Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Phase Composition
3.2. Surface Morphology
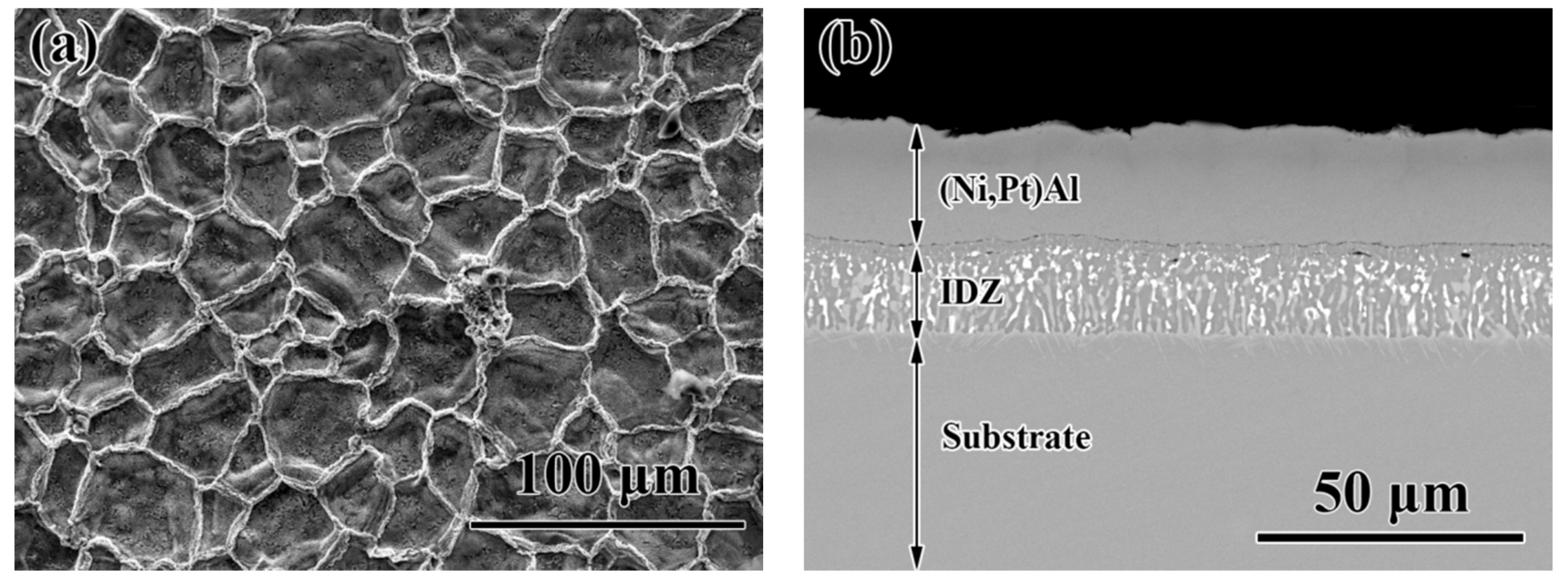
3.3. Cross-Sectional Morphology
4. Discussion
4.1. Oxidation Behavior of SX Superalloy at 980 °C
4.2. Oxidation Behavior of (Ni,Pt)Al Coating at 980 °C
5. Conclusions
- Building of layered oxide on SX superalloy changed constantly. The multi-layered scale mainly consisted of a thick and porous outer layer, which was composed of the non-protective oxide mixture of Cr2O3 + NiCr2O4 + TiO2, and an internal layer of Al2O3. As the oxidation time prolonged, the thickness of the loose outer layer increased and the internal alumina scale became discontinuous, which allowed the inward diffusion of nitrogen and oxygen to form Ta2O5 and TiN, resulting in the aggravation of internal oxidation.
- During oxidation, a continuous Al2O3 scale formed on the surface of the Pt-modified aluminide coating, which held a dual-layer structure with a whisker/blade-like outer layer and a dense internal layer. The long-term existence of θ-Al2O3 whiskers was attributed to the moderate-high temperatures and the addition of Pt. The Pt-modified aluminide coating confirmed its competence in service of a relatively long lifetime.
- In the oxidation process, the β-phase in the outer zone of the Pt-modified aluminide coating gradually converted to the γ′ phase due to aluminum depletion induced by oxidation.
- In short, the Pt-modified aluminide coating showed the best oxidation resistance by forming a continuous Al2O3 scale and possessing a slow degradation rate from β to γ′. It can be used for protecting the hot components of electric gas turbines, small type turbine engines and turboshaft engines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, C.Y.; Yang, Y.F.; Zhang, Z.Y.; Bao, Z.B.; Chen, M.H.; Zhu, S.L.; Wang, F.H. A Zr–doped single–phase Pt–modified aluminide coating and the enhanced hot corrosion resistance. Corros. Sci. 2018, 133, 406–416. [Google Scholar] [CrossRef]
- Liu, G.; Niu, Y.; Wang, W. Oxidation behaviour of aluminide and Pt–modified aluminide coatings on CMSX–4 at 1100 °C. Corros. Sci. Technol. Prot. 2001, 13, 400–404. [Google Scholar]
- Meng, X.X.; Yuwen, P.; Shao, W.; Qu, W.T.; Zhou, C.G. Cyclic oxidation behaviour of Co/Si co–doped β–NiAl coating on nickel based superalloys. Corros. Sci. 2018, 133, 112–119. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Jiang, C.Y.; Yu, C.T.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Preparation and oxidation performance of a low–diffusion Pt–modified aluminide coating with Re–base diffusion barrier. Corros. Sci. 2020, 168, 108582. [Google Scholar] [CrossRef]
- Rafiee, H.; Arabi, H.; Rastegari, S. Effects of temperature and Al–concentration on formation mechanism of an aluminide coating applied on superalloy IN738LC through a single step low activity gas diffusion process. J. Alloys Compd. 2010, 505, 206–212. [Google Scholar] [CrossRef]
- Li, M.F.; Kong, C.; Zhang, J.Q.; Zhou, C.G.; Young, D.J. Oxidation behavior of Ni–Al coating with and without a Ni–Re diffusion barrier in dry CO2 gas at 650 °C. Corros. Sci. 2019, 149, 236–243. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Zhang, Z.Y.; Bao, Z.B.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Hot corrosion behaviour of single–phase platinum–modified aluminide coatings: Effect of Pt content and pre–oxidation. Corros. Sci. 2017, 127, 82–90. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhao, X.F.; Zhao, C.S.; Hao, W.; Wang, X.; Xiao, P. The oxidation performance for Zr–doped nickel aluminide coating by composite electrodepositing and pack cementation. Corros. Sci. 2017, 123, 103–115. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ma, Z.; Dong, S.Z.; Xu, M.M.; Li, S.; Zhang, C.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. High-temperature oxidation behaviour of refurbished (Ni,Pt)Al coating on Ni-based superalloy at 1100 °C. Corros. Sci. 2021, 187, 109521. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Yao, H.R.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Preparation and enhanced oxidation performance of a Hf-doped single-phase Pt-modified aluminide coating. Corros. Sci. 2016, 113, 17–25. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Effect of aluminisation characteristics on the microstructure of single phase β-(Ni,Pt)Al coating and the isothermal oxidation behavior. Corros. Sci. 2016, 106, 43–54. [Google Scholar] [CrossRef]
- Swadźba, R.; Hetmańczyk, M.; Sozańska, M.; Witala, B.; Swadźba, L. Structure and cyclic oxidation resistance of Pt, Pt/Pd-modified and simple aluminide coatings on CMSX-4 superalloy. Surf. Coat. Technol. 2011, 206, 1538–1544. [Google Scholar] [CrossRef]
- Swadźba, R.; Hetmańczyk, M.; Wiedermann, J.; Swadźba, L.; Moskal, G.; Witala, B.; Radwański, K. Microstructure degradation of simple, Pt- and Pt+Pd-modified aluminide coatings on CMSX-4 superalloy under cyclic oxidation conditions. Surf. Coat. Technol. 2013, 215, 16–23. [Google Scholar] [CrossRef]
- Yu, C.T.; Liu, H.; Ullaha, A.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. High–temperature performance of (Ni,Pt)Al coatings on second–generation Ni–base single–crystal superalloy at 1100 °C: Effect of excess S impurities. Corros. Sci. 2019, 159, 108115. [Google Scholar] [CrossRef]
- Liu, H.; Xu, M.M.; Li, S.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Improving cyclic oxidation resistance of Ni3Al-based single crystal superalloy with low-diffusion platinum-modified aluminide coating. J. Mater. Sci. Technol. 2020, 54, 132–143. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals. Corros. Ser. 2008, 1, 219–226. [Google Scholar]
- Alvarado–Orozco, J.M.; Morales–Estrella, R.; Boldrick, M.S.; Ortiz–Merino, J.L.; Konitzer, D.G.; Trápaga–Martínez, G.; Muñoz–Saldaña, J. First Stages of Oxidation of Pt–Modified Nickel Aluminide Bond Coat Systems at Low Oxygen Partial Pressure. Oxid. Met. 2012, 78, 269–284. [Google Scholar] [CrossRef]
- An, T.F.; Guan, H.R.; Sun, X.F.; Hu, Z.Q. Effect of the θ–α–Al2O3 Transformation in Scales on the Oxidation Behavior of a Nickel–Base Superalloy with an Aluminide Diffusion Coating. Oxid. Met. 2000, 54, 301–316. [Google Scholar] [CrossRef]
- Pfennig, A.; Fedelich, B. Oxidation of single crystal PWA 1483 at 950 °C in flowing air. Corros. Sci. 2008, 50, 2484–2492. [Google Scholar] [CrossRef]
- Yang, L.L.; Chen, M.H.; Wang, J.L.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Oxidation of duplex coatings with different thickness ratio of the internal nanocrystalline layer to the outer NiCrAlY one. Corros. Sci. 2018, 143, 136–147. [Google Scholar] [CrossRef]
- Lou, H.; Zhu, S.; Wang, F. Rehealing ability of oxide scales formed on microcrystalline K38G coatings. Oxid. Met. 1995, 43, 317–328. [Google Scholar] [CrossRef]
- Zhao, C.S.; Zhou, Y.H.; Zou, Z.H.; Luo, L.R.; Zhao, X.F.; Guo, F.W.; Xiao, P. Effect of alloyed Lu, Hf and Cr on the oxidation and spallation behavior of NiAl. J. Corros. Sci. 2017, 126, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Oskay, C.; Galetz, M.C.; Murakami, H. Oxide scale formation and microstructural degradation of conventional, Pt–and Pt/Ir–modified NiAl diffusion coatings during thermocyclic exposure at 1100 °C. Corros. Sci. 2018, 144, 313–327. [Google Scholar] [CrossRef]
- Liu, G.; Niu, Y.; Wang, W.; Wu, W.T. Oxidation behaviour of Pt–modified aluminide coatings on IN738 at 1100 °C. J. Chin. Soc. For. Corros. Prot. 2001, 01, 55–59. [Google Scholar]
- Yan, K.; Guo, H.B.; Gong, S.K. High–temperature oxidation behavior of minor Hf doped NiAl allo in dry and humid atmospheres. Corros. Sci. 2013, 75, 337–344. [Google Scholar] [CrossRef]
- Hou, P.Y.; Paulikas, A.P.; Veal, B.W. Stress development and relaxation in Al2O3 during early stage oxidation of β–NiAl. Mater. High. Temp. 2005, 22, 535–543. [Google Scholar] [CrossRef]
- Svensson, H.; Angenete, J.; Stiller, K. Microstructure of oxide scales on aluminide diffusion coatings after short time oxidation at 1050 °C. Surf. Coat. Technol. 2004, 177, 152–157. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behavior of NiAl—Ι. Phase transformations in the alumina scale during oxidation of NiAl and NiAl–Cr alloys. Corros. Sci. 1992, 33, 1667–1690. [Google Scholar] [CrossRef]
- Liu, G.M.; Li, M.S.; Zhu, M.; Zhou, Y.C. Transient of alumina oxide scale on β–NiAl coated on M38G alloy at 950 °C. Intermetallics 2007, 15, 1285–1290. [Google Scholar] [CrossRef]
- Pint, B.A.; Martin, J.R.; Hobbs, L.W. The oxidation mechanism of θ–Al2O3 scales. Solid State Ion. 1995, 78, 99–107. [Google Scholar] [CrossRef]
- Chen, C.F.; Rühle, M. Transient alumina transformation on a sputtered K38G nanocrystalline coating. Surf. Coat. Technol. 2005, 191, 263–266. [Google Scholar] [CrossRef]
- Chen, W.F.; Shan, X.J.; Li, H.; Guo, Y.; Guo, F.W.; Zhao, X.F.; Ni, N.; Xiao, P. Effects of iron and platinum on the isothermal oxidation of β–NiAl overlay coatings fabricated by spark plasma sintering. Surf. Coat. Technol. 2019, 382, 125178. [Google Scholar] [CrossRef]
- De Wit, J.; Van Manen, P. The precious metal effect in high temperature corrosion. Mater. Sci. Forum 1994, 154, 109–118. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Eggeman, A.S.; Brewster, G.; Xiao, P. Pt effect on early stage oxidation behaviour of Pt–diffused γ–Ni/γ′–Ni3Al coatings. Acta Mater. 2020, 189, 232–247. [Google Scholar] [CrossRef]
- Grabke, H.J. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]









| O | Al | Ti | Cr | Ni | |
|---|---|---|---|---|---|
| 1 | 66.64 | 5.58 | 4.87 | 15.25 | 7.57 |
| 2 | 58.76 | 19.62 | 4.11 | 8.17 | 9.34 |
| 3 | 64.23 | 10.58 | 10.00 | 14.47 | 0.72 |
| 4 | 56.29 | 25.19 | 1.88 | 5.81 | 10.83 |
| 5 | 69.06 | 6.02 | 10.62 | 12.44 | 1.86 |
| 6 | 58.59 | 20.02 | 6.40 | 8.63 | 6.36 |
| 7 | 68.21 | 0.65 | 2.76 | 20.20 | 8.18 |
| 8 | 59.49 | 28.57 | 0.70 | 3.78 | 7.46 |
| Oxidation Time | Pt | Ni | Al |
|---|---|---|---|
| 300 h | 3.45 | 54.42 | 42.13 |
| 500 h | 3.87 | 56.63 | 39.50 |
| 1000 h | 3.11 | 58.62 | 38.27 |
| 1500 h | 4.14 | 59.59 | 36.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, S.; Zhang, C.; Xu, N.; Bao, Z. Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature. Crystals 2021, 11, 972. https://doi.org/10.3390/cryst11080972
Li Y, Li S, Zhang C, Xu N, Bao Z. Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature. Crystals. 2021; 11(8):972. https://doi.org/10.3390/cryst11080972
Chicago/Turabian StyleLi, Yanyan, Shuai Li, Chao Zhang, Na Xu, and Zebin Bao. 2021. "Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature" Crystals 11, no. 8: 972. https://doi.org/10.3390/cryst11080972
APA StyleLi, Y., Li, S., Zhang, C., Xu, N., & Bao, Z. (2021). Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature. Crystals, 11(8), 972. https://doi.org/10.3390/cryst11080972






