Effect of Fe2O3 on Electro-Deoxidation in Fe2O3-Al2O3-NaCl-KCl System
Abstract
:1. Introduction
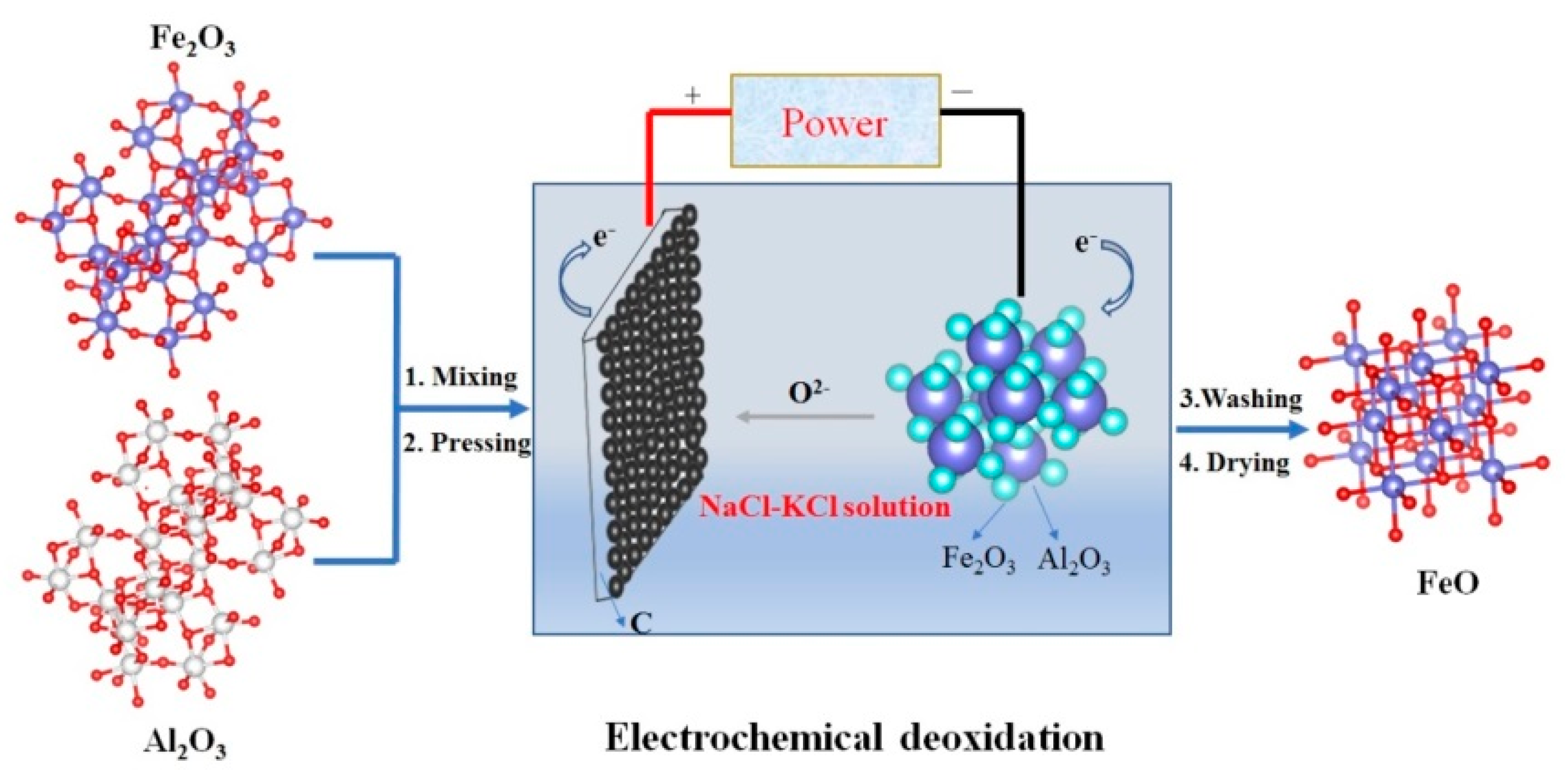
2. Experimental
3. Results and Discussion
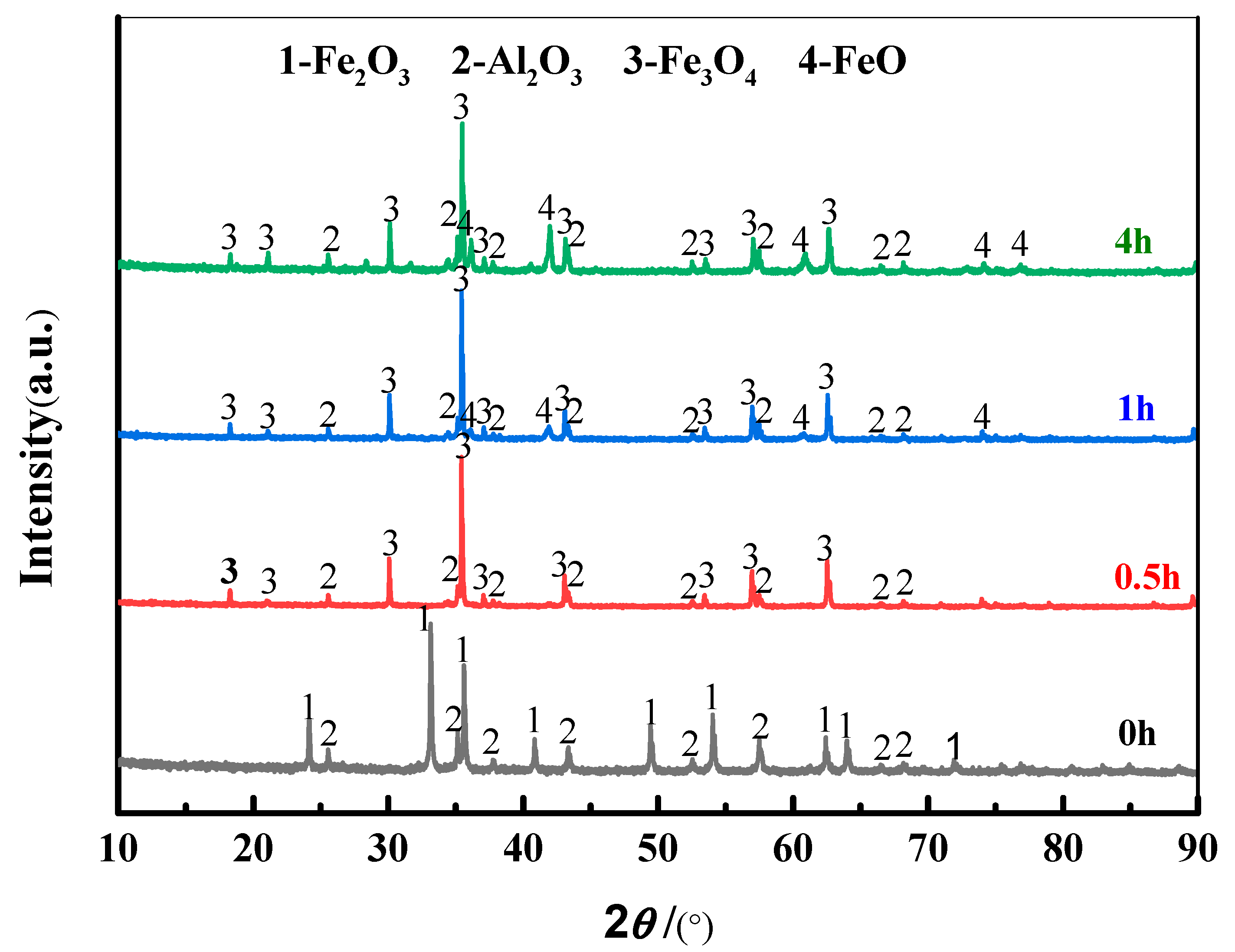
3.1. Thermodynamic Analysis and Electrochemical Reduction of Fe2O3-Al2O3
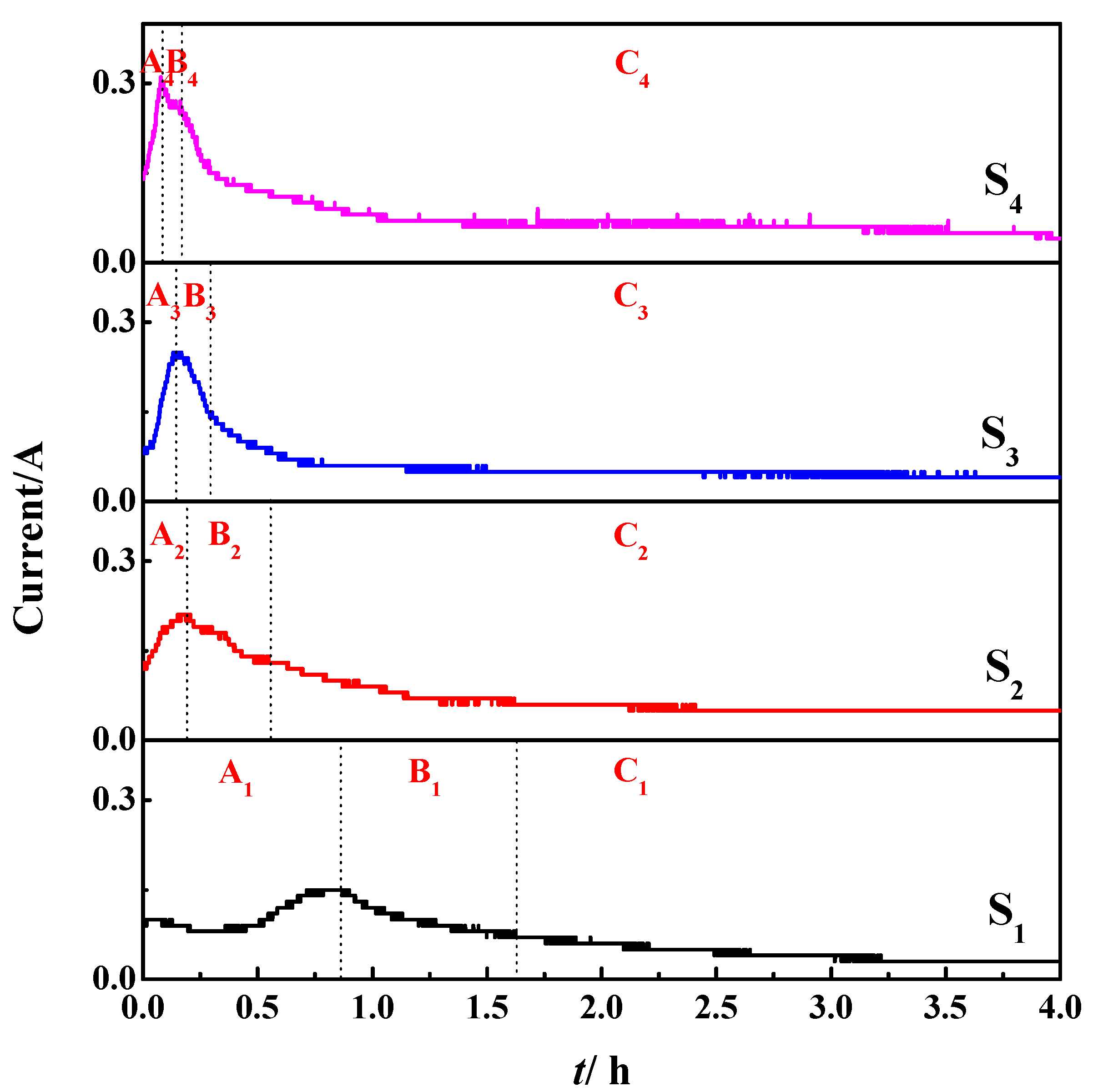
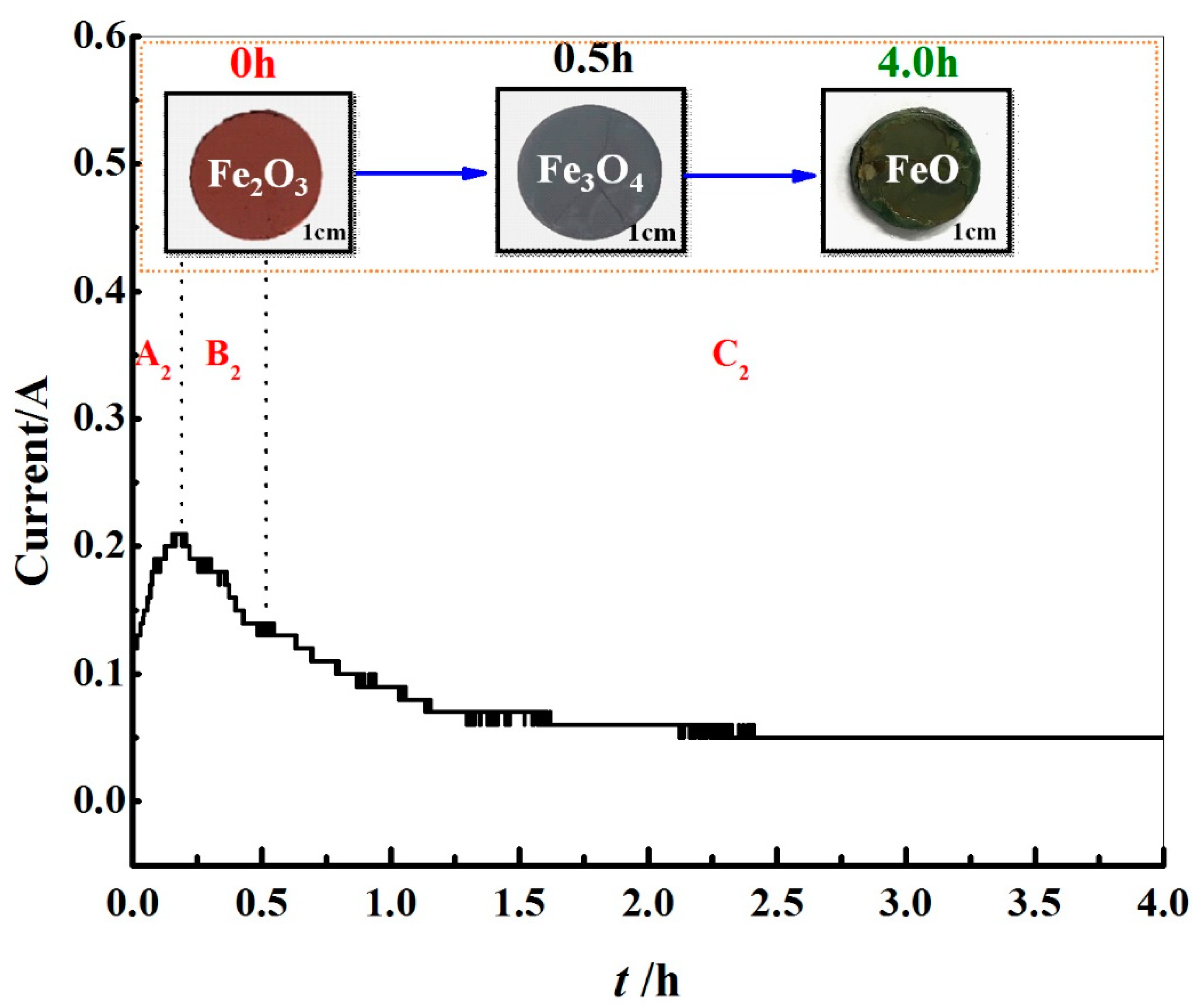
3.2. Effect on Deoxygenation Rate
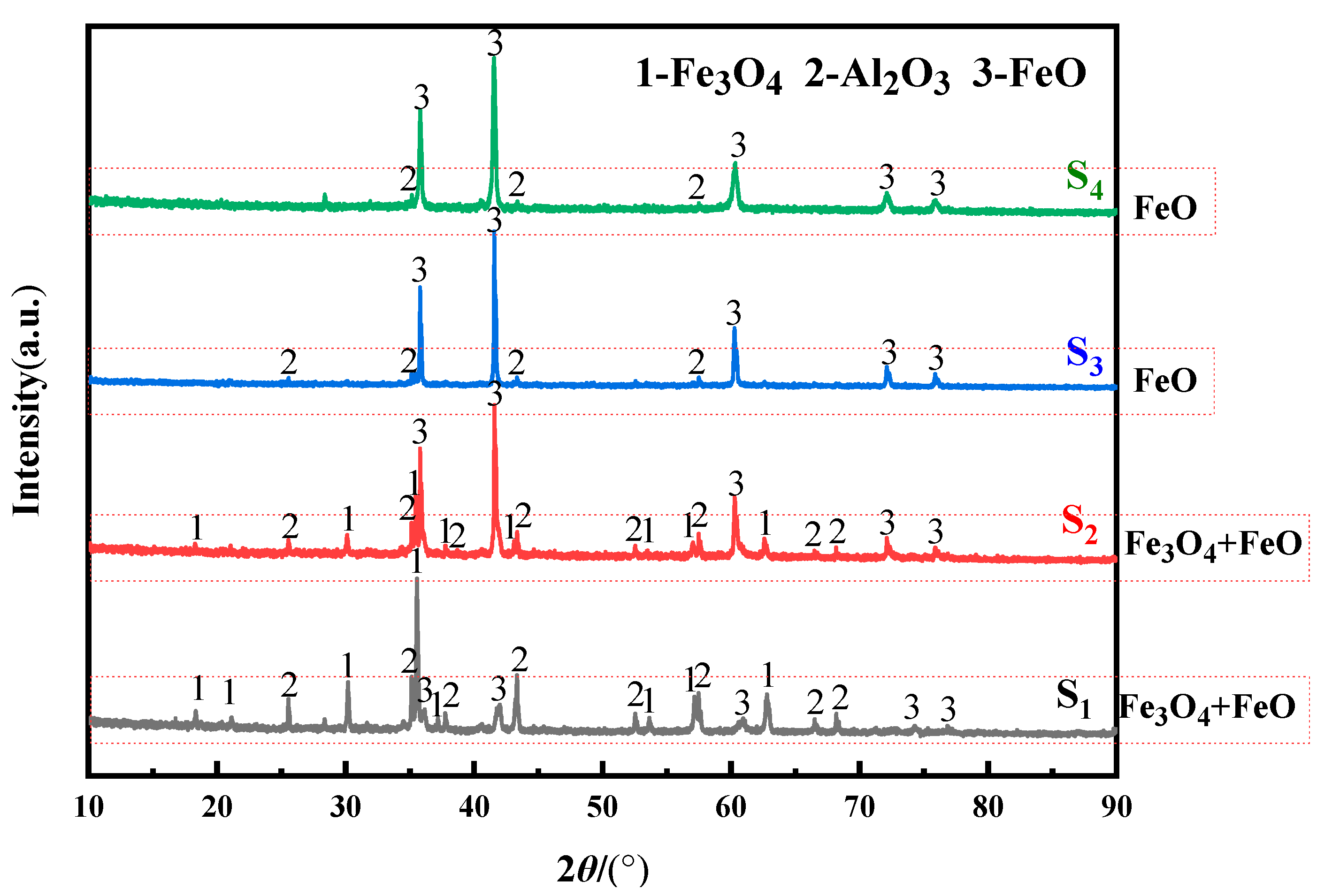
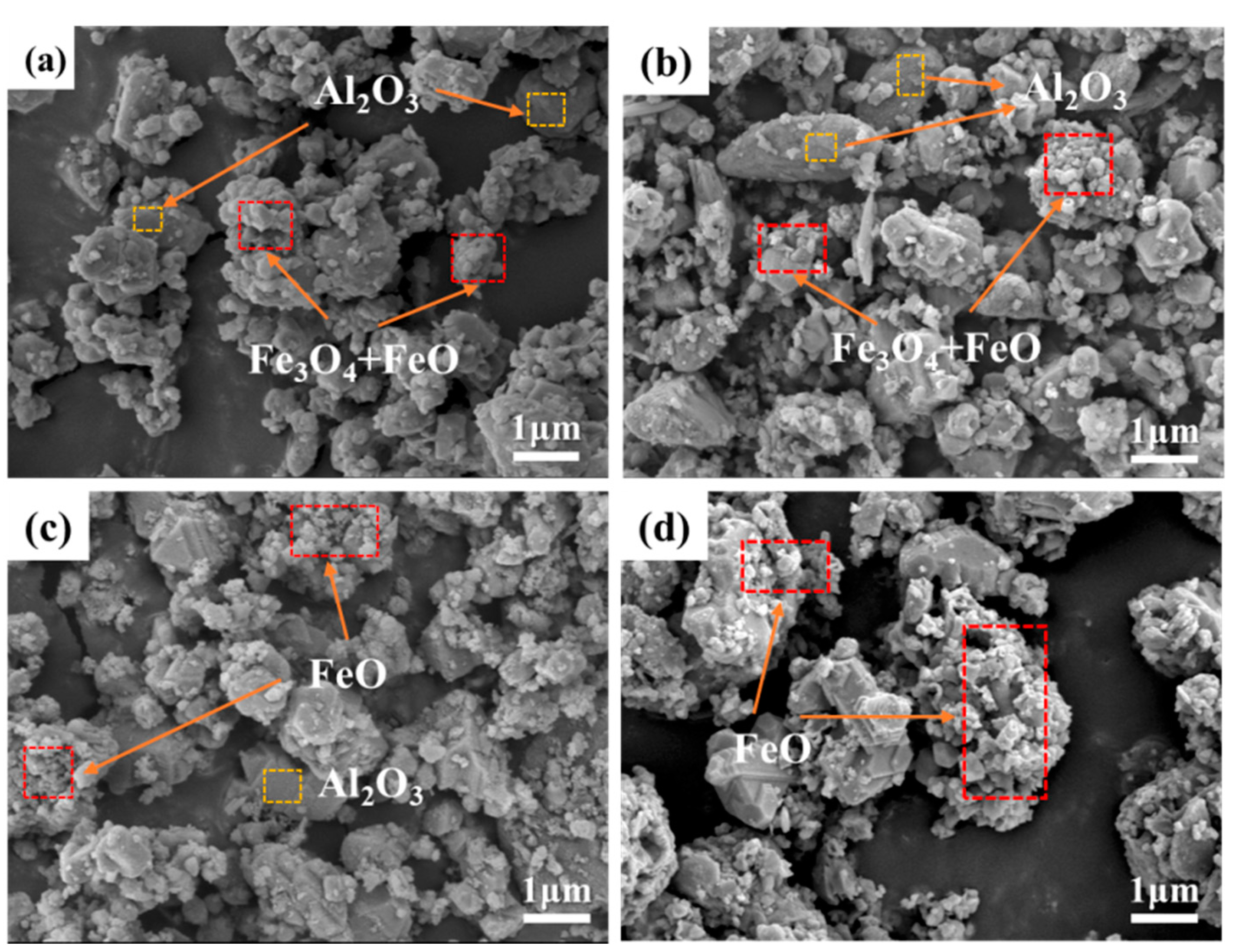
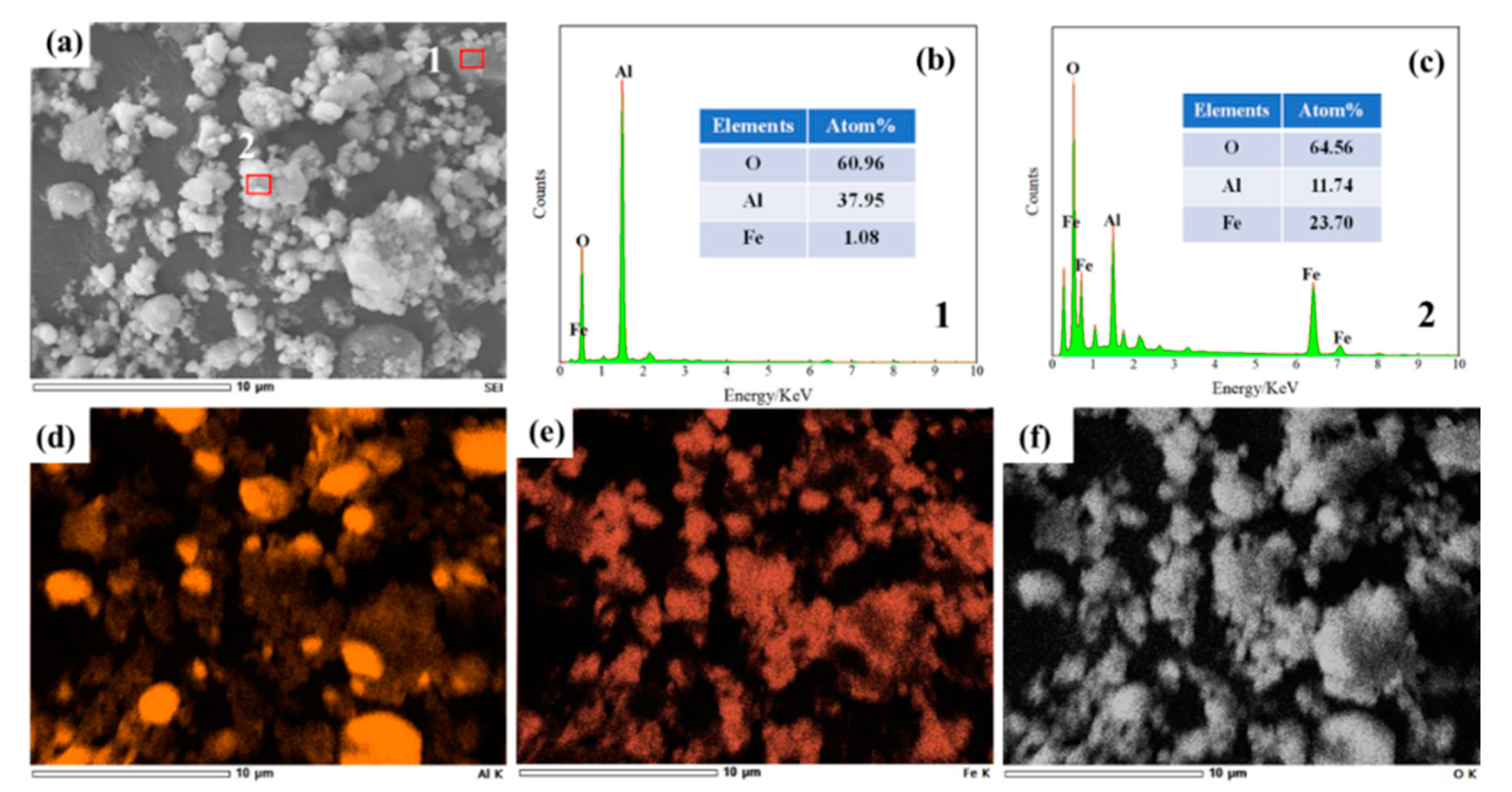
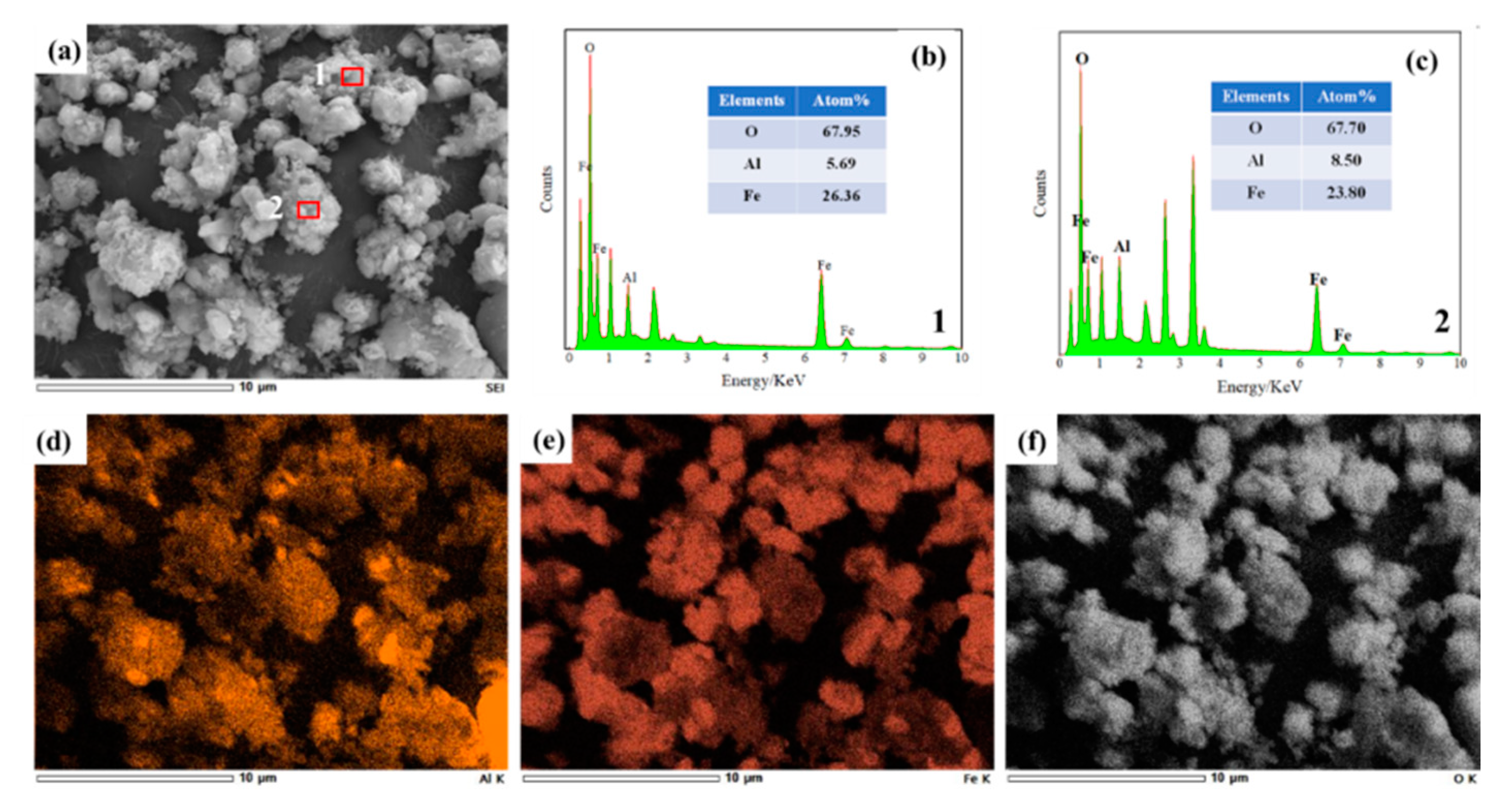
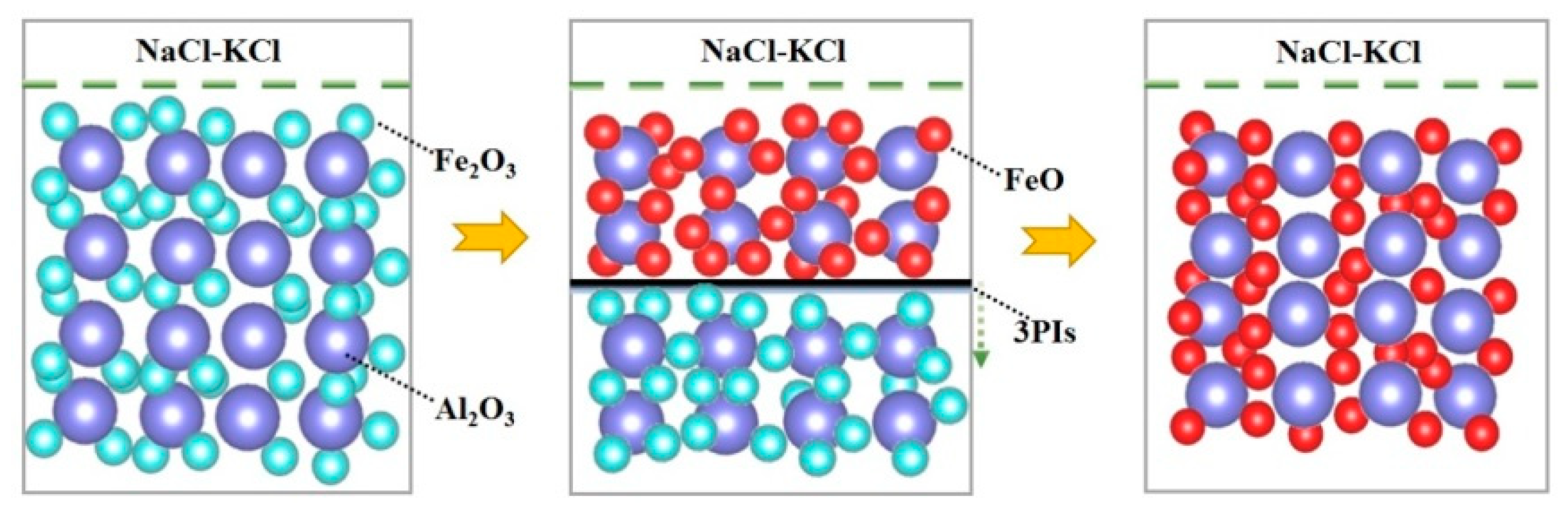
3.3. Effect on Organizational Structure
3.4. Effect Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilization of Bayer red mud. Res. Cons. Rec. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Fan, G.Q.; Wang, M.; Dang, J.; Zhang, R.; Lv, Z.P.; He, W.C.; Lv, X.W. A novel recycling approach for efficient extraction of titanium from high-titanium-bearing blast furnace slag. Waste Manag. 2021, 120, 626–634. [Google Scholar] [CrossRef]
- Wang, N.; Yan, H.Y.; Luo, C.; Li, H. Thermodynamic analysis of preparation of cermet from zinc kiln slag. In TMS 2021 11th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland, 2020; pp. 1023–1032. [Google Scholar]
- Agrawal, S.; Dhawan, N. Investigation of carbothermic microwave reduction followed by acid leaching for recovery of iron and aluminum values from Indian red mud. Miner. Eng. 2020, 159, 106653. [Google Scholar] [CrossRef]
- Mancini, A.; Lothenbach, B.; Geng, G.; Grolimund, D.; Wieland, E. Iron speciation in blast furnace slag cements. Cem. Concr. Res. 2021, 140, 106287. [Google Scholar] [CrossRef]
- Geng, C.; Liu, J.; Wu, S.; Jia, Y.; Du, B.; Yu, S. Novel method for comprehensive utilization of MSWI fly ash through co-reduction with red mud to prepare crude alloy and cleaned slag. J. Hazard. Mater. 2020, 384, 121315. [Google Scholar] [CrossRef] [PubMed]
- Mukiza, E.; Zhang, L.L.; Liu, X.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Luo, C.; Peng, J.; Yan, H.Y.; Liang, J.L. Thermodynamic behavior analysis of Fe-FeAl2O4 cermet prepared by zinc kiln slag. In TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2021; pp. 935–942. [Google Scholar]
- Ma, T. Preparation of Fe-Al-Based Cermet with Red Mud and Properties Study. Master’s Thesis, University of Science and Technology Beijing, Beijing, China, 2017; pp. 52–70. [Google Scholar]
- Ikram, U.H.M.; Mukherjee, P.S.; Khanna, R. Formation of light-weight ferroalloys in the Fe2O3-Al2O3-C system at 1550 C: Influence of silica impurities. Metals 2017, 7, 391. [Google Scholar] [CrossRef] [Green Version]
- Chobtham, C.; Kongkarat, S. Synthesis of hercynite from aluminium dross at 1550 °C: Implication for Industrial Waste Recycling. Mater. Sci. Forum. 2020, 977, 223–228. [Google Scholar] [CrossRef]
- Zhang, R.; Dang, J.; Liu, D.; Lv, Z.P.; Fan, G.Q.; Hu, L.W. Reduction of perovskite-geikielite by methane–hydrogen gas mixture: Thermodynamic analysis and experimental results. Sci. Total. Environ. 2020, 699, 134355. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Saih, Y.; Loptain, S.; Basset, J.M. Catalytic methane decomposition over Fe-Al2O3. ChemSusChem 2016, 9, 1243–1248. [Google Scholar] [CrossRef]
- Mei, D.; Zhao, H.; Yan, S. Kinetics model for the reduction of Fe2O3/Al2O3 by CO in chemical looping combustion. Chem. Eng. Process.-Process Intensif. 2018, 124, 137–146. [Google Scholar] [CrossRef]
- Ohno, K.; Konishi, H.; Watanabe, T.; Ishihara, S.; Kunitomo, K. Effect of pre-reduction degree on softening behavior of simulant sinter Iron ore. ISIJ Int. 2020, 60, 1520–1527. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 2000, 407, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.B.; Woolley, D.E.; Kenney, G.B. Emerging SOM technology for the green synthesis of metals from oxides. JOM 2001, 53, 32–35. [Google Scholar] [CrossRef]
- Ono, K.; Suzuki, R.O. A new concept for producing Ti sponge: Calciothermic reduction. JOM 2002, 54, 59–61. [Google Scholar] [CrossRef]
- Jiao, S.Q.; Zhu, H.M. Novel metallurgical process for titanium production. J. Mater. Res. 2006, 21, 2172–2175. [Google Scholar] [CrossRef]
- Chen, G.Z. Interactions of molten salts with cathode products in the FFC Cambridge Process. Int. J. Miner. Metall. Mater. 2020, 27, 1572–1587. [Google Scholar] [CrossRef]
- Li, H.; Jia, L.; Liang, J.L.; Yan, H.Y.; Cai, Z.Y.; Reddy, R.G. Study on the direct electrochemical reduction of Fe2O3 in NaCl-CaCl2 melt. Int. J. Electrochem. Sci. 2019, 14, 11267–11278. [Google Scholar] [CrossRef]
- Pepper, R.A.; Couperthwaite, S.J.; Millar, G.J. Comprehensive examination of acid leaching behaviour of mineral phases from red mud: Recovery of Fe, Al, Ti, and Si. Miner. Eng. 2016, 99, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Chung, Y.; Park, J.H. Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process. J. Clean. Prod. 2016, 137, 777–787. [Google Scholar] [CrossRef]
- Zhu, X.; Niu, Z.; Li, W.; Zhao, H.; Tang, Q. A novel process for recovery of aluminum, iron, vanadium, scandium, titanium and silicon from red mud. J. Environ. Chem. Eng. 2020, 8, 103528. [Google Scholar] [CrossRef]
- Li, G.; Wang, D.; Chen, Z. Direct reduction of solid Fe2O3 in molten CaCl2 by potentially green process. J. Mater. Sci. Technol. 2009, 25, 767–771. [Google Scholar]
- Mohandas, K.S. Direct electrochemical conversion of metal oxides to metal by molten salt electrolysis: A review. Miner. Process. Extr. Metall. 2013, 122, 195–212. [Google Scholar] [CrossRef]
- Xie, Z.P. Structural Ceramics; Tsinghua University Press: Beijing, China, 2011; pp. 332–356. [Google Scholar]

| Samples | E (Decomposition Voltage) V | T (Temperature) °C | t (Reaction Time) h | mFe2O3/mAl2O3 |
|---|---|---|---|---|
| S1 | 2.3 | 850 | 4 | 1:1 |
| S2 | 2.3 | 850 | 4 | 3:2 |
| S3 | 2.3 | 850 | 4 | 2:1 |
| S4 | 2.3 | 850 | 4 | 4:1 |
| S5 | 2.3 | 850 | 0.5 | 3:2 |
| S6 | 2.3 | 850 | 1 | 3:2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yan, H.; Jing, Z.; Qi, X.; Li, H.; Liang, J. Effect of Fe2O3 on Electro-Deoxidation in Fe2O3-Al2O3-NaCl-KCl System. Crystals 2021, 11, 1026. https://doi.org/10.3390/cryst11091026
Xu Y, Yan H, Jing Z, Qi X, Li H, Liang J. Effect of Fe2O3 on Electro-Deoxidation in Fe2O3-Al2O3-NaCl-KCl System. Crystals. 2021; 11(9):1026. https://doi.org/10.3390/cryst11091026
Chicago/Turabian StyleXu, Yanke, Hongyan Yan, Zhenwei Jing, Xiwei Qi, Hui Li, and Jinglong Liang. 2021. "Effect of Fe2O3 on Electro-Deoxidation in Fe2O3-Al2O3-NaCl-KCl System" Crystals 11, no. 9: 1026. https://doi.org/10.3390/cryst11091026
APA StyleXu, Y., Yan, H., Jing, Z., Qi, X., Li, H., & Liang, J. (2021). Effect of Fe2O3 on Electro-Deoxidation in Fe2O3-Al2O3-NaCl-KCl System. Crystals, 11(9), 1026. https://doi.org/10.3390/cryst11091026





