Effects of Trace Amounts of Mn, Zr and Sc on the Recrystallization and Corrosion Resistance of Al-5Mg Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructure Observation
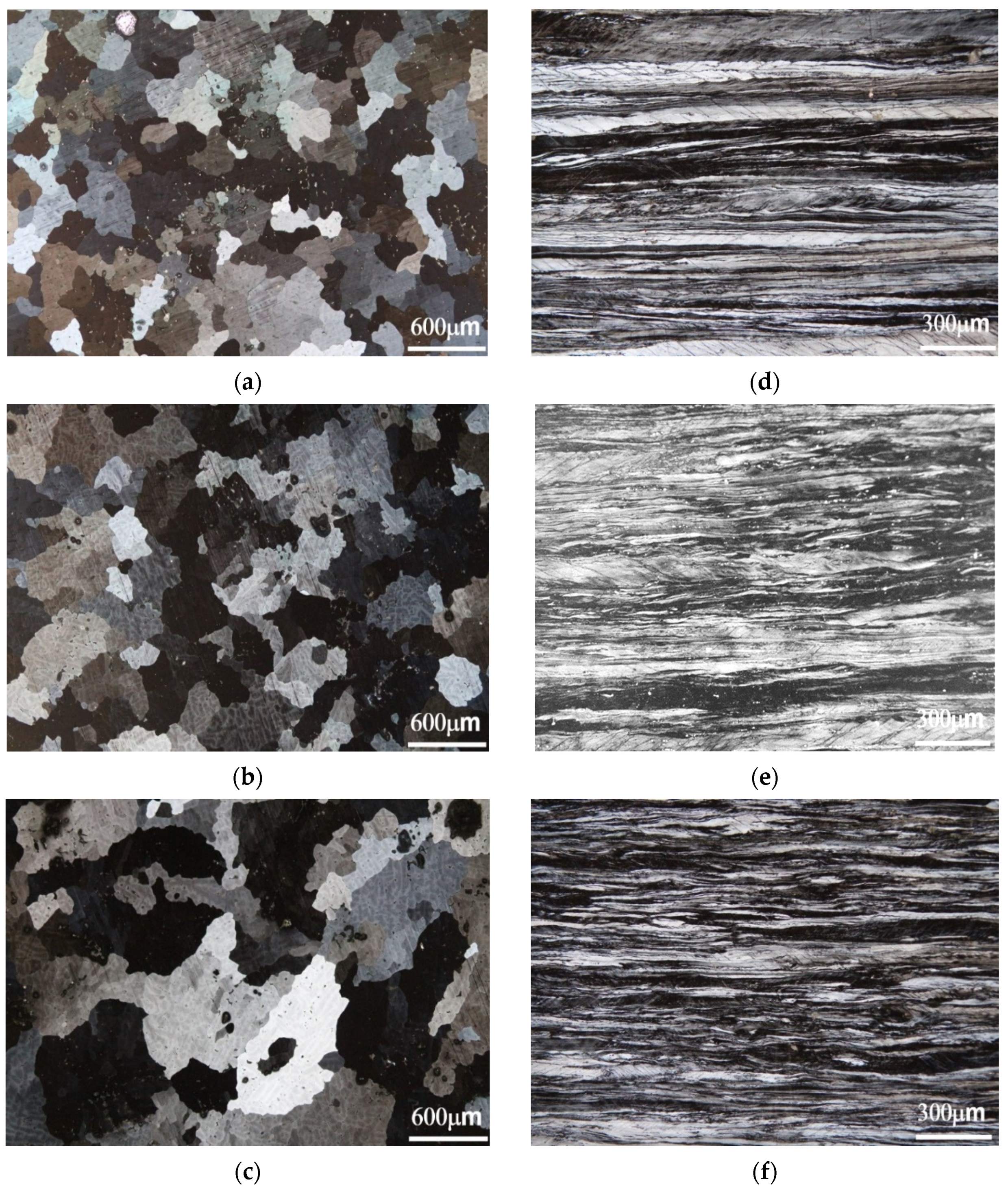
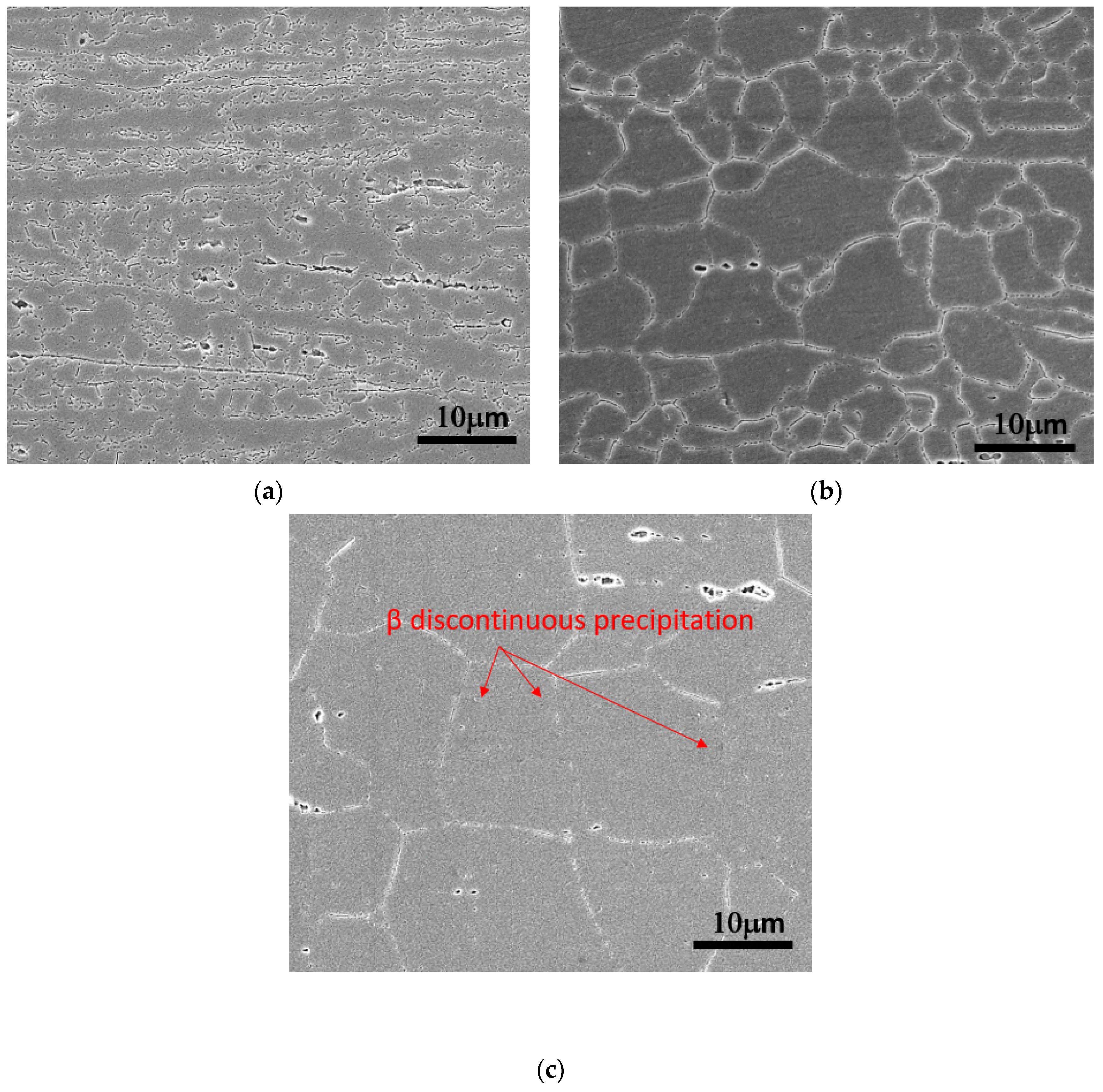
3.1.1. Optical Metallographic Observations
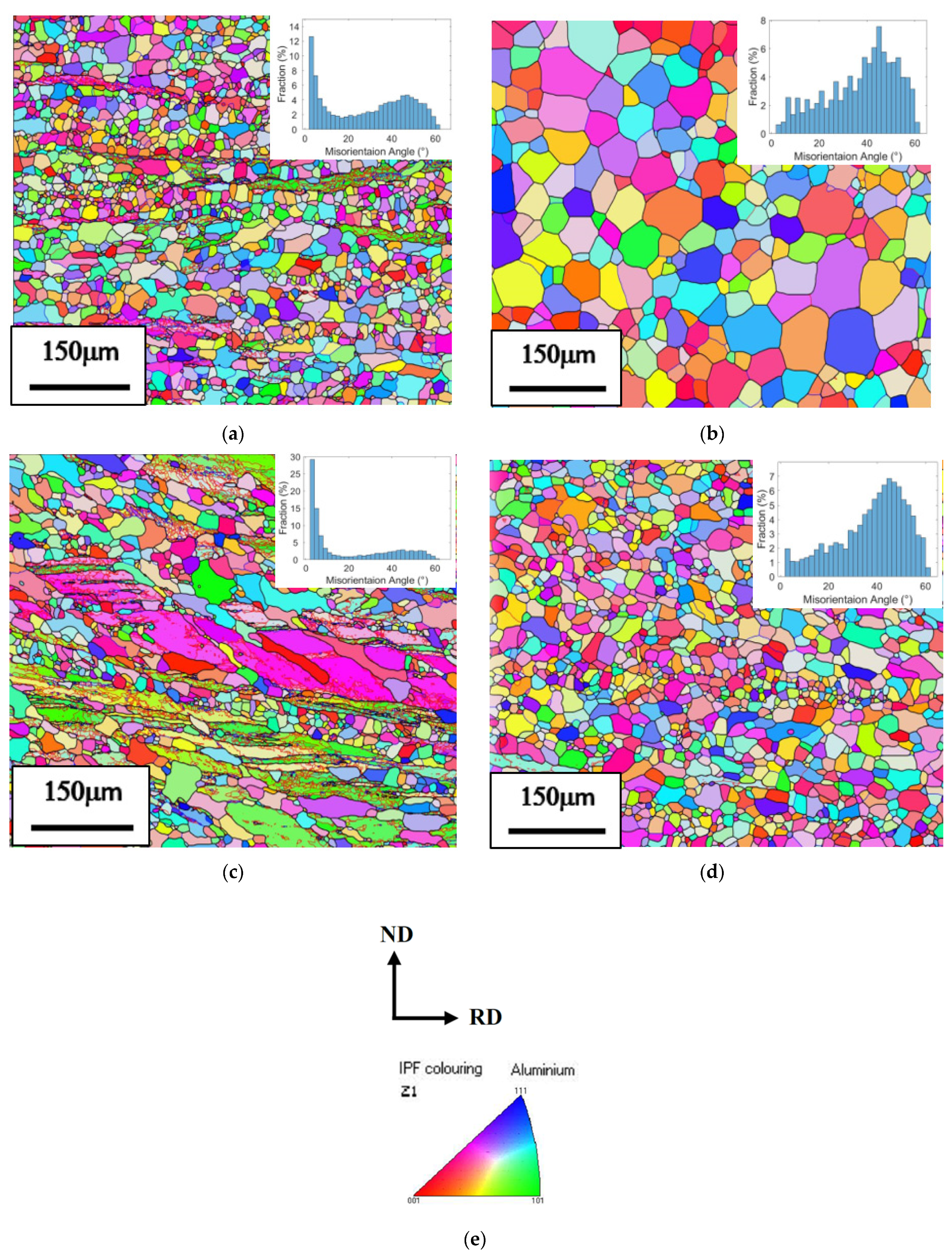
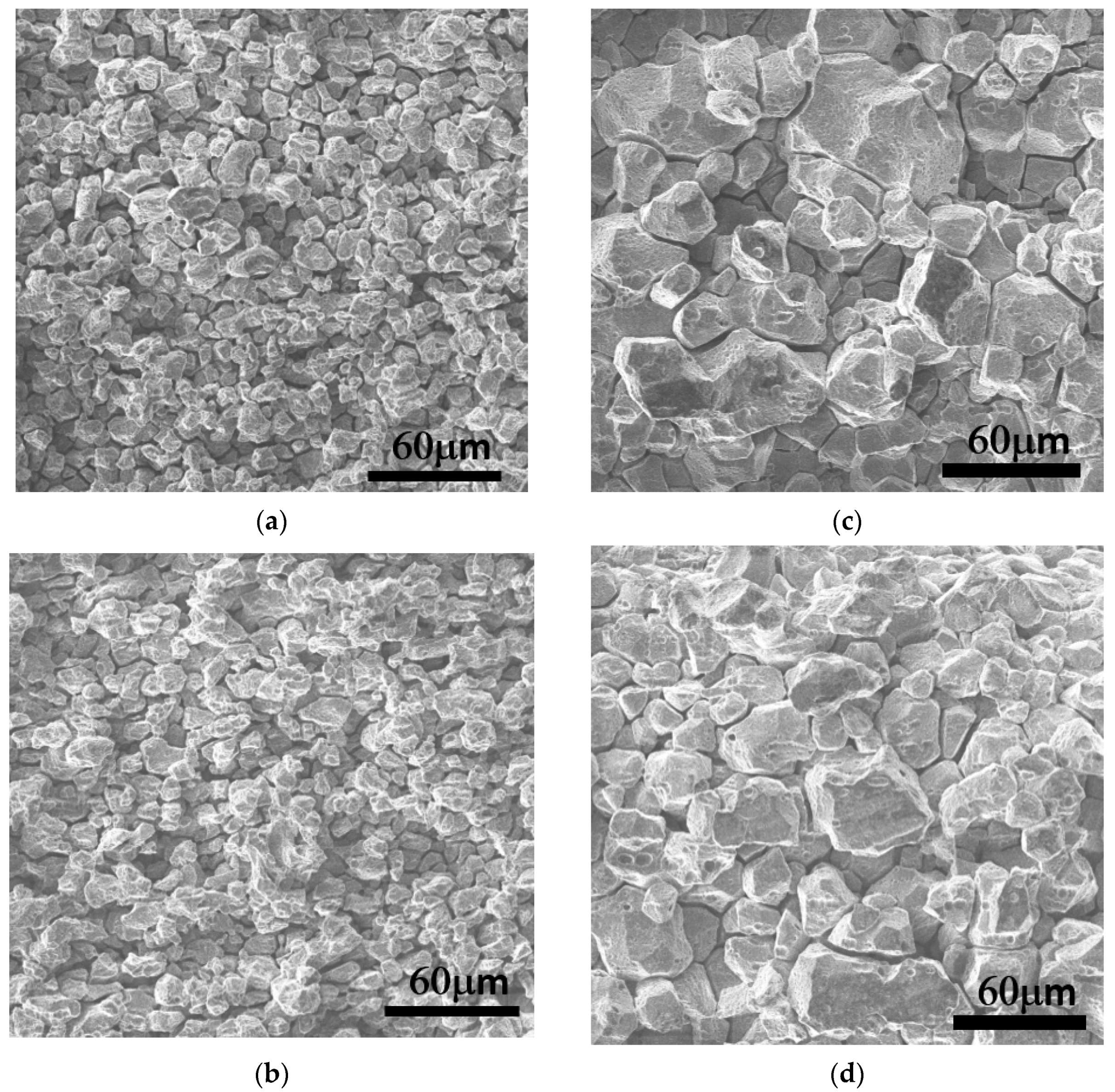
3.1.2. Recrystallization Analysis
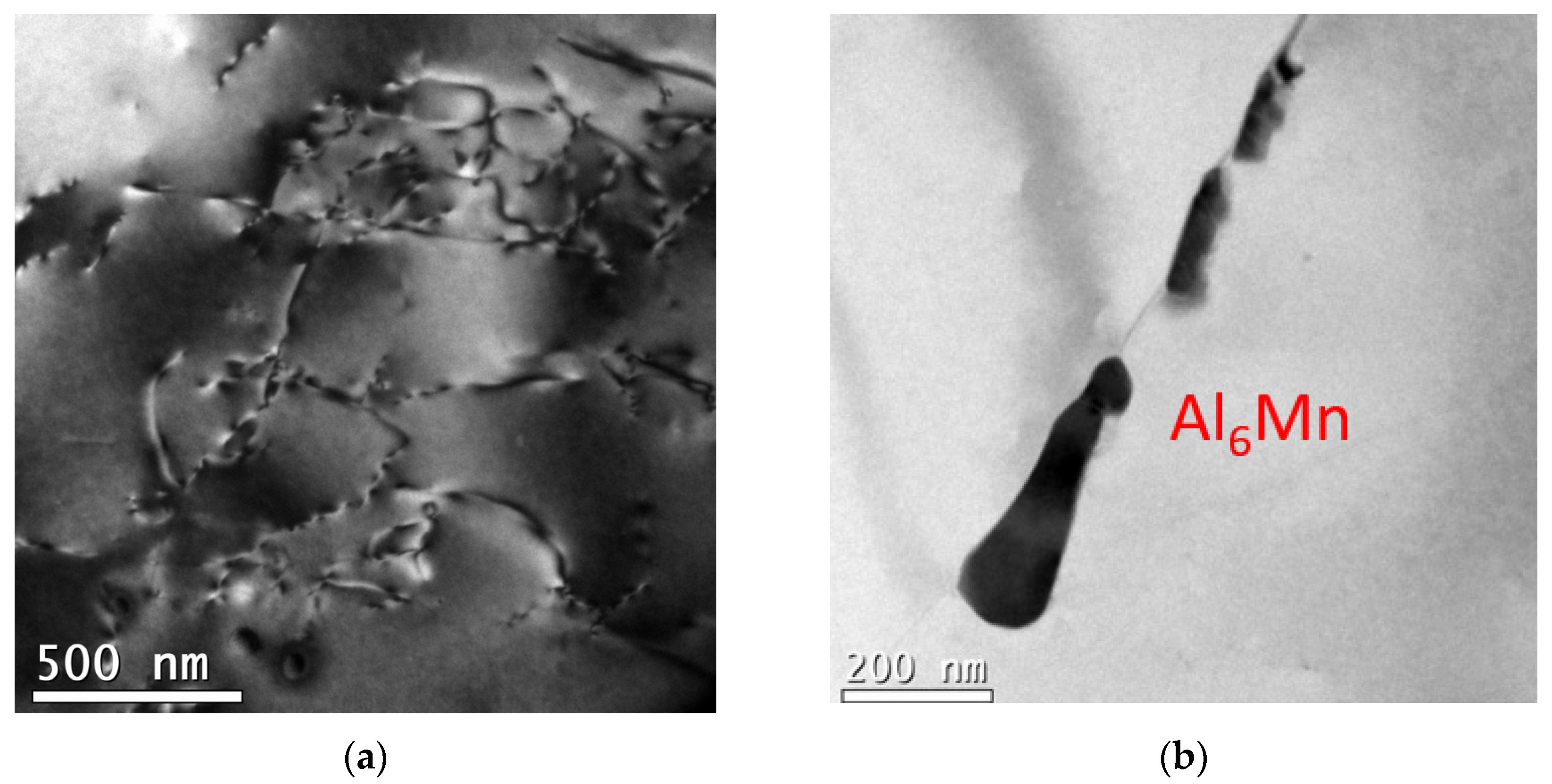
3.2. TEM Precipitation Analysis
3.3. Corrosion Pproperties
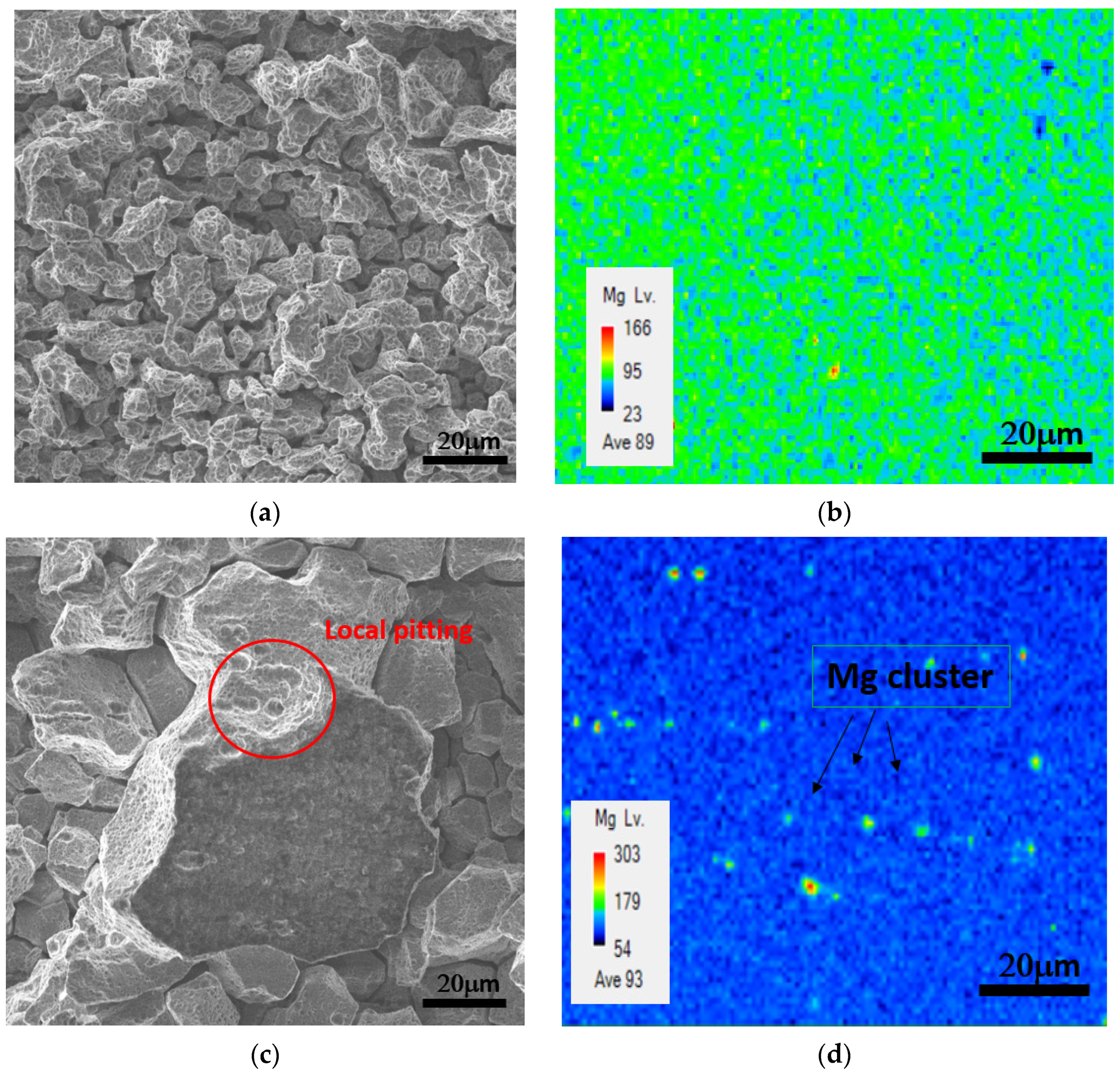
3.3.1. Surface Morphology
3.3.2. ASTM G67 NAMLT (Nitric Acid Mass Loss Test)
3.3.3. Corrosion Morphology Analysis
4. Conclusions
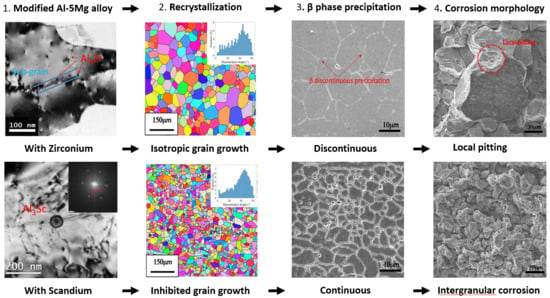
- After individual process annealing, the high temperature thermally stable dispersed Al3Zr and Al3Sc phases in the boundary matrices of the Al-5Mg alloys inhibited recrystallization and grain growth. Although Al3Zr particles were smaller and denser than Al3Sc particles, the dispersed Al3Sc particle phase was still better than the Al3Zr particle phase in inhibiting the recrystallization and thermal stability.
- In the initial stage of recrystallization of alloy A(0.1Mn) and alloy B(0.1Zr), the ASTM G67 mass losses of the two alloys were the most serious. They had serious susceptibility (larger than 25 mg/cm2) to intergranular corrosion. However, as the temperature of the process annealing rose to 450 °C, the recrystallized grains began to grow, and the G67 susceptibility to intergranular corrosion obviously decreased (less than 25 mg/cm2). By contrast, though alloy C(0.05Sc) could excellently inhibit recrystallization, the ASTM G67 mass loss of alloy C was over 25 mg/cm2, and it became susceptible to intergranular corrosion.
- As the temperature of the process annealing rose, The β-Mg2Al3 phase precipitation images of alloy A(0.1Mn, AA5356) and alloy B(0.1Zr) changed from continuous precipitation to local aggregation. In consequence, the corrosion images of the alloys transformed from intergranular corrosion to local pitting corrosion, thereby improving the corrosion resistance of the alloys.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Aluminum and Aluminum Alloys. In ASM Specialty Handbook; ASM International Materials Park: Novelty, OH, USA, 1994; pp. 33, 59, 61, 580. [Google Scholar]
- McMahon, M.E.; Haines, R.L.; Steiner, P.J.; Schulte, J.M.; Fakler, S.E.; Burns, J.T. Beta phase distribution in Al-Mg alloys of varying composition and temper. Corros. Sci. 2020, 169, 108618. [Google Scholar] [CrossRef]
- ASTM G67-04 Standard Test Method for Determining the Susceptibility to Intergranular Corrosion of 5XXX Series Aluminum Alloys by Mass Loss After Exposure to Nitric Acid(NAMLT Test). Available online: https://www.asminternational.org/home/-/journal_content/56/33542825/ASTMSTDTESTG0067-04/PUBLICATION-STANDARD-TEMPLATE (accessed on 7 July 2021).
- Gupta, R.K.; Zhang, R.; Davies, C.H.J.; Birbilis, N. Influence of Mg Content on the Sensitization and Corrosion of Al-xMg(-Mn) Alloys. Corrosion 2013, 69, 1081–1087. [Google Scholar] [CrossRef]
- Oguocha, I.N.A.; Adigun, O.J.; Yannacopoulos, S. Effect of sensitization heat treatment on properties of Al-Mg alloy AA5083-H116. J. Mater. Sci. 2008, 43, 4208–4214. [Google Scholar] [CrossRef]
- Yan, J.; Hodge, A.M. Study of β precipitation and layer structure formation in Al 5083: The role of dispersoids and grain boundaries. J. Alloys Compd. 2017, 703, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Jiang, F.; Long, M.; Jiang, J.; Liu, H.; Tong, M. Effect of annealing temperature on microstructure, mechanical properties and corrosion behavior of Al-Mg-Mn-Sc-Zr alloy. Appl. Surf. Sci. 2020, 514, 146081. [Google Scholar] [CrossRef]
- Scotto, D.A.D.; Gaies, J.; Golumbfskieb, W.; Taheri, M.L. Grain boundary misorientation dependence of β phase precipitation in an Al–Mg alloy. Scr. Mater. 2017, 76, 81–84. [Google Scholar] [CrossRef]
- Miyake, Y.; Sato, Y.; Teranishi, R.; Kaneko, K. Effect of heat treatments on the microstructure and formability of Al-Mg-Mn-Sc-Zr alloy. Micron 2017, 101, 151–155. [Google Scholar] [CrossRef]
- Zhang, R.; Gupta, R.K.; Davies, C.H.J.; Hodge, A.M.; Tort, M.; Xia, K.; FBirbilis, N. The Influence of Grain Size and Grain Orientation on Sensitization in AA5083. Corrosion 2016, 72, 160–168. [Google Scholar] [CrossRef]
- Aldalur, E.; Suárez, A.; Veiga, F. Metal transfer modes for Wire Arc Additive Manufacturing Al-Mg alloys: Influence of heat input in microstructure and porosity. J. Mater. Process. Technol. 2021, 297, 117271. [Google Scholar] [CrossRef]
- Huang, X.; Baroux, B. Effect of minor Sc on microstructure and mechanical properties of Al–Zn–Mg–Zr alloy metal–inert gas welds. J. Alloys Compd. 2015, 629, 197–207. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.; Mochugovskiy, A.; Levchenko, V.; Tabachkova, N.; Mufalo, W.; Portnoy, V. Precipitation behavior of L12Al3Zr phase in Al-Mg-Zr alloy. Mater. Charact. 2018, 139, 30–37. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Knipling, K.E.; Seidma, D.N.; Dunand, D.C. Ambient- and high-temperature mechanical properties of isochronally aged Al-0.06Sc, Al-0.06Zr and Al-0.06Sc-0.06Zr (at.%) alloys. Acta Mater. 2011, 59, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Jiang, F.; Zhang, M.; Tang, Z.; Tong, M. Recrystallization behavior of Al-Mg-Mn-Sc-Zr alloy based on two different deformation ways. Mater. Lett. 2020, 265, 127455. [Google Scholar] [CrossRef]
- Liu, J.; Yao, P.; Zhao, N.Q.; Shi, C.S.; Li, H.J.; Li, X.; Xi, D.S.; Yang, S. Effect of minor Sc and Zr on recrystallization behavior and mechanical properties of novel Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2016, 657, 717–725. [Google Scholar] [CrossRef]
- Goswami, R.; Spanosa, G.; Pao, P.S.; Holtz, R.L. Precipitation behavior of the β phase in Al-5083. Mater. Sci. Eng. A 2010, 527, 1089–1095. [Google Scholar] [CrossRef]
- Popović, M.; Romhanji, E. Characterization of microstructural changes in an Al-6.8wt.% Mg alloy by electrical resistivity measurements. Mater. Sci. Eng. A 2008, 492, 460–467. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, Y.; Morris, J.G. The Effect of Mg Precipitaion on the Mechanical Properties of 5XXX Aluminum Al-loys. Mater. Sci. Eng. A 2005, 392, 136–144. [Google Scholar] [CrossRef]
- Shi, C.; Chen, G. Effect of Zr addition on hot deformation behavior and microstructural evolution of AA7150 aluminum alloy. Mater. Sci. Eng. A 2014, 596, 183–193. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al-Zr and Al-Zr-Ti alloys during isothermal aging at 375–425 °C. Acta Mater. 2008, 56, 114–127. [Google Scholar] [CrossRef]
- Sun, P.L.; Zhao, Y.H.; Tseng, T.Y.; Suc, J.R.; Lavernia, E.J. The influence of cooling rate on the microstructures and mechanical properties in ultrafine grained aluminum processed by hot rolling. Mater. Sci. Eng. A 2010, 527, 5287–5294. [Google Scholar] [CrossRef]




| Com. | Mg | Mn | Zr | Sc | Fe | Si | Al | |
|---|---|---|---|---|---|---|---|---|
| Alloy | ||||||||
| A(0.1Mn) | 5.22 | 0.09 | N.D. | N.D. | 0.07 | 0.06 | Rem. | |
| B(0.1Zr) | 5.10 | N.D. | 0.11 | N.D. | 0.08 | 0.04 | Rem. | |
| C(0.05Sc) | 5.23 | N.D. | N.D. | 0.05 | 0.08 | 0.03 | Rem. | |
| State Alloy | 250 °C | 350 °C | 450 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grain Size (μm2) | Aspect | R.R. | Result | Grain Size (μm2) | Aspect | R.R. | Result | Grain Size (μm2) | Aspect | R.R. | Result | |
| A(0.1Mn) | N.D. | 3.11 | 28% | Reco. | 243.4 | 1.44 | 88.3% | C. Recry | 1034.1 | 1.56 | 91.9% | A. growth |
| B(0.1Zr) | N.D. | 4.50 | 26.5% | Reco. | 221.2 | 1.61 | 67.3% | P. Recry | 1005.3 | 1.51 | 89.4% | I. growth |
| C(0.05Sc) | N.D. | 3.23 | 19.7% | Reco. | 152.9 | 1.64 | 41.3% | P. Recry | 201.2 | 1.55 | 90.8% | C. Recry |
| State Alloy | 250 °C | 350 °C | 450 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Annealing | Sensitization | ICS * | Annealing | Sensitization after Annealing | ICS | Annealing | Sensitization after Annealing | ICS | |
| A(0.1Mn) | 1.8 (0.2) | 24.9 (0.9) | No | 1.9 (0.3) | 65.2 (2.3) | YES | 1.5 (0.2) | 23.0 (0.8) | NO |
| B(0.1Zr) | 2.8 (0.3) | 22.0 (1.2) | No | 2.3 (0.2) | 50.3 (1.8) | YES | 3.1 (0.3) | 21.5 (1.0) | NO |
| C(0.05Sc) | 3.2 (0.5) | 23.6 (1.1) | No | 3.8 (0.4) | 58.8 (2.2) | YES | 3.4 (0.2) | 59.2 (2.5) | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-L.; Chiu, Y.-C.; Pan, T.-A.; Chen, M.-C. Effects of Trace Amounts of Mn, Zr and Sc on the Recrystallization and Corrosion Resistance of Al-5Mg Alloys. Crystals 2021, 11, 926. https://doi.org/10.3390/cryst11080926
Lee S-L, Chiu Y-C, Pan T-A, Chen M-C. Effects of Trace Amounts of Mn, Zr and Sc on the Recrystallization and Corrosion Resistance of Al-5Mg Alloys. Crystals. 2021; 11(8):926. https://doi.org/10.3390/cryst11080926
Chicago/Turabian StyleLee, Sheng-Long, Yang-Chun Chiu, Tse-An Pan, and Mien-Chung Chen. 2021. "Effects of Trace Amounts of Mn, Zr and Sc on the Recrystallization and Corrosion Resistance of Al-5Mg Alloys" Crystals 11, no. 8: 926. https://doi.org/10.3390/cryst11080926