface Anal. 1990, 16, 309–314. [Google Scholar] [CrossRef]
- Choudhury, T.; Saied, S.; Sullivan, J.; Abbot, A. Reduction of oxides of iron, cobalt, titanium and niobium by low-energy ion bombardment. J. Phys. D Appl. Phys. 1989, 22, 1185. [Google Scholar] [CrossRef]
- Godfroid, T.; Gouttebaron, R.; Dauchot, J.; Leclere, P.; Lazzaroni, R.; Hecq, M. Growth of ultrathin Ti films deposited on SnO2 by magnetron sputtering. Thin Solid Films 2003, 437, 57–62. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahé, E. Structure and composition of passive titanium oxide films. Mater. Sci. Eng. B 1997, 47, 235–243. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, D.; Wang, R.; Feng, Z.; Zuo, Z.; Qin, S.; Liu, H.; Xu, X. The dielectric and photochromic properties of defect-rich BaTiO3 microcrystallites synthesized from Ti2O3. Mater. Sci. Eng. B 2012, 177, 639–644. [Google Scholar] [CrossRef]
- Siemensmeyer, B.; Bade, K.; Schultze, J. XPS and electrochemical studies of thin TiN layers. Ber. Bunsenges. Für Phys. Chem. 1991, 95, 1461–1469. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Wei, Y.; Liu, C. Conductor formation through phase transformation in Ti-oxide thin films. J. Electron. Mater. 2012, 41, 166–171. [Google Scholar] [CrossRef]
- Chowdari, B.; Rao, G.S.; Lee, G. XPS and ionic conductivity studies on Li2O–Al2O3–(TiO2 or GeO2)–P2O5 glass-ceramics. Solid State Ion. 2000, 136, 1067–1075. [Google Scholar] [CrossRef]
- Liu, Q. Study on the Technology of Microwave Roasting-Acid and Alkali Leaching of High Titanium Slag. Master’s Thesis, Kunming Univerity Science Technol, Yunnan, China, April 2017. [Google Scholar]
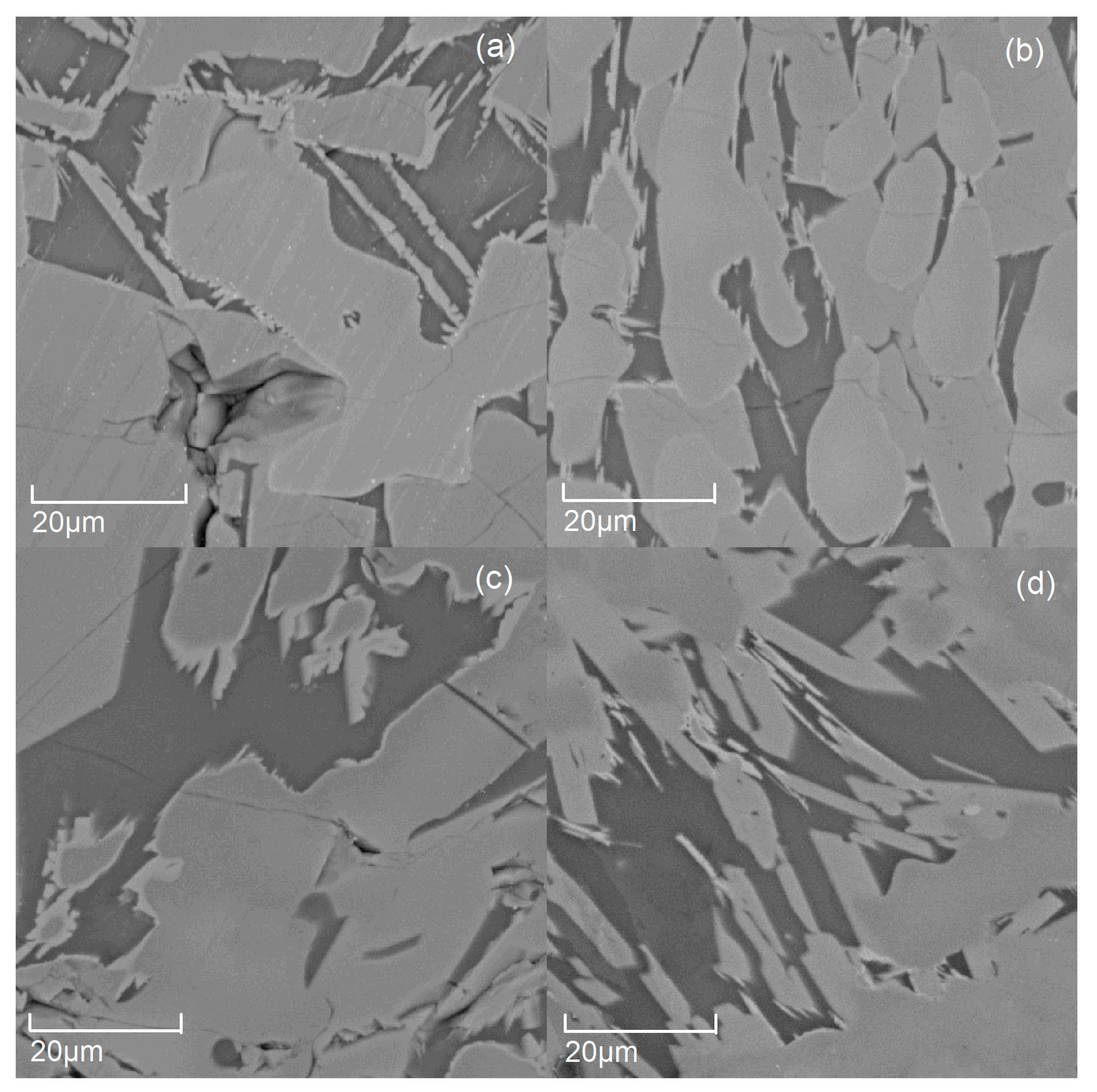
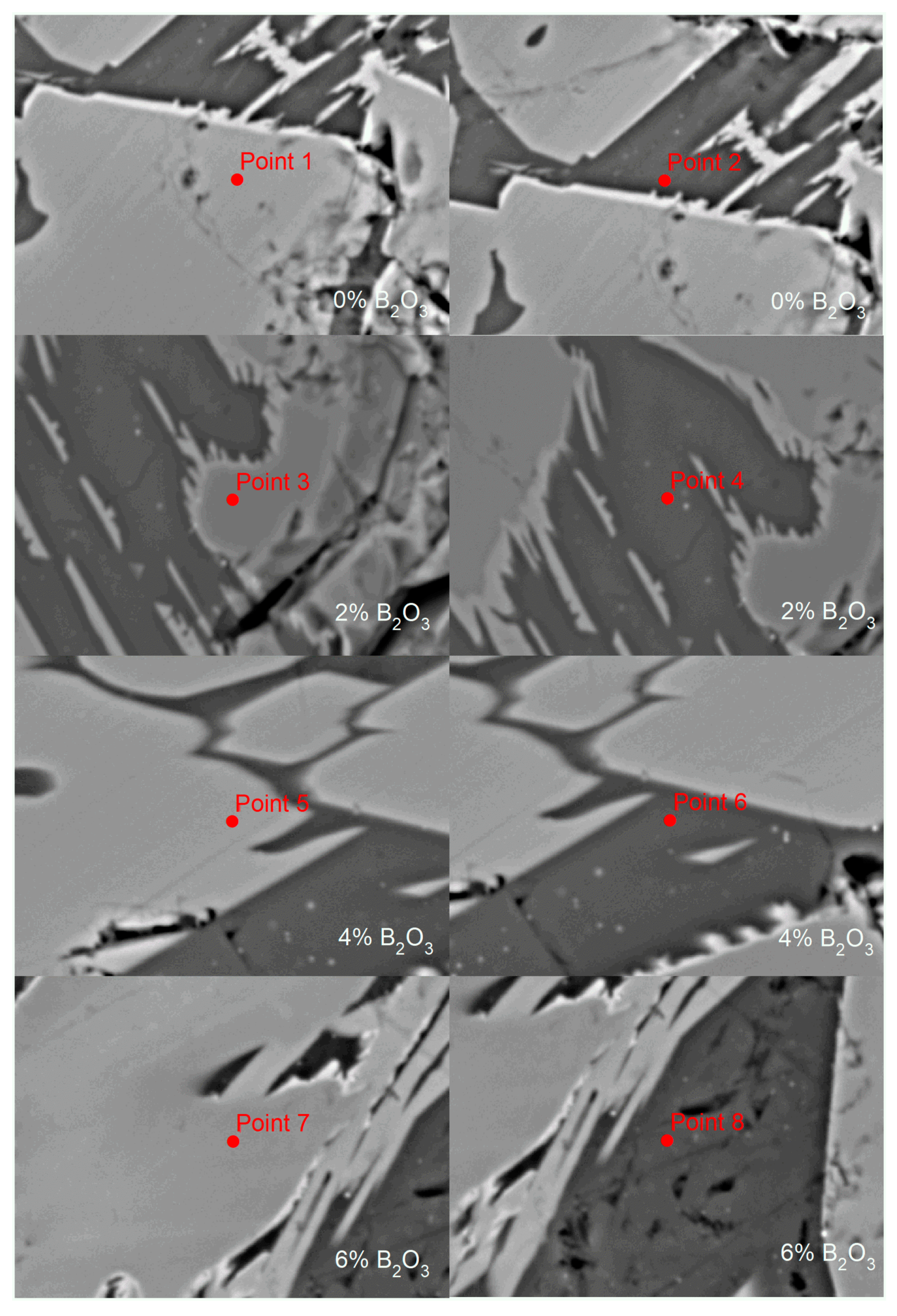

| Point | TiO2 | CaO | MgO | B2O3 | Al2O3 | MnO | Fe2O3 | SiO2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 74.36 | 0.10 | 3.63 | 0.00 | 2.49 | 1.21 | 18.12 | 0.09 |
| 2 | 6.76 | 10.34 | 1.56 | 0.00 | 10.51 | 3.39 | 16.42 | 51.03 |
| 3 | 98.54 | 0.25 | 0.00 | 0.13 | 0.09 | 0.09 | 0.81 | 0.10 |
| 4 | 3.79 | 7.76 | 3.00 | 19.67 | 9.17 | 3.50 | 19.35 | 33.77 |
| 5 | 97.69 | 0.22 | 0 | 0 | 0.09 | 0.18 | 1.69 | 0.09 |
| 6 | 5.26 | 5.43 | 3.65 | 24.71 | 8.18 | 4.22 | 25.90 | 25.61 |
| 7 | 99.43 | 0.09 | 0.00 | 0.00 | 0.10 | 0.05 | 0.29 | 0.03 |
| 8 | 3.90 | 4.77 | 4.71 | 27.30 | 8.56 | 4.22 | 23.42 | 23.12 |
| B2O3 Addition Amount (%) | Content | Ti4+2p1/2 | Ti3+2p1/2 | Ti4+2p3/2 | Ti3+2p3/2 |
|---|---|---|---|---|---|
| 0 | binding energy (eV) | 464.5 | 463.8 | 458.9 | 458.3 |
| peak area | 4491.3 | 452.5 | 8982.7 | 905.0 | |
| proportion (%) | 90.85 | 9.15 | 90.85 | 9.15 | |
| 2 | binding energy (eV) | 464.5 | 463.7 | 458.9 | 458.2 |
| peak area | 5998.2 | 427.3 | 11996.5 | 854.7 | |
| proportion (%) | 93.35 | 6.65 | 93.35 | 6.65 | |
| 4 | binding energy (eV) | 464.5 | 463.6 | 458.9 | 458.1 |
| peak area | 4883.2 | 228.2 | 9766.3 | 456.3 | |
| proportion (%) | 95.54 | 4.46 | 95.54 | 4.46 | |
| 6 | binding energy (eV) | 464.5 | 463.6 | 458.9 | 458.1 |
| peak area | 4818.3 | 0.05 | 9636.6 | 0.1 | |
| proportion (%) | 100 | 0 | 100 | 0 |
| Researcher | Ti2O3 (eV) | TiO2 (eV) | ||||
|---|---|---|---|---|---|---|
| Ti2p1/2 | Ti2p3/2 | Peak Spacing | Ti2p1/2 | Ti2p3/2 | Peak Spacing | |
| Albert F. Carley et al. [29] | 462.5 | 457.5 | 5.0 | 465.0 | 459.0 | 6.0 |
| B. Siemensmeyer et al. [30] | 463.6 | 457.9 | 5.7 | 465.2 | 459.4 | 5.8 |
| T. Choudhury et al. [31] | 463.4 | 457.6 | 5.8 | 465.0 | 459.1 | 5.9 |
| T. Godfroid et al. [32] | 463.1 | 457.2 | 5.9 | 464.7 | 458.8 | 5.9 |
| J. Pouilleau et al. [33] | 463.1 | 457.4 | 5.7 | 464.8 | 459.1 | 5.7 |
| Yu Qian et al. [34] | 462.8 | 457.0 | 5.8 | 464.0 | 458.1 | 5.9 |
| B. Siemensmeyer et al. [35] | 463.4 | 457.7 | 5.7 | 465.0 | 459.2 | 5.8 |
| Y. S. Liu et al. [36] | 463.4 | 457.6 | 5.8 | 465.0 | 459.1 | 5.9 |
| B. V. R. Chowdari et al. [37] | 464.2 | 458.7 | 5.5 | 465.3 | 459.8 | 5.5 |
| Liu Qianqian et al. [38] | 463.7 | 458.2 | 5.5 | 464.8 | 459.3 | 5.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Wang, R.; Xu, Z.; Duan, H.; Chen, D. Migration and Enrichment Behaviors of Ca and Mg Elements during Cooling and Crystallization of Boron-Bearing Titanium Slag Melt. Crystals 2021, 11, 888. https://doi.org/10.3390/cryst11080888
Fan H, Wang R, Xu Z, Duan H, Chen D. Migration and Enrichment Behaviors of Ca and Mg Elements during Cooling and Crystallization of Boron-Bearing Titanium Slag Melt. Crystals. 2021; 11(8):888. https://doi.org/10.3390/cryst11080888
Chicago/Turabian StyleFan, Helin, Ruixiang Wang, Zhifeng Xu, Huamei Duan, and Dengfu Chen. 2021. "Migration and Enrichment Behaviors of Ca and Mg Elements during Cooling and Crystallization of Boron-Bearing Titanium Slag Melt" Crystals 11, no. 8: 888. https://doi.org/10.3390/cryst11080888
APA StyleFan, H., Wang, R., Xu, Z., Duan, H., & Chen, D. (2021). Migration and Enrichment Behaviors of Ca and Mg Elements during Cooling and Crystallization of Boron-Bearing Titanium Slag Melt. Crystals, 11(8), 888. https://doi.org/10.3390/cryst11080888






