Abstract
Crystalline hydrate of double cesium europium sulfate [CsEu(H2O)3(SO4)2]·H2O was synthesized by the crystallization from an aqueous solution containing equimolar amounts of 1Cs+:1Eu3+:2SO42− ions. Anhydrous salt CsEu(SO4)2 was formed as a result of the thermal dehydration of the crystallohydrate. The unusual effects observed during the thermal dehydration were attributed to the specific coordination of water molecules in the [CsEu(H2O)3(SO4)2]·H2O structure. The crystal structure of [CsEu(H2O)3(SO4)2]·H2O was determined by a single crystal X-ray diffraction analysis, and the crystal structure of CsEu(SO4)2 was obtained by the Rietveld method. [CsEu(H2O)3(SO4)2]·H2O crystallizes in the monoclinic system, space group P21/c (a = 6.5574(1) Å, b = 19.0733(3) Å, c = 8.8364(2) Å, β = 93.931(1)°, V = 1102.58(3) Å3). The anhydrous sulfate CsEu(SO4)2 formed as a result of the thermal destruction crystallizes in the monoclinic system, space group C2/c (a = 14.327(1) Å, b = 5.3838(4) Å, c = 9.5104(6) Å, β = 101.979(3) °, V = 717.58(9) Å3). The vibration properties of the compounds are fully consistent with the structural models and are mainly determined by the deformation of non-rigid structural elements, such as H2O and SO42−. As shown by the diffused reflection spectra measurements and DFT calculations, the structural transformation from [CsEu(H2O)3(SO4)2]·H2O to CsEu(SO4)2 induced a significant band gap reduction. A noticeable difference of the luminescence spectra between cesium europium sulfate and cesium europium sulfate hydrate is detected and explained by the variation of the extent of local symmetry violation at the crystallographic sites occupied by Eu3+ ions, namely, by the increase in inversion asymmetry in [CsEu(H2O)3(SO4)2]·H2O and the increase in mirror asymmetry in CsEu(SO4)2. The chemical shift of the 5D0 energy level in cesium europium sulfate hydrate, with respect to cesium europium sulfate, is associated with the presence of H2O molecules in the vicinity of Eu3+ ion.
1. Introduction
The unusual electron configuration of rare-earth elements (REE) results in the specific chemical and physical properties of their compounds widely applied in glass and ceramic industries [1,2,3,4,5,6,7,8,9,10], nuclear engineering [11,12,13], electronic and photonic systems [14,15,16,17,18,19,20,21,22,23,24,25,26]. The 4f electrons of rare-earth elements are completely shielded by the filled 5s and 5p shells. The screening effect leads to the fact that the binding field only slightly affects the electrons of the 4f shell and it leads to the appearance of narrow absorption bands in the electronic spectra [7,27,28,29,30]. Unpaired f electrons determine not only the valence characteristics of rare-earth elements and spectroscopic parameters of their compounds, but also magnetic properties. Accordingly, in many REE compounds, paramagnetic and ferromagnetic effects were observed [31,32,33,34,35,36].
Among the REE elements, europium compounds are of particular interest, since Eu3+ ions provide efficient photoluminescence in the red spectral range highly needed for creating white LEDs with similar to daylight emission characteristics [37,38,39,40,41,42,43,44,45,46]. In recent years, a large number of studies related to the synthesis and properties of crystal phosphors doped with Eu3+ ions have appeared. However, in such systems, the doping level is usually very low and, often, the distribution of Eu3+ ions in the corresponding crystallographic positions is not obvious. For this reason, in complex compounds, it is difficult to clearly determine the relation between the coordination and spectroscopic parameters of Eu3+ ions in the host lattice. In such a situation, the compounds with a stoichiometric content of europium ions have attracted the increasing attention of researchers [37,38,47,48,49,50,51,52,53]. Self-activated phosphors are characterized by an almost complete absence of structural defects, and the precise determination of the crystal structure makes it possible to evaluate the relations between the Eu3+ ion coordination in the lattice and spectroscopic characteristics of the compound. Simple europium stoichiometric compounds with tetrahedral MO4 units, where M = Mo, W and S, were thoroughly studied and their applicability as highly efficient polyfunctional materials was shown [54,55,56,57,58,59,60,61,62,63,64,65,66,67]. At the same time, the properties of complex compounds of monovalent cations and rare-earth elements with tetrahedral anions are presented quite sporadically in the literature [37,48,49,50,51,53,68,69,70,71,72]. The structures and some properties of several double molybdates and tungstates with general composition AEu(MO4)2 (A = Li, Na, K, Cs, Rb, Ag+; M = Mo, W) were investigated in the past and the examples of such contributions can be found elsewhere [72,73,74,75,76]. Contrary to that, the characterizations of the complex sulfate compounds of europium and monovalent cations are very scarce in the literature [53,68,69,71,77,78,79,80,81]. The present study is aimed at the observation of structural, thermal and spectroscopic characteristics of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2. To the best of our knowledge, these sulfates have not been considered up to now. However, a structural similarity can be assumed between [CsEu(H2O)3(SO4)2]·H2O and earlier reported sulphate tetrahydrates ALn(SO4)2 4H2O (A = Rb, Cs, Tl, NH4) because of the similarity in the ionic radii of Cs+ and these A+ ions [69,80,81,82,83,84,85,86].
2. Methods and Materials
In the synthesis, the solutions of CsNO3, Eu(NO3)3 and H2SO4 were used as starting materials. For the solution preparation, twice distilled deionized water was used. The volumes of liquids were measured using glass pipettes and cylinders with the accuracy of 0.1 mL. Solid reagents were weighed on an analytical balance with the accuracy of 0.1 mg.
An europium nitrate solution was prepared using Eu2O3 (99.995%, TDM-96 Ltd., Russia). To remove carbonate and europium hydroxide impurities occasionally formed during their storage, the commodity oxide was calcined at 900 °C for 12 h, after which it was cooled to room temperature in a desiccator over silica gel. Subsequently, the calcined europium oxide, mass: 17.5963 g was transferred into a flask, and 21.6 mL of concentrated nitric acid (C(HNO3) = 14.6 mol/L, ρ = 1.3956 g/cm3, ultrapure, Vekton Ltd., Nizhegorodsky, Russia) was added in small portions to the europium oxide. The mixture was carefully stirred until the oxide was completely dissolved according to equation:
Eu2O3 + 6HNO3→2Eu(NO3)3 + 3H2O
After the dissolution, the solution volume in the flask was adjusted to the mark with deionized water and mixed well for homogeneity. A sulfuric acid solution with the molar concentration of 2 mol/L was prepared by diluting concentrated sulfuric acid. To make this, 50 mL of water was poured into a 100.00 mL volumetric flask, then 11.17 mL of concentrated sulfuric acid (C(H2SO4) = 17.9 mol/L, ρ = 1.8349 g/cm3, ultrapure, Vekton Ltd., Nizhegorodsky, Russia) was carefully poured in small portions, avoiding a strong heating of the solution. After this, the solution was naturally cooled to room temperature and the volume was adjusted to the mark with deionized water.
[CsEu(H2O)3(SO4)2]·H2O was obtained by a slow crystallization of the solution containing stoichiometric amounts of ions. For this, in a glass beaker, 10 mL of the CsNO3 (C(Cs+) = 1 mol/L) solution, 10 mL of the Eu(NO3)3 (C(Eu3+) = 1 mol/L) solution and 10 mL of the H2SO4 (C(SO42−) = 2 mol/L) solution were mixed. The mixed solution was inserted into a desiccator over silica gel at 25 °C. In 12 h, the crystals precipitated from the mother liquor fell out in the reaction mixture. They were extracted, washed with ice water, pressed between filter paper sheets and dried in an empty desiccator to a constant weight. Anhydrous sulfate CsEu(SO4)2 was obtained by calcining the hydrate [CsEu(H2O)3(SO4)2]·H2O in a muffle furnace at the temperature of 500 °C for 10 h in the air. The CsEu(SO4)2 double sulfate was synthesized only in the powder form.
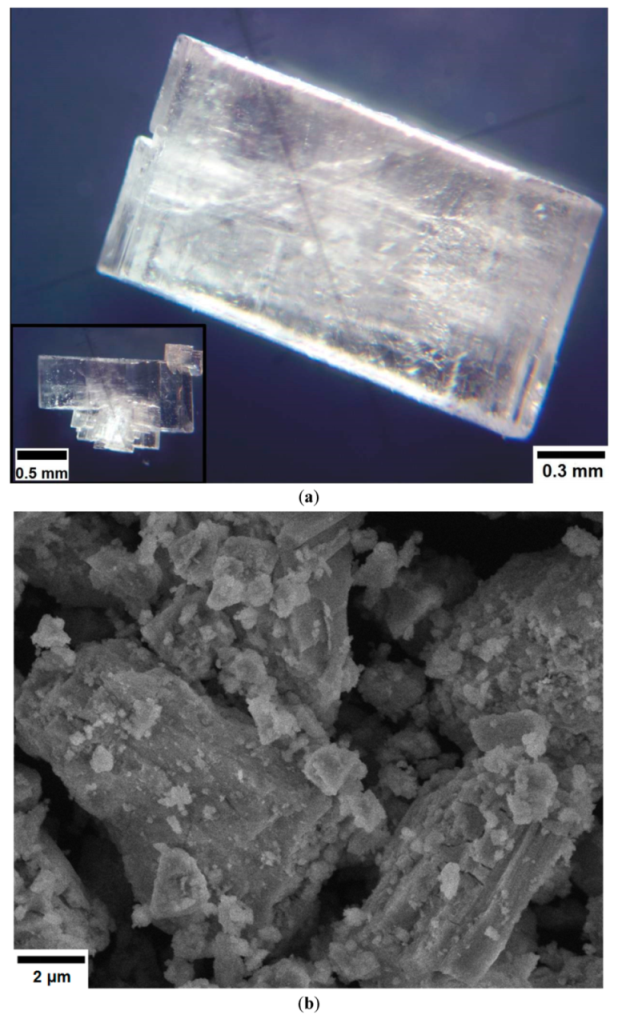
The photo images of the obtained single crystals of [CsEu(H2O)3(SO4)2]·H2O, as observed with the use of optical microscope, are shown in Figure 1a. As it is seen, the crystals were transparent and they were well faceted, and that was a robust indicator of their high structural quality, as it was earlier observed for different materials [79,87,88,89]. The crystals were partly twinned due to the existence of several crystallization centers. The SEM pattern of CsEu(SO4)2 particles is given in Figure 1b. The product mainly contained loose aggregates. Such type of the particle micromorphology is commonly formed in powder compounds fabricated by the high-temperature decomposition process due to gas release effects [90,91].
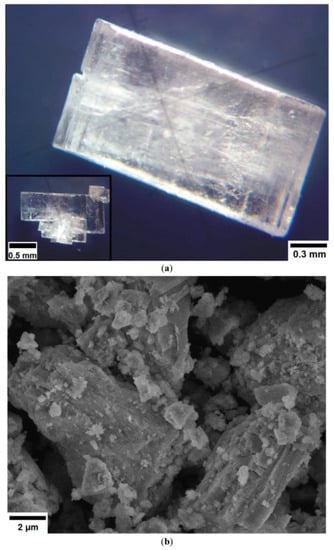
Figure 1.
(a) Photo images of selected [CsEu(H2O)3(SO4)2]·H2O crystals and (b) an SEM pattern of the CsEu(SO4)2 powder.
The optical microscopy images of the crystals were fixed with an MS-2 microscope (State Optical Institute, Saint Petersburg, Russia) in reflected unpolarized light. The SEM patterns were exhibited using a JEOL JSM-6510LV scanning electron microscope. To avoid the surface charging effects, the powder samples were deposited on a conductive substrate (carbon tape) and covered with a nanometer gold layer (99.9%).
The single crystal X-ray diffraction data from [CsEu(H2O)3(SO4)2]·H2O were recorded by a SMART APEXII diffractometer (Mo Kα, λ = 0.7106 Å) at T = 102(2) K. The orientation matrixes and cell parameters were calculated and refined by 45,124 reflections. The main information about the crystal data, data collection and refinement are reported in Table 1. The program APEXII (Bruker) was used to integrate the reflex intensities. Space group P21/c was obtained by the analysis of extinction rules and intensity statistics obtained from all reflections. The multiscan absorption correction of reflection intensities was performed by the APEXII software (Bruker, 2003–2008, Karlsruhe, Germany). Then, the intensities of equivalent reflections were averaged. The structure was solved by the direct methods using package SHELXS and refined in the anisotropic approach for non-hydrogen atoms using the SHELXL program [92]. All hydrogen atoms of H2O molecules were found via Fourier difference maps and, further, they were refined in a constrained mode. The structure test for the presence of other missing elements of symmetry and possible voids was produced using the PLATON program [93]. The DIAMOND program was used for the crystal structure plotting [94].

Table 1.
Main parameters of processing and Rietveld refinement of the [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 powder samples.
The powder diffraction data of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 for Rietveld analysis were collected at room temperature with a Bruker D8 ADVANCE powder diffractometer (Cu-Kα radiation) and linear VANTEC detector. The step size of 2θ was 0.02°, and the counting time was 5 s per step. The Rietveld refinement was performed by using TOPAS 4.2 [95]. The structural parameters of [CsEu(H2O)3(SO4)2]·H2O determined by the single crystal analysis were used as a basis in powder pattern Rietveld refinement. For the CsEu(SO4)2 sample, all peaks were indexed by monoclinic cell (C2/c) with the parameters close to those of RbEu(SO4)2 [96]. Therefore, the crystal structure of RbEu(SO4)2 was taken as a starting model for Rietveld refinement, and, in the structure, the Rb ion was replaced by the Cs ion. The refinement was stable and gave low R-factors. The crystallographic data were deposited in Cambridge Crystallographic Data Centre (CSD# 2102324-2102325). The data can be downloaded from the site (www.ccdc.cam.ac.uk/data_request/cif) (accessed on 10 August 2021).
The Fourier-transformed infrared spectroscopy (FTIR) measurements were carried out with the use of a Fourier Transform Infrared Spectrometer FSM 1201 (Infraspek Ltd., Saint Petersburg, Russia). The sample for the investigation was prepared as a tablet with the addition of annealed KBr. The Raman spectra were recorded using an i-Raman Plus spectrometer (B&W Tek, Lubeck, Germany) at a laser excitation wavelength of 785 nm. The diffuse reflectance spectra were measured on a UV-2600 spectrophotometer (Shimadzu, Kyoto, Japan) equipped by the ISR-2600Plus attachment with an integrating sphere. The optical bandgap was estimated on the base of the measurements of diffuse reflectance spectra.
The calculation of electronic bandgap structures of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 was performed by using the DFT (density functional theory) method as implemented in the CASTEP code [97]. On-the-fly generated norm-conserving potentials were used and 5s5p6s, 4f5s5p6s, 3s3p, 2s2p and 1s electrons were treated as the valence ones for Cs, Eu, S, O and H, respectively. The self-consistent field tolerance was set to 2.0 10−7 eV/atom. The energy cutoff was chosen as 1143 eV for both compounds and superimposed by the 3 × 1 × 2 k-point grid, in the case of [CsEu(H2O)3(SO4)2]·H2O, and by the 4 × 4 × 2 k-point grid in the case of CsEu(SO4)2. The local density approximation based on the Perdew and Zunger [98] parameterization of the numerical results of Ceperley and Alder [99] was used. The Hubbard U energy term Uf = 6 eV for the Eu 4f orbital was applied.
The thermal analysis was carried out in the argon flow with the use of a Simultaneous Thermal Analysis (STA) equipment 499 F5 Jupiter NETZSCH (NETZSCH Holding, Selb, Germany). The powder samples were inserted into alumina crucibles. The heating rate was 3 K/min. For the enthalpy determination, the equipment was initially calibrated with the use of standard metal substances, such as In, Sn, Bi, Zn, Al, Ag, Au, Ni. The heat effect peaks were determined with the package Proteus 6 2012.
The luminescence spectra under room temperature were registered on a HORIBA Jobin Yvon T64000 triple spectrometer with the spectral resolution 2.1 cm−1 using the excitation from the GaN laser at 410 nm and the power of 5 mW on the sample. The microscope based on Olympus BX-41 with the Olympus LMPlanFl 50 × objective lens f = 10.2 mm with numerical aperture N.A. = 0.5 was used. The unfocused laser radiation illuminated the small sample powder quantity tangentially. The angle between incident laser light and the registered luminescence was about 60 degrees.
3. Results and Discussions
3.1. Crystal, Vibrational and Electronic Structure
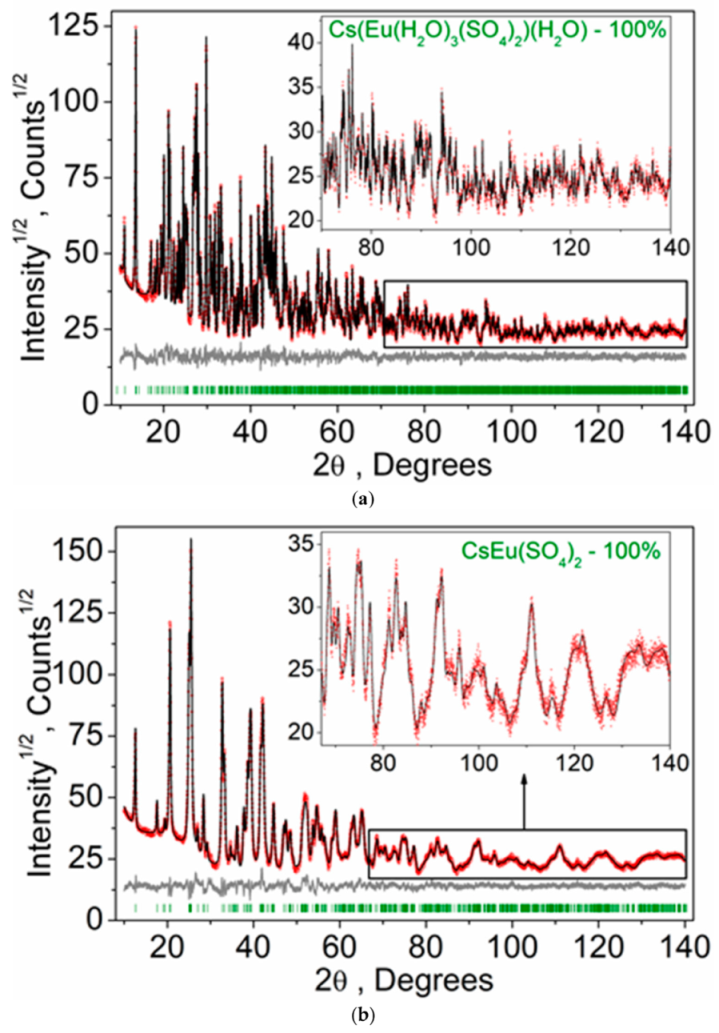
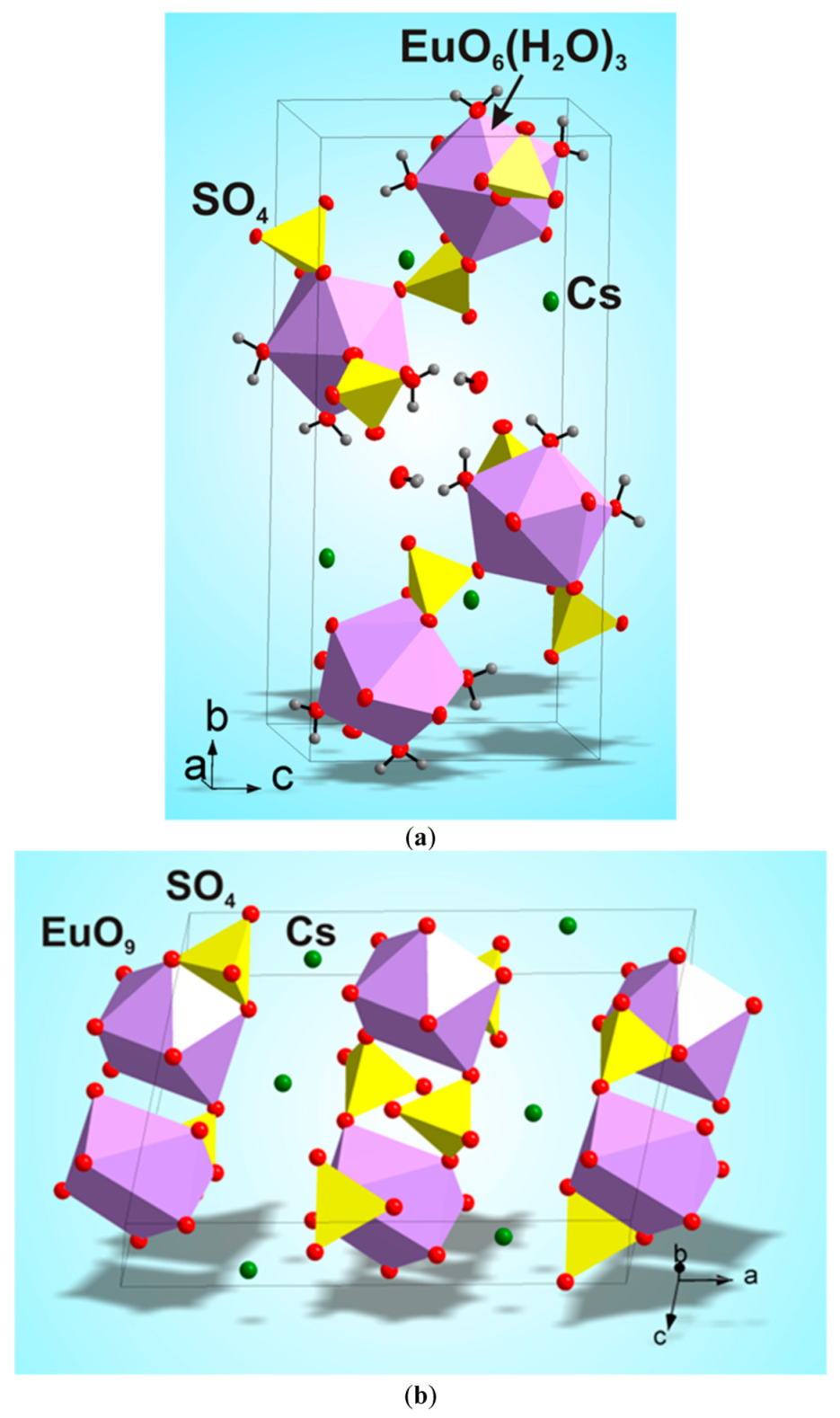
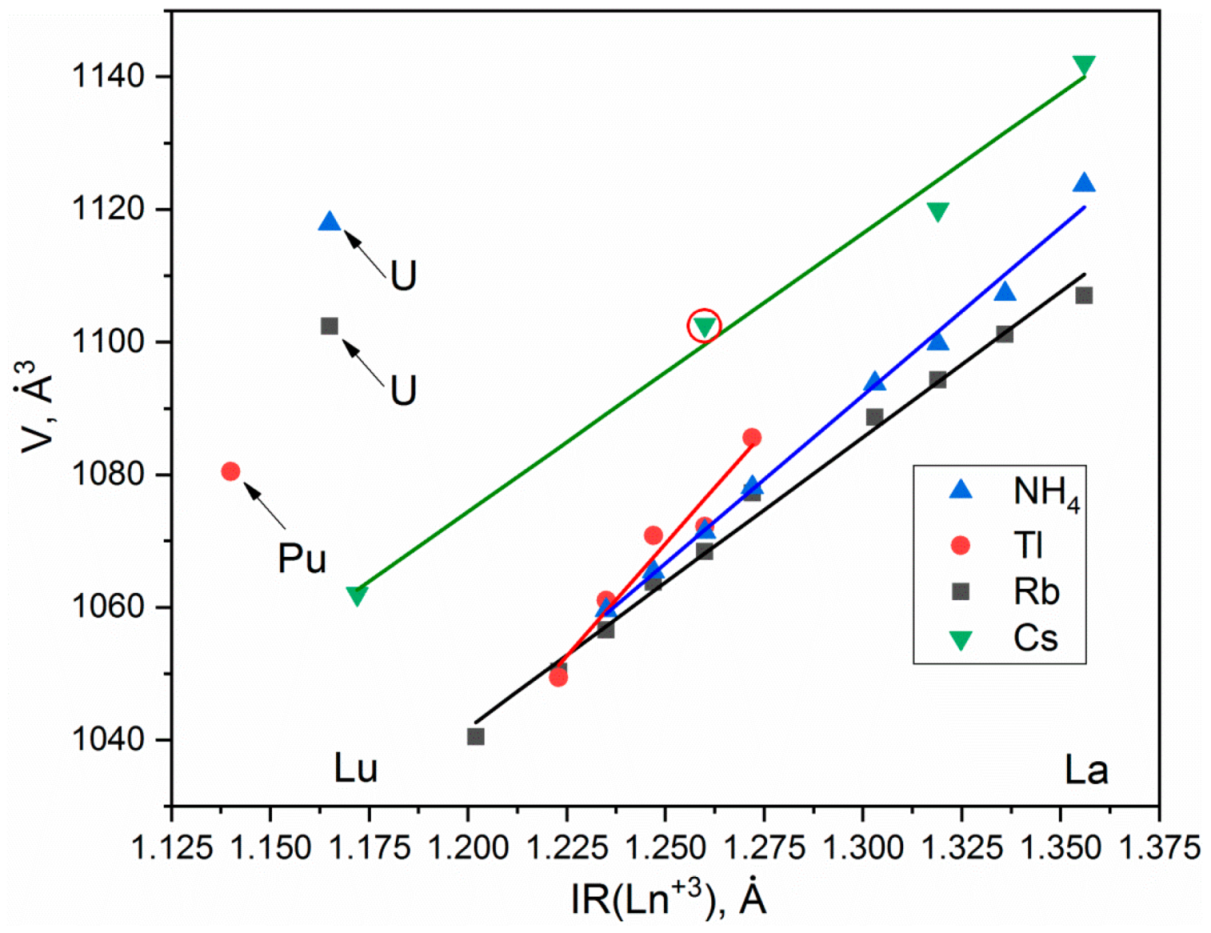
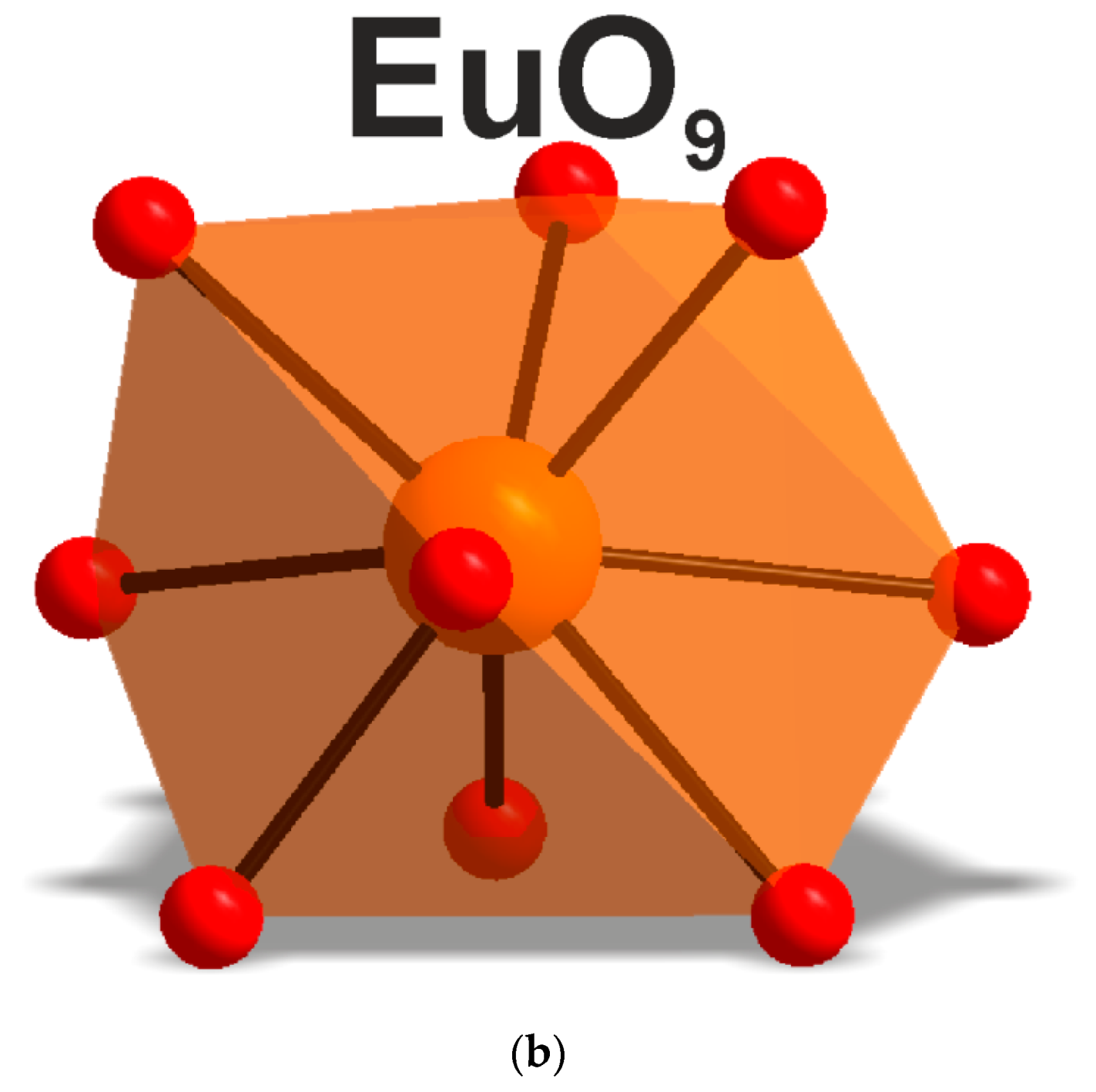
According to the single crystal and powder diffraction analysis (Figure 2a, Table 1 and Tables S1–S5), [CsEu(H2O)3(SO4)2]·H2O crystallized in the monoclinic space group P21/c. The grown crystals did not contain any foreign crystalline impurity. The asymmetric part of the unit cell contained one Cs+ ion, one Eu3+ ion, two S6+ ions, eight O2− ions and four H2O molecules. The Cs+ ion in [CsEu(H2O)3(SO4)2]·H2O was coordinated by 13 O2− ions forming a complex polyhedron. The Cs+ ion was coordinated by four Eu ions, six SO4 tetrahedra and two H2O molecules. Each Eu3+ ion was coordinated by six O2− ions and three H2O molecules forming a EuO6(H2O)3 three-capped trigonal prism (Figure 3a). The EuO6(H2O)3 polyhedron was joined with two SO42− tetrahedra by nodes and edges, respectively, forming, in total, a 2D net. The tridentate bridge–chelate μ2 coordination of the anion towards Eu atoms was observed. One H2O molecule was not coordinated to any metal and it should be considered as an isolated one. It was interesting to consider the stability of this type of structure in reference to the metal ion substitution. The collection of the known compounds [A(Ln,Ac)(H2O)3(SO4)2]·H2O is presented in Table S6 (see Supplementary Materials) and the dependence of unit cell volume VA on the ion radius IR of the Ln or Ac element is shown in Figure 4 [69,80,81,82,83,84,85,86]. It was evident that only such big-sized cations as A = NH4, Tl, Rb and Cs provided a stable monoclinic structure. In this crystal family, the upper limit of VA = 1142.11 Å3 was reached in [CsLa(H2O)3(SO4)2]·H2O, but the lower limit was unclear. At least, it was below or equal to VA = 1040.5 Å3 obtained in [RbEr(H2O)3(SO4)2]·H2O. Up to now, the dominant part of the [A(Ln,Ac)(H2O)3(SO4)2]·H2O crystals was synthesized for lanthanide elements and only three compounds were reported on for actinide elements. However, the monoclinic crystals [AAc(H2O)3(SO4)2]·H2O were reported on for all A cations, except for Cs, and it indicated that the search for new compounds among actinides is a promising field for future research activities. As seen in Figure 4, the VA(IR) dependences could be well approximated by the linear functions specific for each A element: VNH3 = 506.67 IR + 433.28, VTl = 677.64⋅IR + 222.52, VRb = 438.36⋅IR + 515.74 and VCs = 420.25 IR + 570.12. These functions could be used for the prediction of unit cell volumes of other presently unknown crystals [ALn(H2O)3(SO4)2]·H2O. For example, in [ALn(H2O)3(SO4)2]·H2O, all possible VCs values should be in the range of VCs = 1062.69−1142.11 Å3.
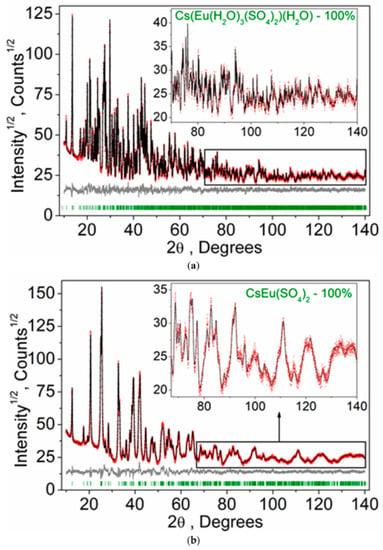
Figure 2.
Difference Rietveld plots of (a) [CsEu(H2O)3(SO4)2]·H2O and (b) CsEu(SO4)2.
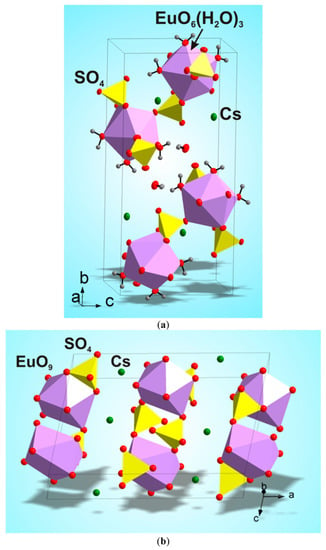
Figure 3.
Crystal structures of (a) [CsEu(H2O)3(SO4)2]·H2O and (b) CsEu(SO4)2. The unit cells are outlined.
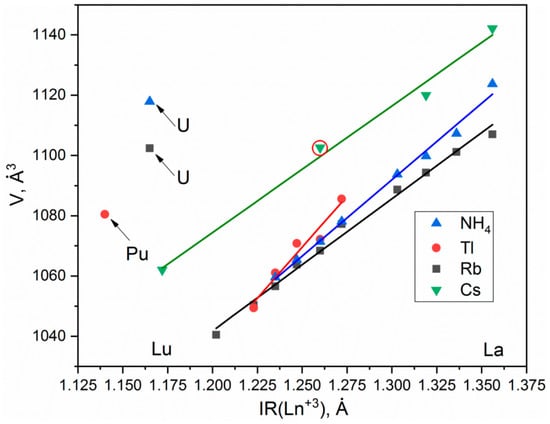
Figure 4.
Unit cell volume dependences on the ionic radius of Ln3+ or Ac3+ ion for compounds [Cs(Ln,Ac)(H2O)3(SO4)2]·H2O. The point of [CsEu(H2O)3(SO4)2]·H2O is highlighted by a red circle.
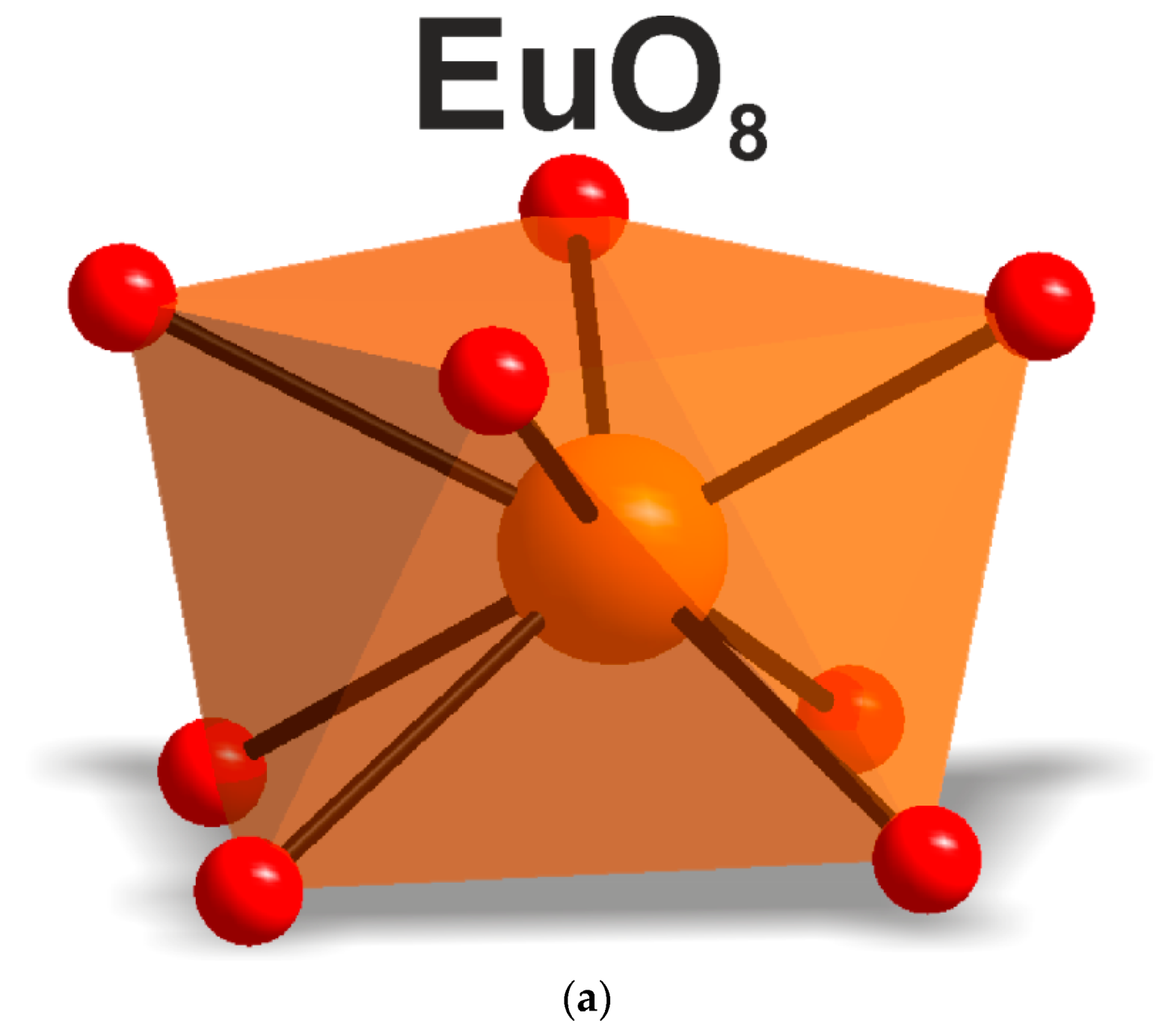
According to the results of the powder diffraction analysis (Figure 1b, Table 1 and Tables S7 and S8), CsEu(SO4)2 crystallizes in the monoclinic space group C2/c. As it is seen in Figure 3b, the structure was of layered type. There were a half of Eu, a half of Cs ions and one SO4 group in the asymmetric part of the unit cell. The Cs+ ion in CsEu(SO4)2 was coordinated by 14 O− ions forming a hexagonal dipyramid. In the CsEu(SO4)2 structure, the Cs+ ion was coordinated by six Eu ions and eight SO4 tetrahedra. Each Eu3+ ion was coordinated by six sulfate groups SO42− via oxygen atoms. Two sulfate groups were chelately coordinated, while the rest were monodentate, resulting in the formation of a two-capped trigonal prism, and the coordination number of europium was equal to eight (Figure 3b). The tetradentate bridge–chelate μ3 coordination mode of the anion towards Eu3+ was observed for CsEu(SO4)2. The structure of CsEu(SO4)2 was isostructural to that of RbEu(SO4)2 [96].
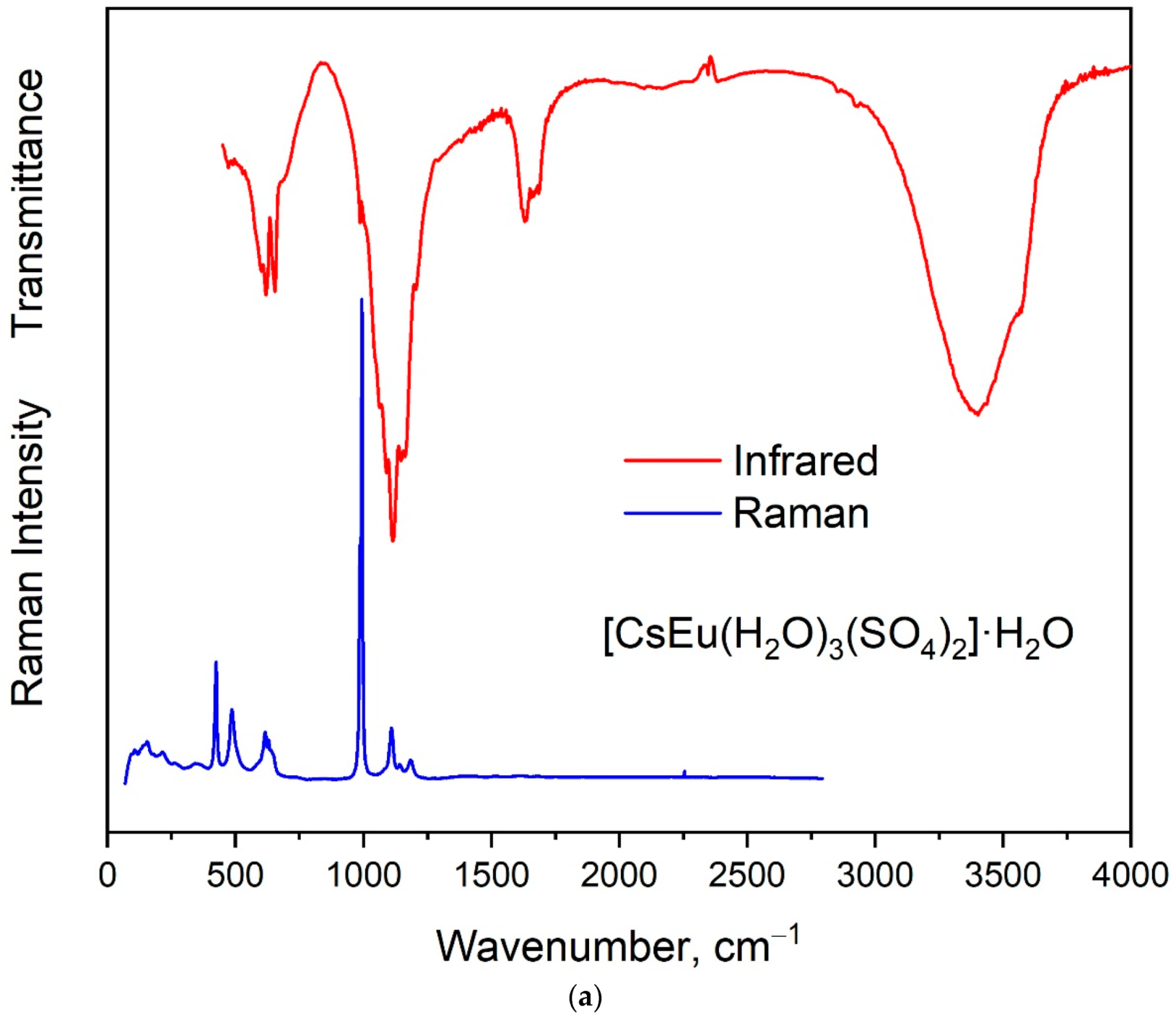
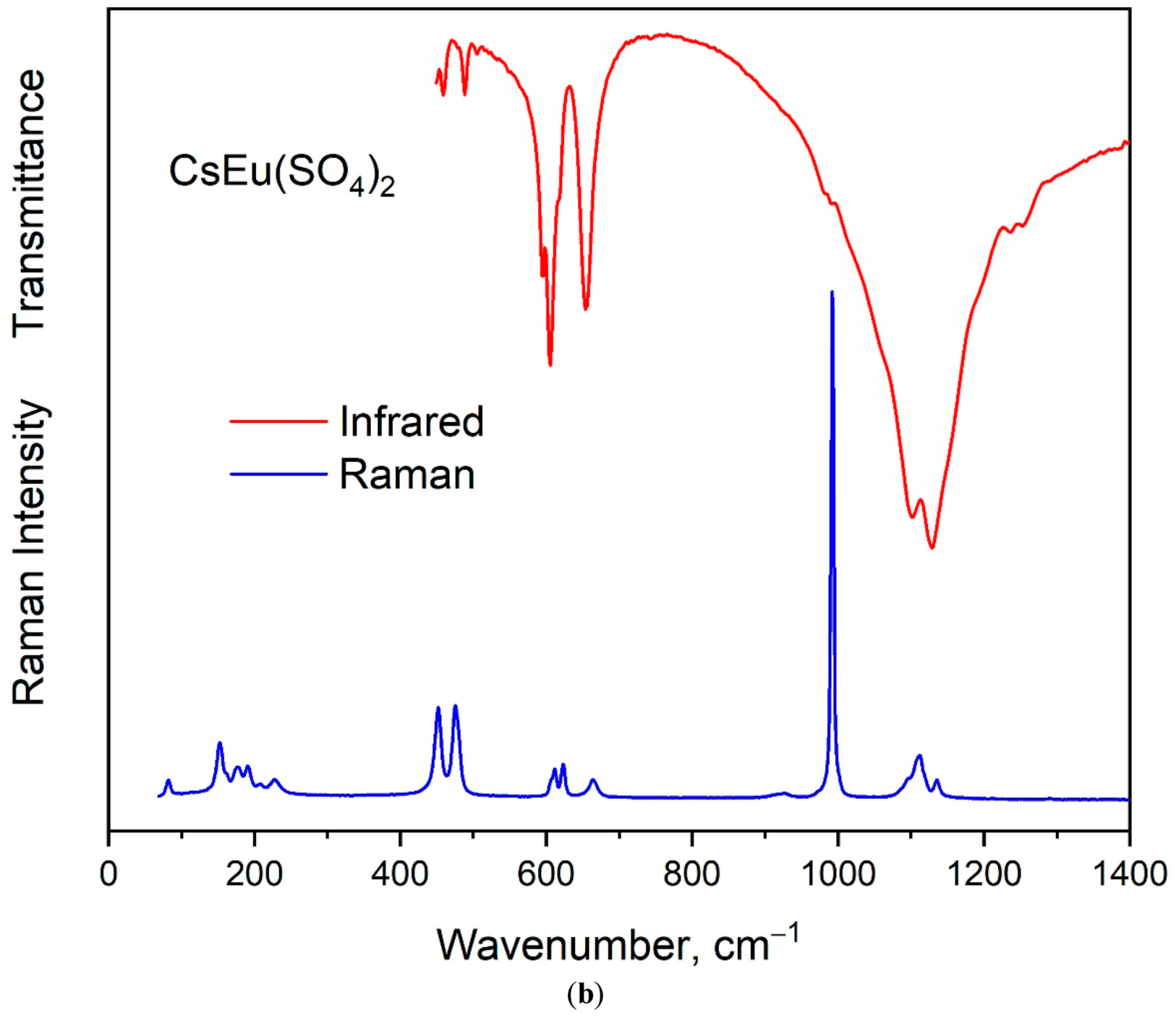
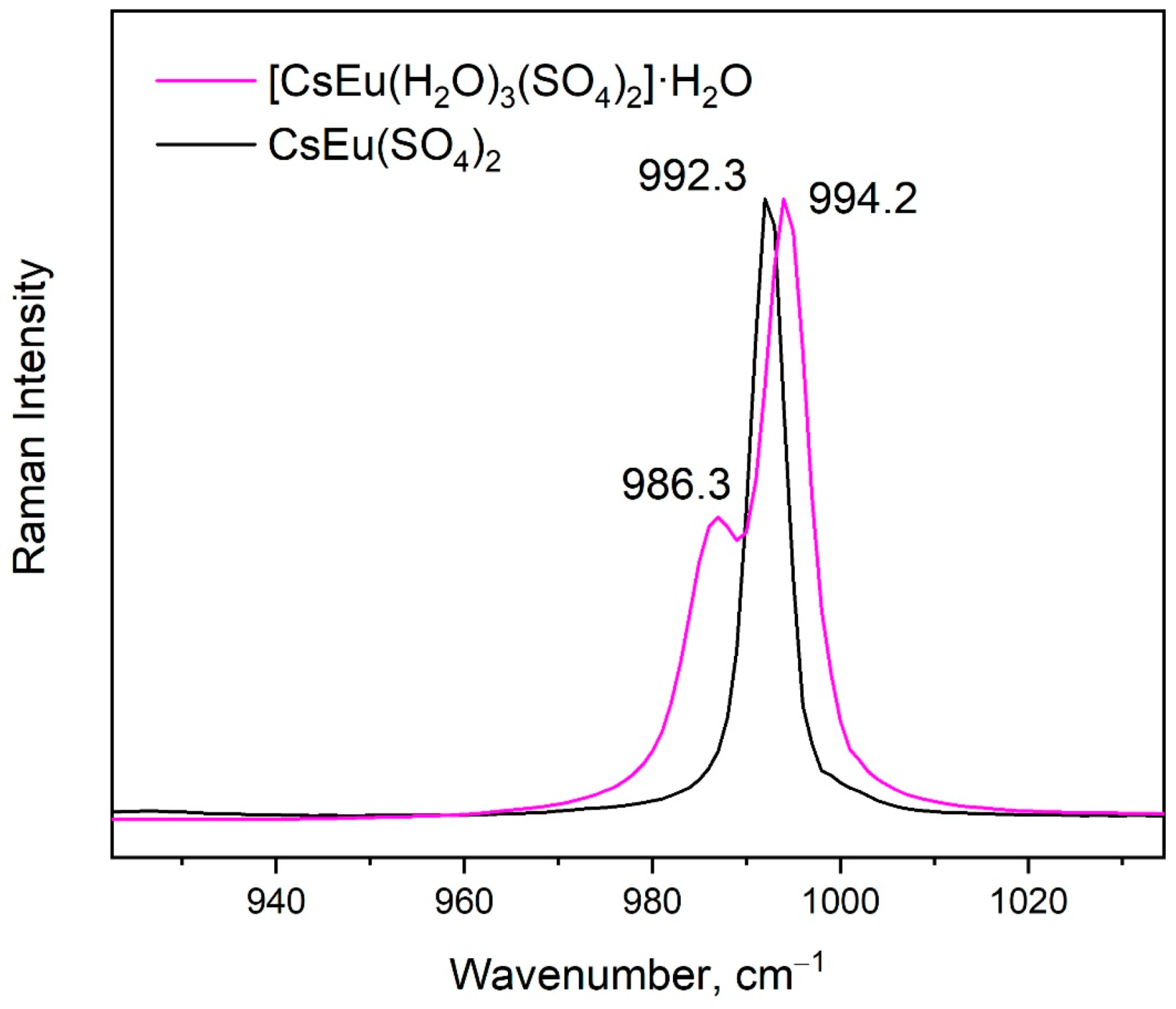
The vibrational spectra of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 are shown in Figure 5a,b, respectively. The normal vibrational modes of free (SO4)2− ions had the wavenumbers of 450, 611, 983 and 1105 cm−1 for ν2, ν4, ν1 and ν3 vibrations, respectively [100]. The correlation for the internal vibrational modes of free sulfate ion and its site symmetry in the lattice and crystal symmetry for the investigated compounds are given in Table 2. Both sulfates had the same factor group symmetry and (SO4)2− units occupied the identical symmetry sites. According to the structure refinement results, CsEu(SO4)2 was characterized by only one crystallographically independent SO4 tetrahedron, while [CsEu(H2O)3(SO4)2]·H2O had two independent SO4 units in its structure. Thus, the number of bands in the Raman and Infrared spectra in the regions of (SO4)2− vibrations should have been twice as big in [CsEu(H2O)3(SO4)2]·H2O than in CsEu(SO4)2. This relation is clearly seen in Figure 6, where one strong band was found in the region of ν1 symmetric stretching vibrations (970-1100 cm−1) of SO4 tetrahedra in the case of CsEu(SO4)2 and two bands in the case of [CsEu(H2O)3(SO4)2]·H2O. The ν3 vibrations were observed in the range of 1020–1250 cm−1. The ν4 vibrational modes (antisymmetric bending) were located between 575 and 690 cm−1. The ν2 symmetric bending vibrations were found in the range of 400–520 cm−1. The strong multicomponent band of H2O vibrations was observed in the Infrared spectrum of [CsEu(H2O)3(SO4)2]·H2O, as seen in Figure 5a. The spectral bands from 1550 to 1750 cm−1 were related to the H–O–H bending vibrations, while a wide band over 3000–3700 cm−1 appeared due to the symmetric O–H stretching. The total set of the Raman and Infrared modes observed in the experiment and their wavenumbers are presented in Table S9.
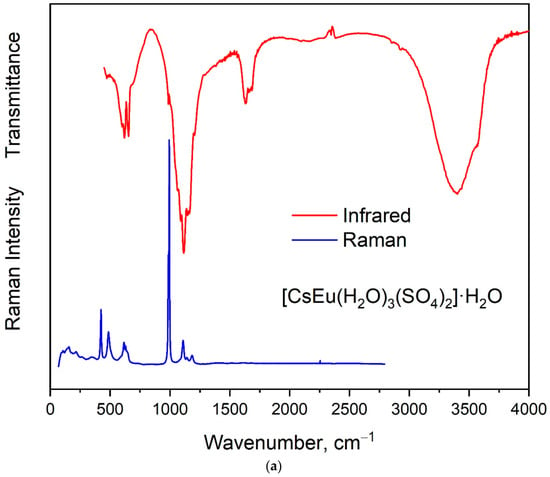
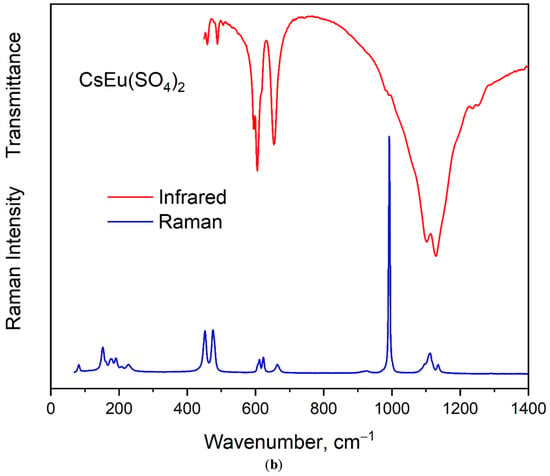
Figure 5.
Infrared and Raman spectra of (a) [CsEu(H2O)3(SO4)2]·H2O and (b) CsEu(SO4)2.

Table 2.
Correlation between molecular symmetry, site symmetry and factor group symmetry for SO42− vibrations in [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2.
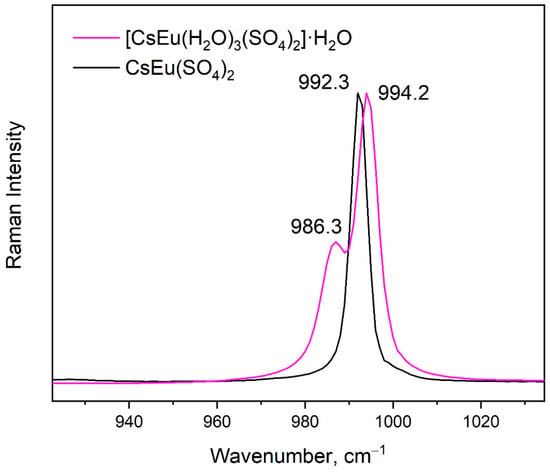
Figure 6.
Comparison of the Raman spectra of [CsEu(H2O)3(SO4)2]·H2O (purple curve) and CsEu(SO4)2 (black curve) in the range of sulfate tetrahedra stretching.
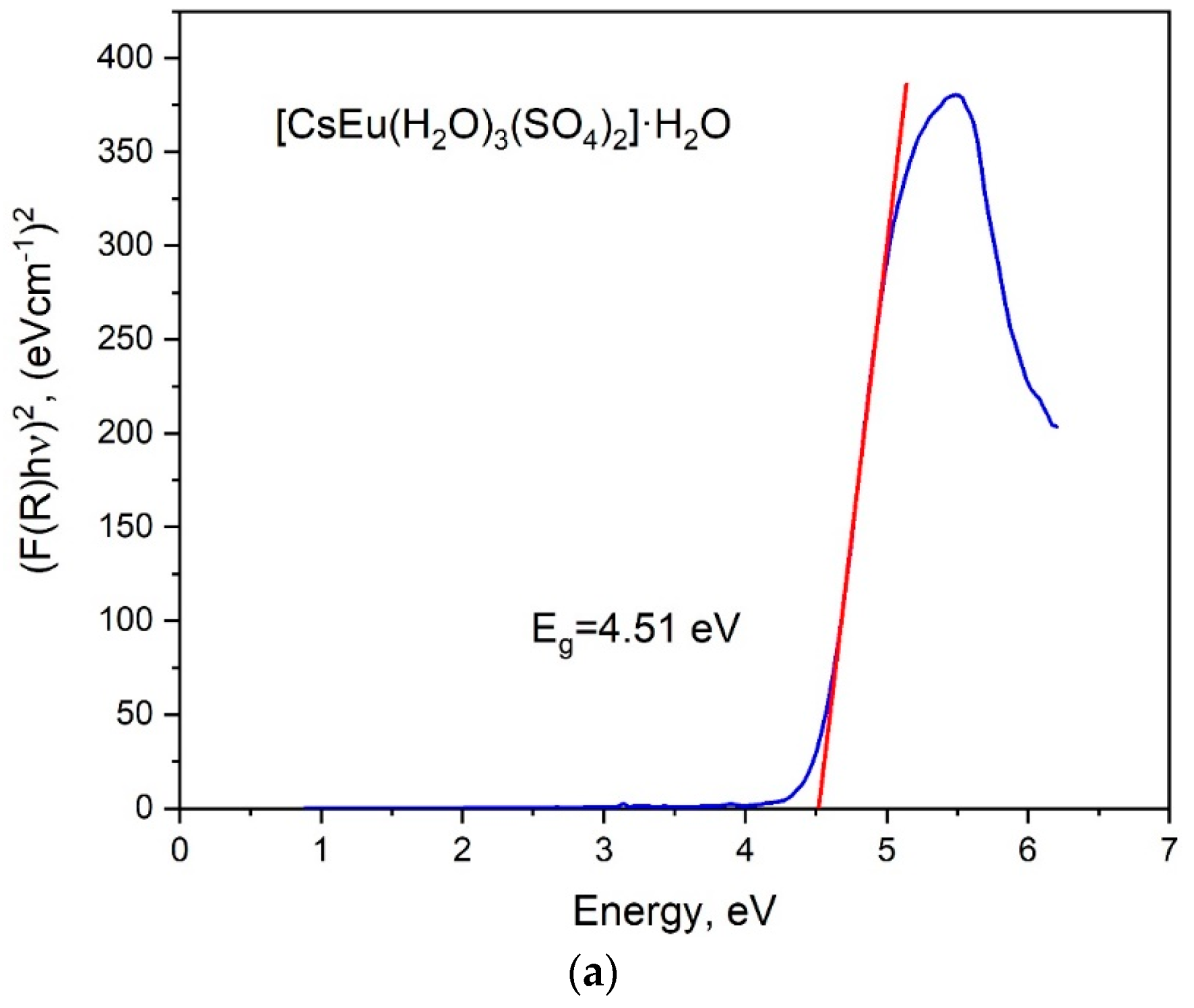
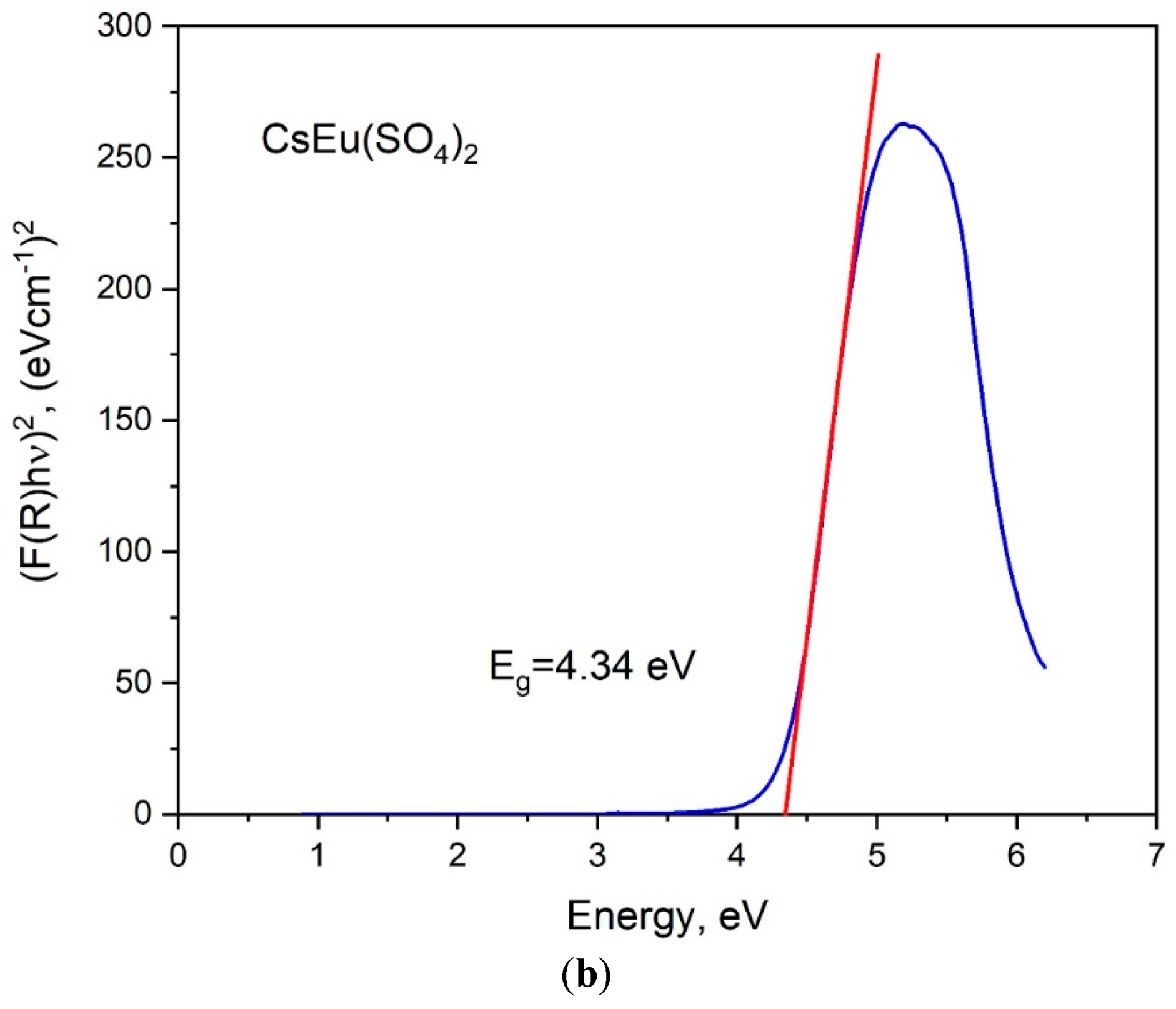
The band gap energies of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 were determined from the UV reflectance spectra with the use of the Kubelka–Munk function: F(R) = K/S = (1 − R)/2R, where K is the absorption coefficient, S is the scattering coefficient and R is the material reflectance. The Tauc plots [101], where the Kubelka–Munk function (F(R)hν)n was dependent on photon energy hν, are shown in Figure 7. The nature of electronic transition was determined by the exponent factor n = 2 or 1/2 for direct or indirect electronic transitions, respectively. As it is seen in Figure 7a,b, the linear function extrapolation to the abscissa axis was successfully reached in the case of n = 2, and the direct band gap values were determined as those equal to 4.51 and 4.34 eV for [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2, respectively.
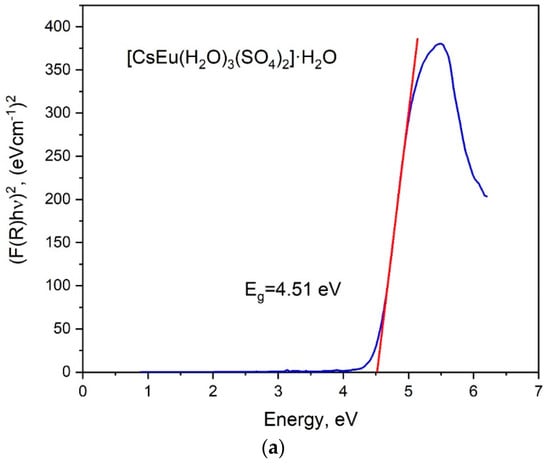
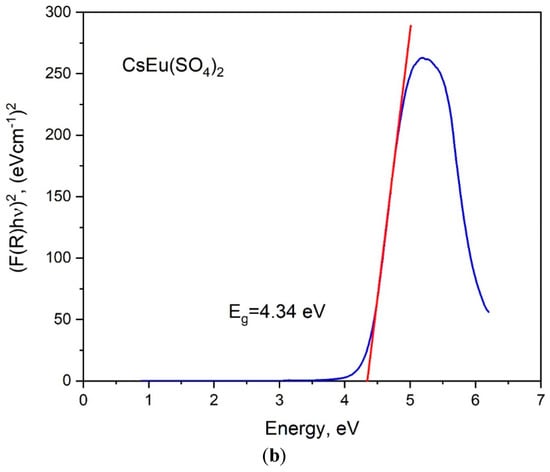
Figure 7.
Tauc plots for (a) [CsEu(H2O)3(SO4)2]·H2O and (b) CsEu(SO4)2.
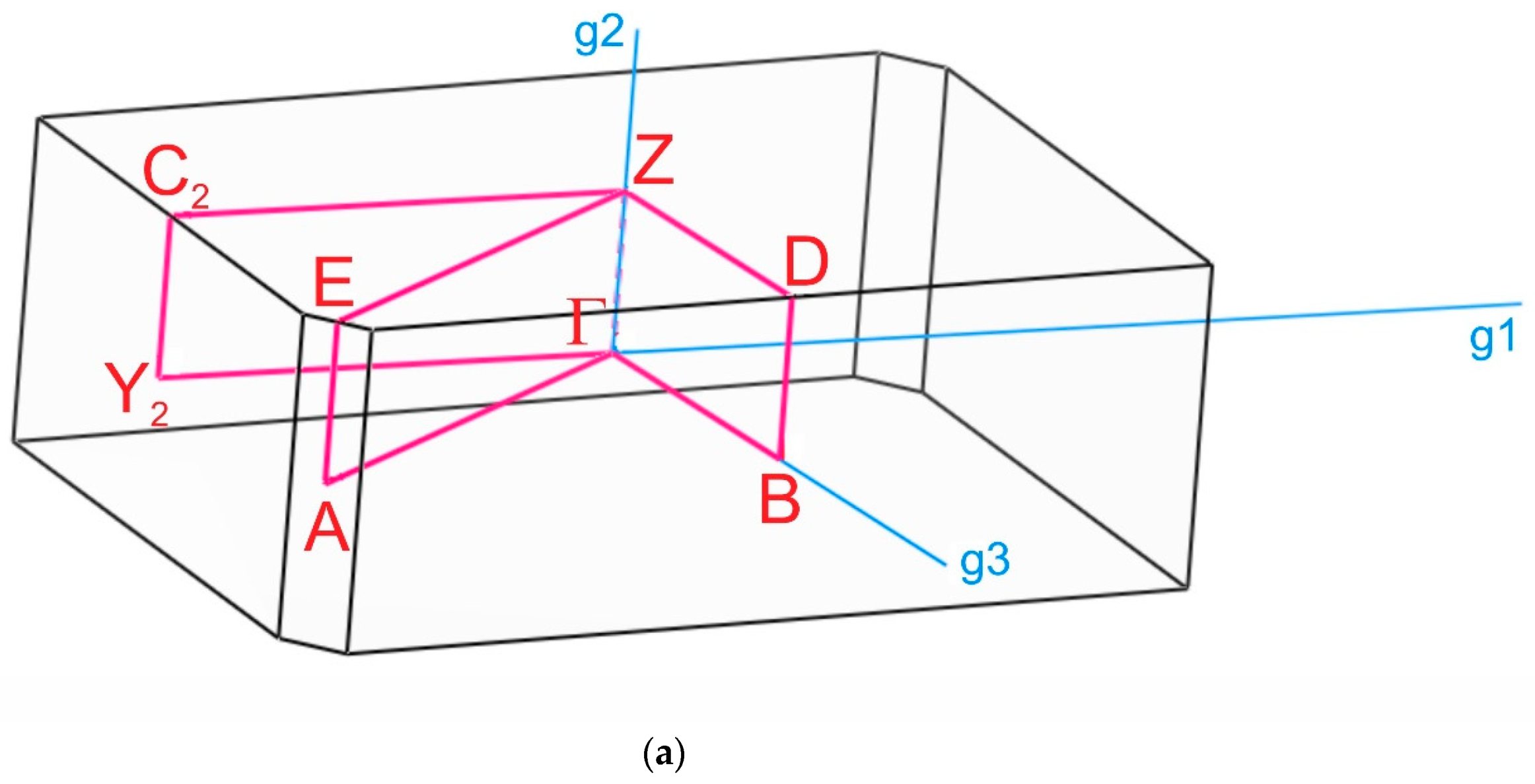
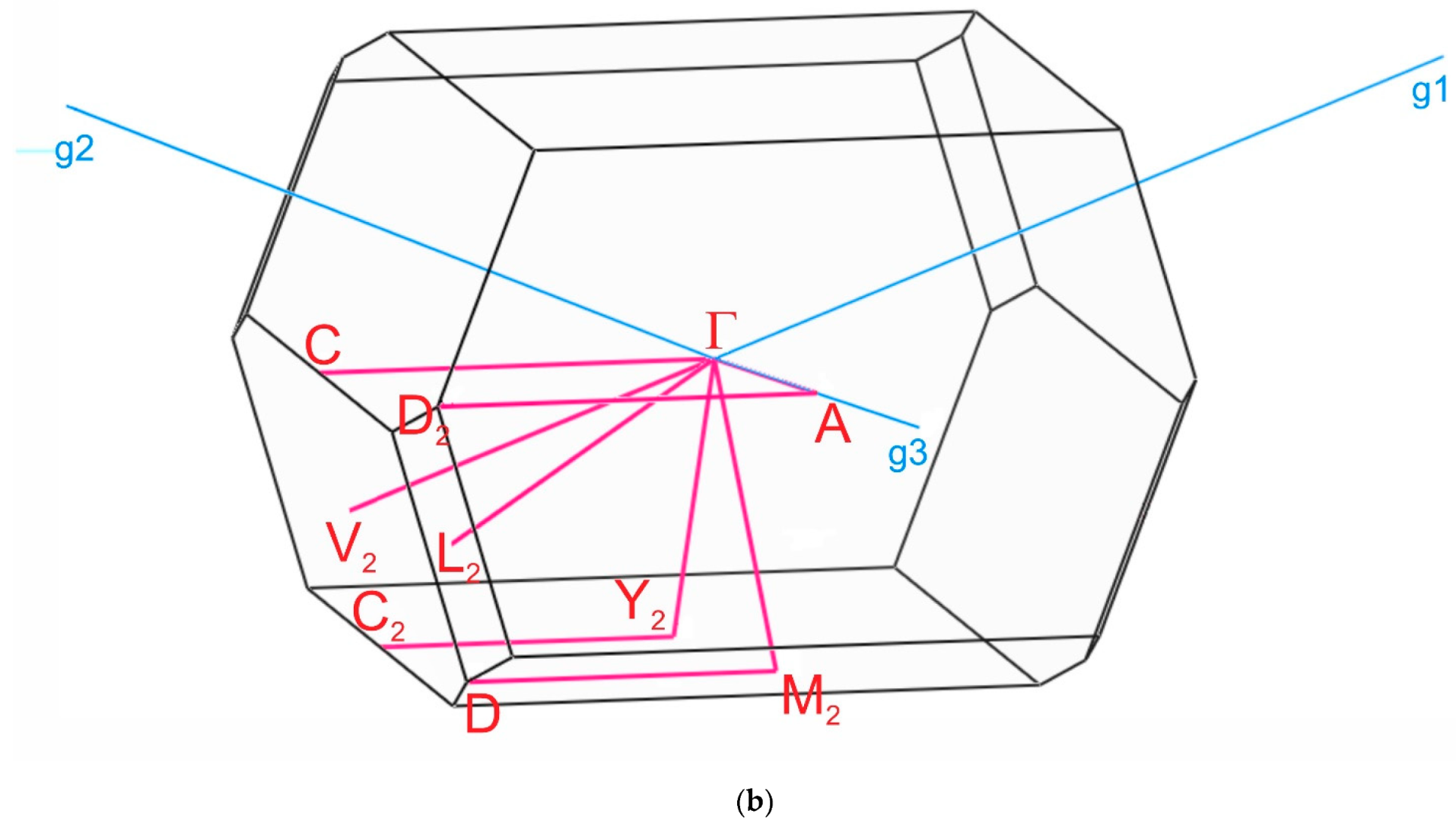
The paths along the Brillouin zone for the investigated compounds (Figure 8), chosen as a set of specific line segments connecting distinctive BZ points, should be written as: Γ–Z–D–B–Γ–A–E–Z–C2–Y2–Γ for [CsEu(H2O)3(SO4)2]·H2O and Γ–C|C2–Y2–Γ–M2–D|D2–A–Γ|L2–Γ–V2 for CsEu(SO4)2 [102]. The coordinates of these points were: Γ(0, 0, 0), Z(0, 0.5, 0), D(0, 0.5, 0.5), B(0, 0, 0.5), A(−0.5, 0, 0.5), E(−0.5, 0.5, 0.5), C2(−0.5, 0.5, 0), Y2(−0.5, 0, 0) for [CsEu(H2O)3(SO4)2]·H2O (Figure 8a) and Γ(0,0,0), C(−0.287, 0.287, 0), C2(−0.713, −0.287, 0), Y2(−0.5, −0.5, 0), M2(−0.5, −0.5, 0.5), D(−0.725, −0.275, 0.5), D2(−0.275, 0.275, 0.5), A(0, 0, 0.5), L2(−0.5, 0, 0.5), V2(−0.5, 0, 0) for CsEu(SO4)2 (Figure 8b).
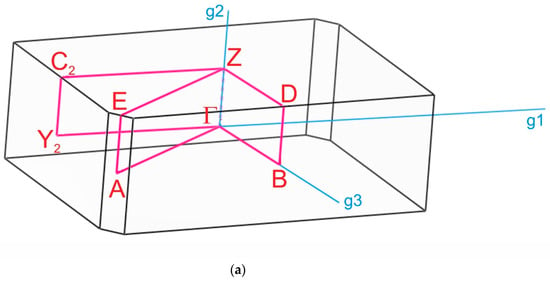
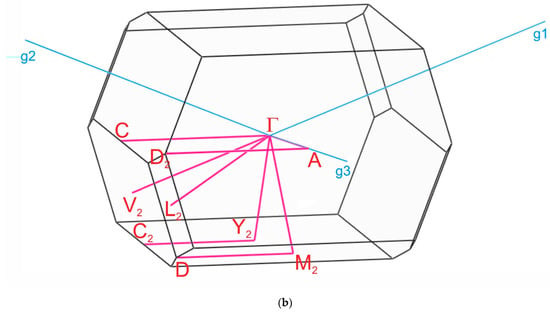
Figure 8.
Brillouin zones of (a) [CsEu(H2O)3(SO4)2]·H2O and (b) CsEu(SO4)2.
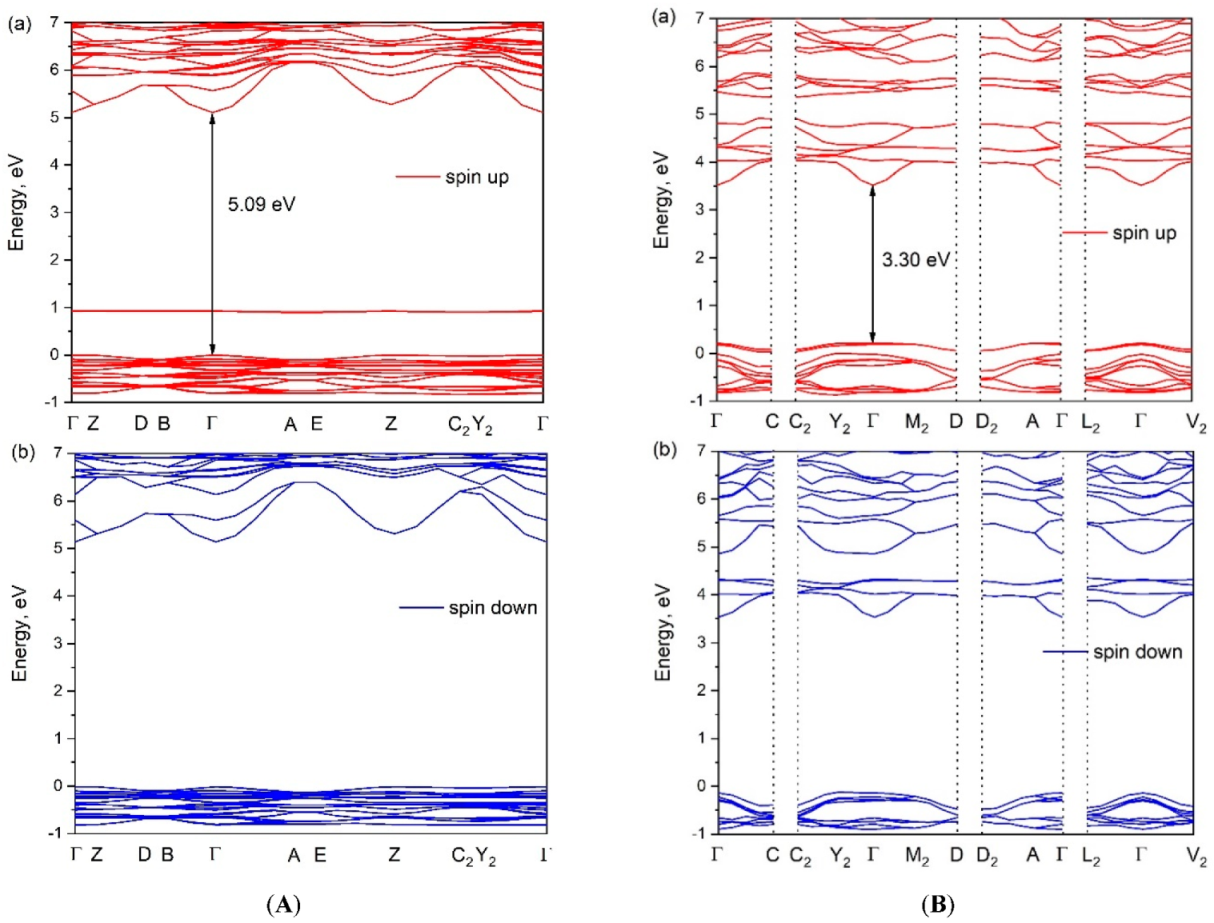
The calculated electronic band structures of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 are shown in Figure 9. The bandgap calculated value was determined as the difference between the valence band top (VBT) and the conduction band bottom (CBB). As europium is a lanthanide, the band structure was presented as spin up and spin down components. The VBT and CBB points of [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 were located in the center of the Brillouin zone (see Figure 9) and, thus, we can say that both compounds were direct band gap materials. The calculated bandgap value for [CsEu(H2O)3(SO4)2]·H2O was 5.09 eV, while, for CsEu(SO4)2, Eg = 3.30 eV. Thus, the transformation from the hydrate to the anhydrous compound reduced the bandgap value in the pair of sulfates.
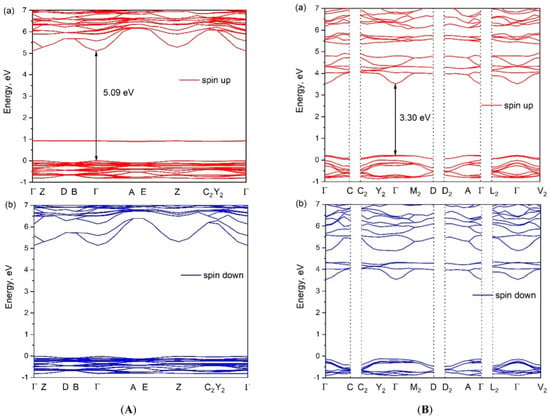
Figure 9.
The electronic band structures of (A) [CsEu(H2O)3(SO4)2]·H2O and (B) CsEu(SO4)2.
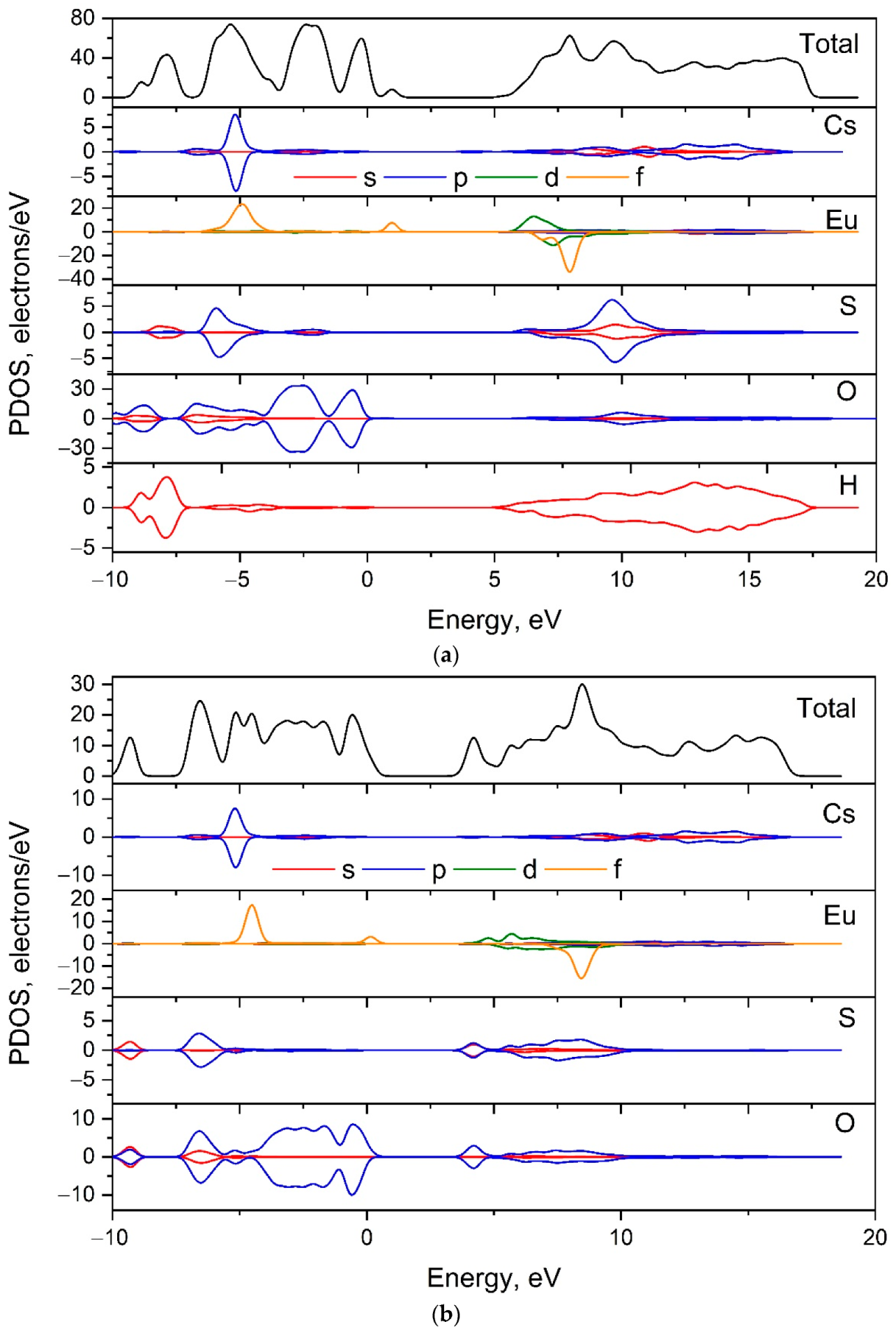
The partial density of states (PDOS) for [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 are shown in Figure 10 and the contribution of each type of atoms can be considered. It can be stated that the valence band top in both compounds was governed by the p electrons of oxygen, while the conduction band bottom was formed by the d electrons of Eu3+ ions. The small peak related to the f-electron state of Eu3+ ions appeared near the Fermi level in both cases.
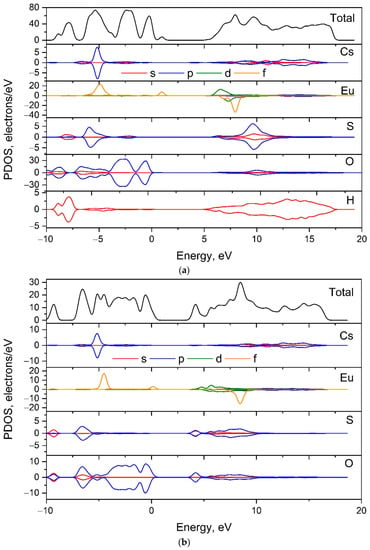
Figure 10.
Electronic partial density of states in (a) [CsEu(H2O)3(SO4)2]·H2O and (b) CsEu(SO4)2.
3.2. Thermochemical Properties
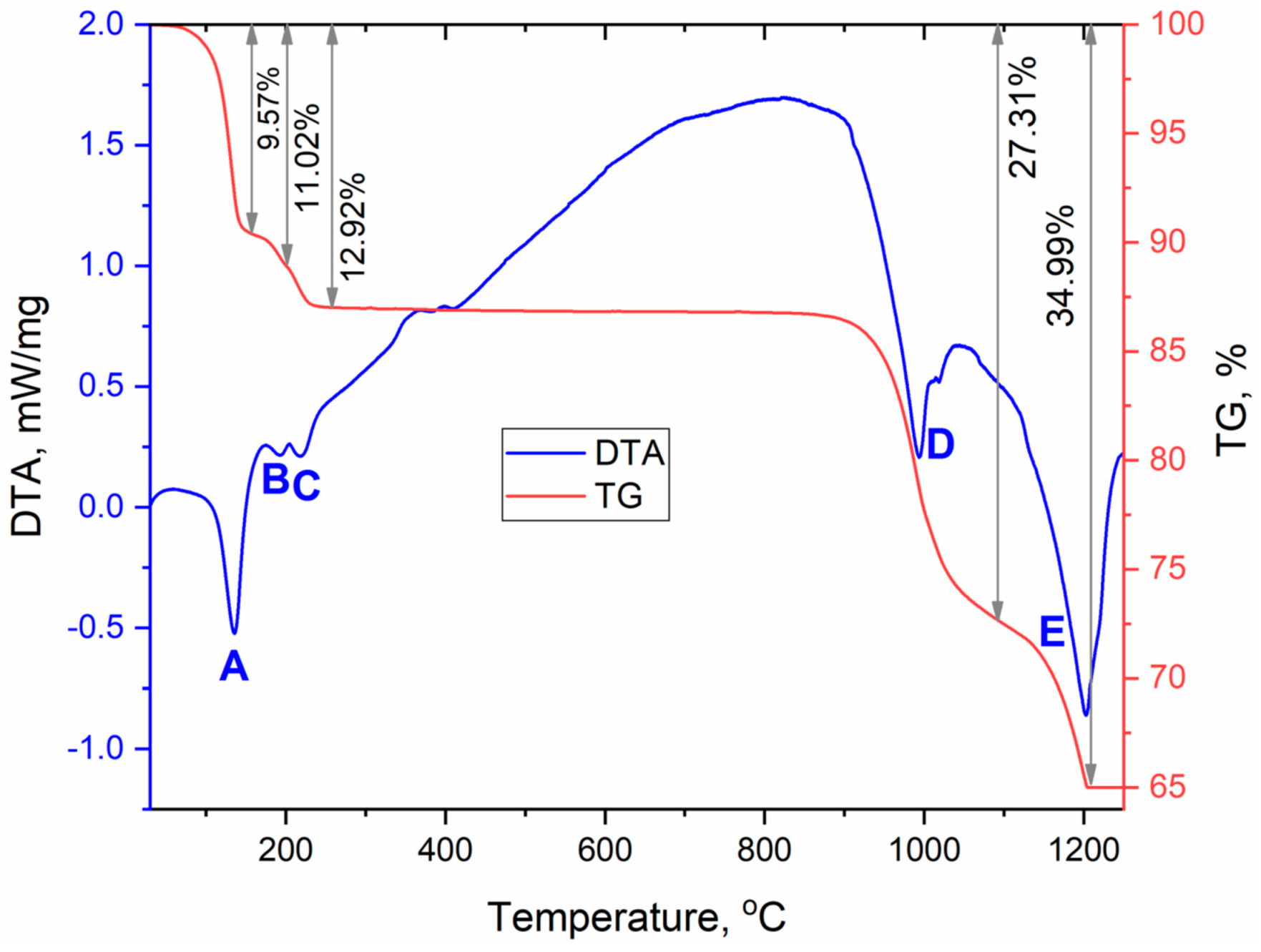
Since anhydrous sulfate CsEu(SO4)2 was formed as a result of the [CsEu(H2O)3(SO4)2]·H2O dehydration, a full-scale study of thermochemical properties can be performed based only on the thermal analysis data shown for [CsEu(H2O)3(SO4)2]·H2O in a wide temperature range (Figure 11, Table 3). The [CsEu(H2O)3(SO4)2]·H2O dehydration proceeded in three stages and led to the formation of anhydrous sulfate CsEu(SO4)2. In the first stage, three water molecules were pinched off (effect A). The remaining water molecule was firmly bound in the structure and the dehydration process occurred in two stages, which corresponded to the formations of a hemihydrate (effect B) and anhydrous salt (effect C), respectively. Anhydrous sulfate CsEu(SO4)2 was stable up to 800 °C, and, at higher temperatures, a two-stage decomposition was observed. At the first stage (effect D), the decomposition into simple sulfates and decomposition of europium (III) sulfate occurred with the formation of europium oxysulfate Eu2O2SO4. At the second stage (effect E), the europium oxysulfate decomposition took place. Thus, the final thermal destruction product at ~1200 °C was a mixture of cesium sulfate and europium oxide. This destruction mechanism resembled that of AgEu(SO4)2 [53], but, in the case of AgEu(SO4)2, the decomposition effects of the complex sulfate and those of europium sulfate were differentiated.
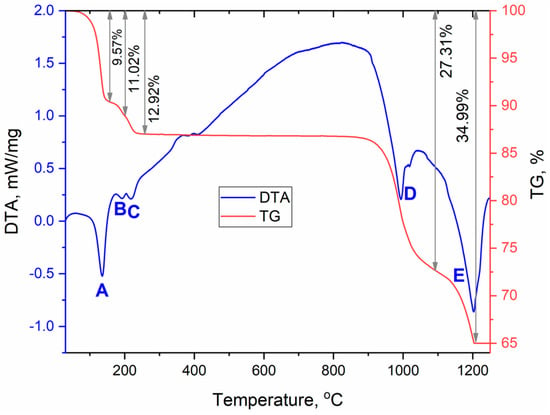
Figure 11.
DSC/TG curves recorded for [CsEu(H2O)3(SO4)2]·H2O.

Table 3.
Thermal effects in [CsEu(H2O)3(SO4)2]·H2O.
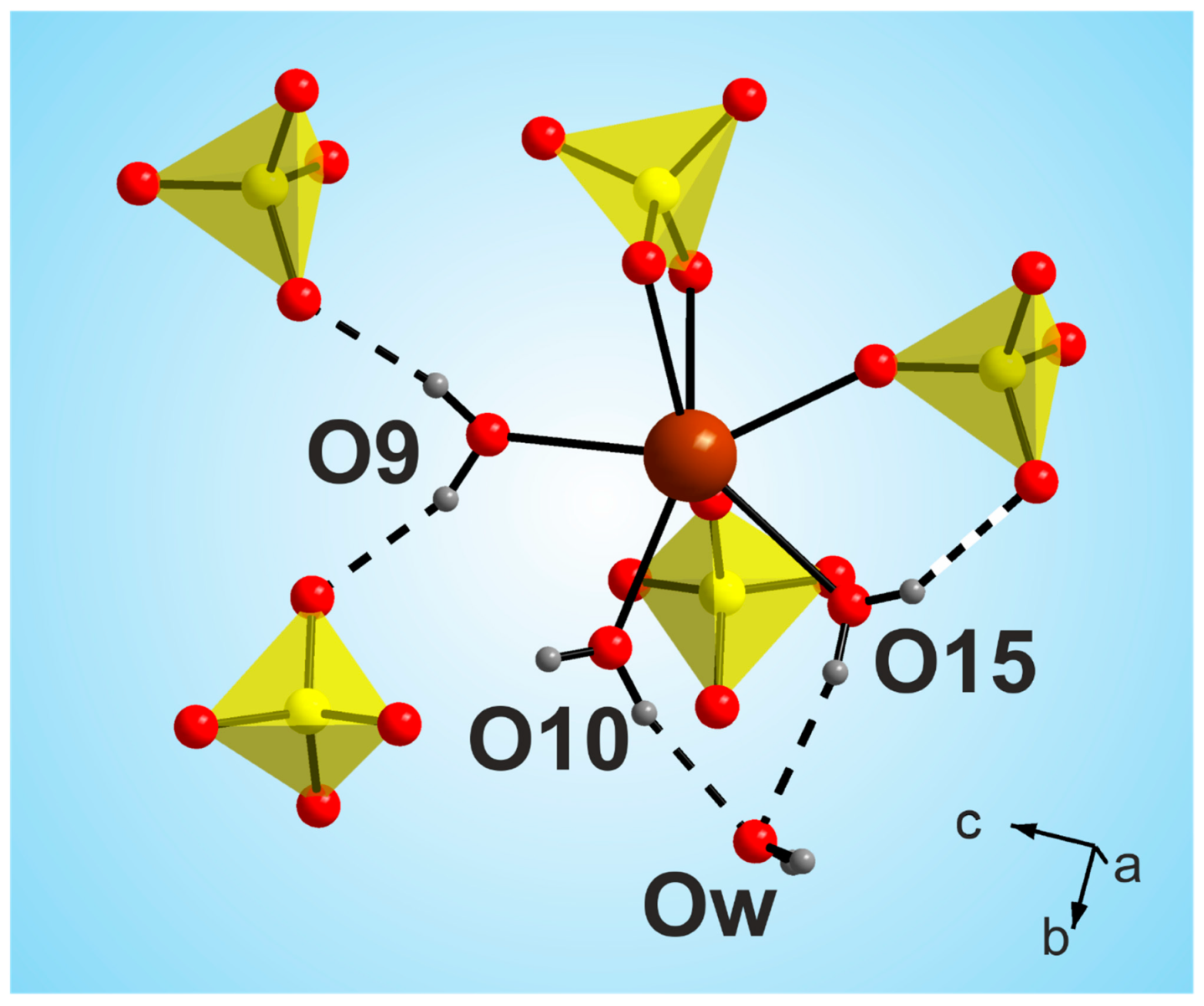
The most interesting feature of the [CsEu(H2O)3(SO4)2]·H2O dehydration process was the unusual water molecules evaporation order, which seemed to be impossible on the base of the crystal structure, where three water molecules were coordinated to the europium atom, and one water molecule was in the void of the crystal structure. Commonly, in solids under heating, water molecules in voids are lost first and, then, coordinated water molecules are evaporated. However, in [CsEu(H2O)3(SO4)2]·H2O, the order was opposite. To explain this phenomenon, it is necessary to consider in detail the coordination of water molecules in the structure and the system of hydrogen bonds shown in Figure 12. It was obvious that the detachment of an uncoordinated water molecule would cause the destabilization of the molecules bound to the O10 and O15 atoms, and it determined the pinching off of these three molecules in one stage. At the same time, the water molecule bound to the O9 atom was very tightly coordinated by the europium polyhedron and two sulfate tetrahedra, and this fact determined its increased stability. It could be intriguing to compare the thermal dehydration processes in [CsEu(H2O)3(SO4)2]·H2O and other isostructural compounds listed in Table S6. However, to our best knowledge, the results of the thermochemical analysis are available only for [Tl(Ln,Ac)(H2O)3(SO4)2]·H2O [69]. In [Tl(Ln,Ac)(H2O)3(SO4)2]·H2O, three water molecules were evaporated first, and, at the second stage, the fourth water molecule was lost. Thus, the dehydration routes in [Tl(Ln,Ac)(H2O)3(SO4)2]·H2O and [CsEu(H2O)3(SO4)2]·H2O were different. Unfortunately, a detailed analysis of the different behavior of these crystals was impossible because only the cell parameters were reported for [Tl(Ln,Ac)(H2O)3(SO4)2]·H2O [69], and their crystal structures remain unknown.
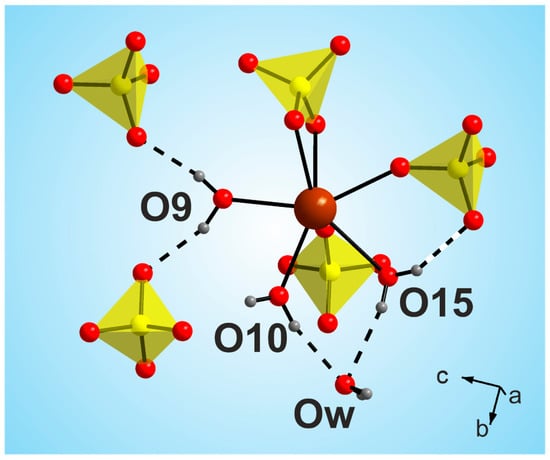
Figure 12.
Coordination of water molecules in the [CsEu(H2O)3(SO4)2]·H2O structure.
3.3. Luminescence Properties
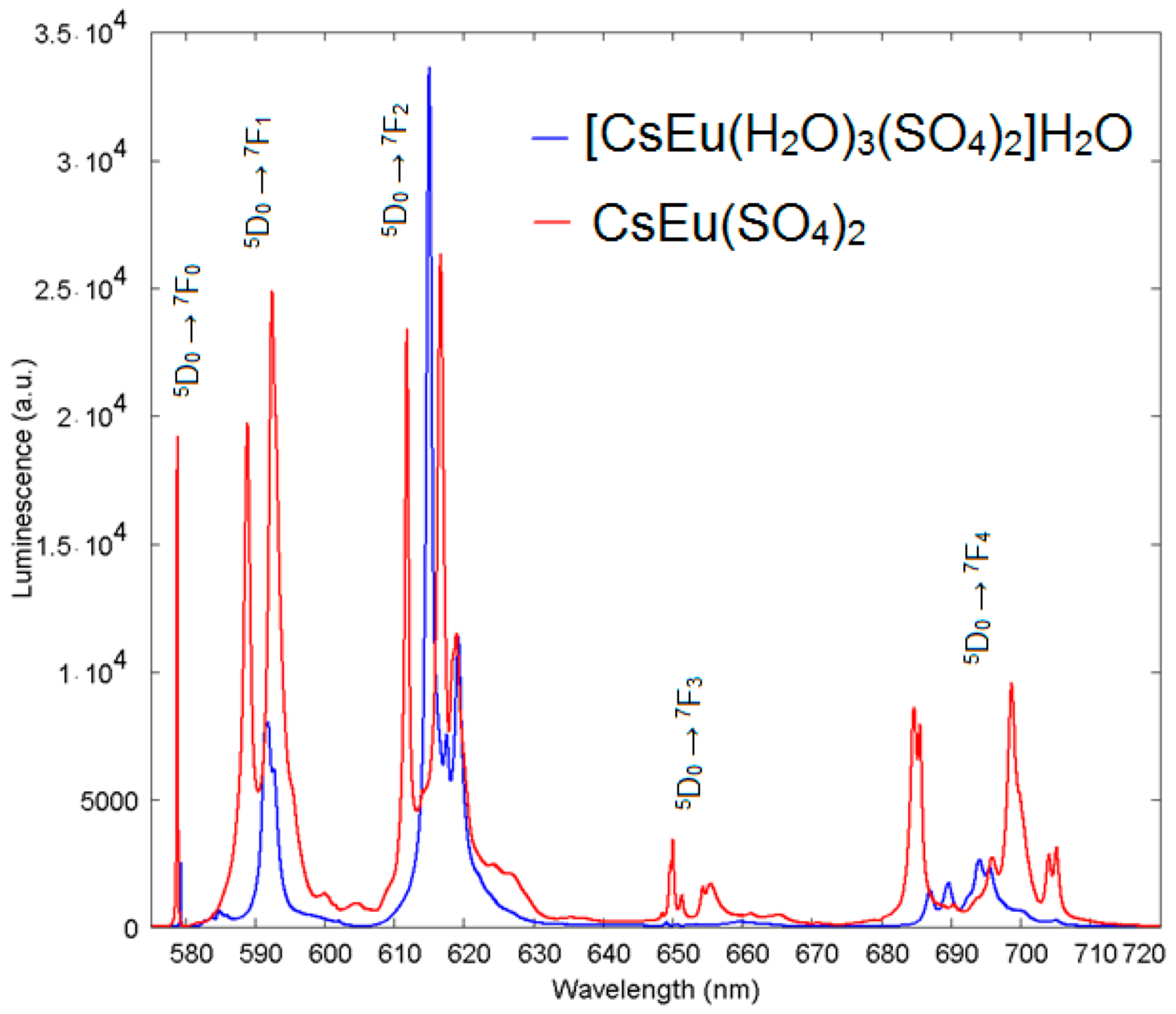
The exciting radiation at 410 nm used for luminescent measurements fell into the resonance with the transition from the ground state 7F0 to the 5D3 state of the Eu3+ ion. The luminescence from 5D3, 5D2 and 5D1 states was negligible, as compared to that from the 5D0 state. The spectra of luminescence from the 5D0 state are presented in Figure 13 for both cesium europium sulfate and cesium europium sulfate hydrate. Both crystals belong to the monoclinic symmetry class but to different space groups (C2/c and P21/c, correspondingly), and the luminescent spectra of the Eu3+ ion drastically differed. The local symmetry of the Eu3+ ion in cesium europium sulfate was C2, while in cesium europium sulfate hydrate it was C1. This difference seemed to be of minor importance; however, from the spectra, additional features of the local environment could be deduced. The amplitudes of luminescent bands at the magnetic dipole 5D0→7F1 transition and at the crystal-field-induced 5D0→7F2 transition were almost equal, and that indicated a relatively low deviation from the inversion symmetry at the Eu3+ ion site in cesium europium sulfate (Figure 14a). Alternatively, the crystal-field-induced 5D0→7F2 transition confidently dominated in cesium europium sulfate hydrate, indicating a much larger violation of the inversion symmetry at the Eu site in this hydrate crystal (Figure 14b). Using the Judd–Ofelt analysis (see, e.g., paper by Kolesnikov et al. [103]), the radiative lifetime of Eu ion in cesium europium sulfate hydrate was 2.27 times smaller than in cesium europium sulfate due to a larger violation of inversion symmetry specified above. At the same time, the ultranarrow line amplitude of at the 5D0→7F0 transition in cesium europium sulfate was of the same order of magnitude as the amplitude of magnetic dipole transition that evidenced a relatively stronger extent of the mirror symmetry violation at the Eu site in cesium europium sulfate, with respect to that in cesium europium sulfate hydrate.
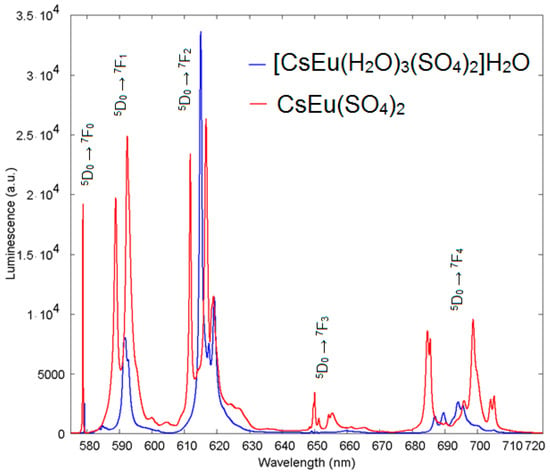
Figure 13.
Emission spectra of [CsEu(H2O)3(SO4)2]·H2O (blue) and CsEu(SO4)2 (red).
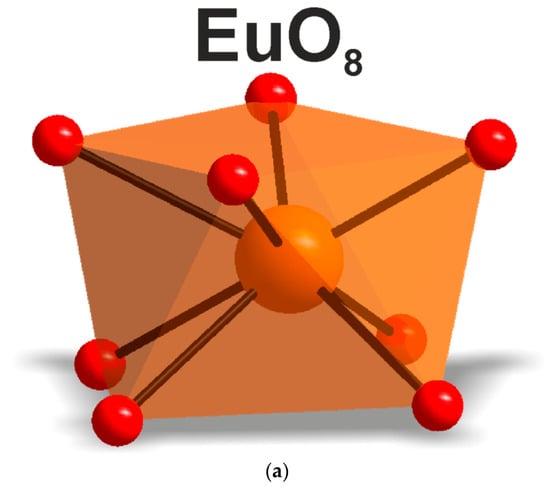
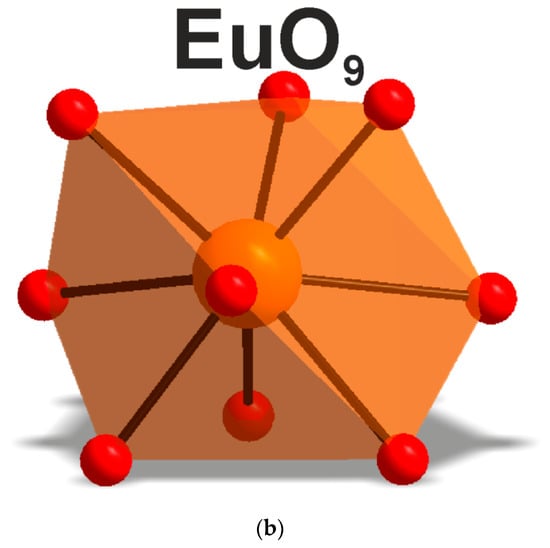
Figure 14.
Local environment of the Eu3+ ion in (a) CsEu(SO4)2 and (b) [CsEu(H2O)3(SO4)2]·H2O.
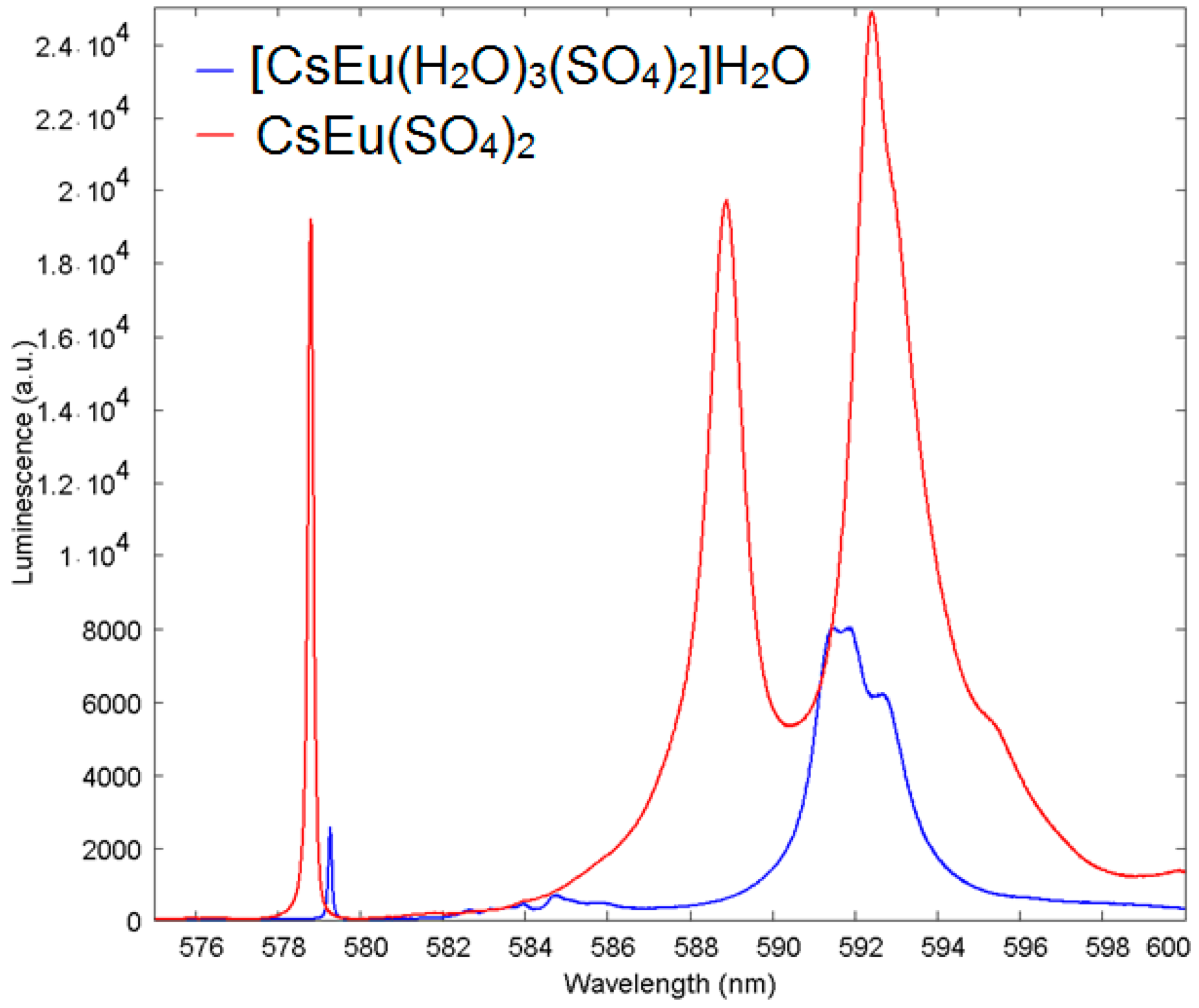
The extent of the chemical shift of the ultranarrow Eu line induced by the presence of H2O molecules in the vicinity of the Eu site in cesium europium sulfate hydrate, with respect to cesium europium sulfate, is illustrated in more detail in Figure 15. The ultranarrow line position in cesium europium sulfate was at 578.8 nm, while in cesium europium sulfate hydrate it shifted to 579.3 nm.
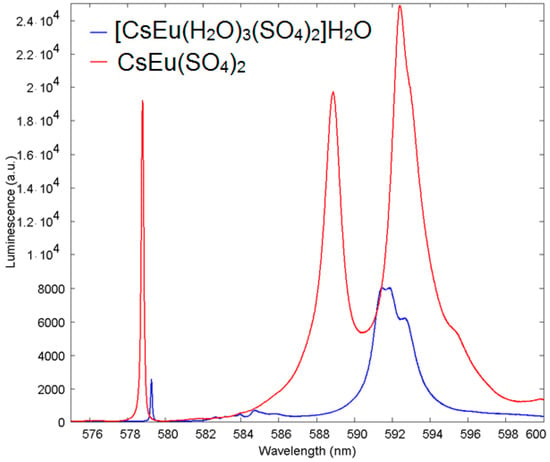
Figure 15.
Luminescence spectra of Eu3+ in [CsEu(H2O)3(SO4)2]·H2O (blue) with respect to CsEu(SO4)2 (red) in the vicinity of the ultranarrow line 5D0-7F0 demonstrating the shift of ultranarrow line.
4. Conclusions
Thus, two new double sulfates [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 were obtained and systematically investigated. The method of simple crystallization of their aqueous solution made it possible to obtain high quality single crystals of [CsEu(H2O)3(SO4)2]·H2O. The thermal dehydration provided the powder of anhydrous double sulfate CsEu(SO4)2 with a high stoichiometry, which is unattainable in a solid-phase reaction between simple sulfates. Both sulfates crystallized in a monoclinic system, but in different space groups, and it led to a significant difference in their vibrational, optical and luminescent properties. The band gap decreased on the transition from [CsEu(H2O)3(SO4)2]·H2O to CsEu(SO4)2. The thermochemical behavior of crystalline hydrate, which seemed illogical at first sight, was well explained by a detailed examination of the coordination of water molecules in the structure. A decisive aspect was found by the consideration of a system of hydrogen bonds, leading to an increased stability of one water molecule in the structure. The noticeable difference of the luminescence spectra between cesium europium sulfate and cesium europium sulfate hydrate was found and explained by the variation of the extent of local symmetry violation at the crystallographic sites occupied by Eu3+ ions, namely, the inversion symmetry and mirror symmetry. The chemical shift of the 5D0 energy level in cesium europium sulfate hydrate, with respect to cesium europium sulfate, was associated with the presence of H2O molecules in the vicinity of the Eu3+ ion.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11091027/s1, Table S1: Crystallographic data and main parameters of single crystal processing and refinement, Table S2: Coordinates of atoms and equivalent isotropic displacement parameters of Cs(Eu(H2O)3(SO4)2)⋅H2O after single crystal refinement, Table S3: Main bond lengths of Cs(Eu(H2O)3(SO4)2)⋅H2O, as obtained from single crystal refinement, Table S4: Fractional atomic coordinates and isotropic displacement parameters (Å2) of Cs(Eu(H2O)3(SO4)2)⋅(H2O) after Rietveld refinement of powder pattern, Table S5: Main bond lengths (Å) of Cs(Eu(H2O)3(SO4)2)⋅(H2O) obtained after Rietveld refinement of powder pattern, Table S6: Cell parameters of known compounds [A(Ln,Ac)(H2O)3(SO4)2]·H2O, A = NH4, Tl, Rb, Cs, Table S7: Fractional atomic coordinates and isotropic displacement parameters (Å2) of CsEu(SO4)2, Table S8: Main bond lengths (Å) of CsEu(SO4)2, Table S9: Raman and Infrared bands (cm−1) observed in [CsEu(H2O)3(SO4)2]·H2O and CsEu(SO4)2 and their assignments, related cif and checkcif files.
Author Contributions
Conceptualization, Y.G.D. and V.V.A.; methodology, I.A.R.; formal analysis, M.S.M. and A.S.O.; data curation, Y.G.D., A.S.K. and N.O.A.; writing—original draft preparation, Y.G.D., M.S.M., A.S.O. and A.S.A.; writing—review and editing, V.V.A.; supervision, O.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Russian Foundation for Basic Research (grant 19-33-90258\19).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, J.B.; Marshall, D.B.; Housley, R.M.; Morgan, P.E. Machinable ceramics containing rare-earth phosphates. J. Am. Ceram. Soc. 1998, 81, 2169–2175. [Google Scholar] [CrossRef]
- Mortier, M.; Monteville, A.; Patriarche, G.; Mazé, G.; Auzel, F. New progresses in transparent rare-earth doped glass-ceramics. Opt. Mater. 2001, 16, 255–267. [Google Scholar] [CrossRef]
- Tanabe, S.; Hayashi, H.; Hanada, T.; Onodera, N. Fluorescence properties of Er3+ ions in glass ceramics containing LaF3 nanocrystals. Opt. Mater. 2002, 19, 343–349. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Santos, L.; Almeida, R. Rare-earth-doped transparent glass ceramics. Comptes Rendus Chim. 2002, 5, 845–854. [Google Scholar] [CrossRef]
- Mortier, M.; Bensalah, A.; Dantelle, G.; Patriarche, G.; Vivien, D. Rare-earth doped oxyfluoride glass-ceramics and fluoride ceramics: Synthesis and optical properties. Opt. Mater. 2007, 29, 1263–1270. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Zhang, G.; Xie, J.; Han, J.; Zhao, X. Crystallization properties of magnesium aluminosilicate glass-ceramics with and without rare-earth oxides. J. Non-Cryst. Solids 2015, 419, 1–5. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Bu, Y.; Liu, C.-S.; Liu, T.; Yan, X. Optical temperature sensing of rare-earth ion doped phosphors. RSC Adv. 2015, 5, 86219–86236. [Google Scholar] [CrossRef]
- Jianbei, Q.I.U.; Qing, J.I.A.O.; Dacheng, Z.H.O.U.; Zhengwen, Y. Recent progress on upconversion luminescence enhancement in rare-earth doped transparent glass-ceramics. J. Rare Earths 2016, 34, 341–367. [Google Scholar]
- Atuchin, V.; Aleksandrovsky, A.; Molokeev, M.; Krylov, A.; Oreshonkov, A.; Zhou, D. Structural and spectroscopic properties of self-activated monoclinic molybdate BaSm2(MoO4)4. J. Alloy Compd. 2017, 729, 843–849. [Google Scholar] [CrossRef]
- Zou, Z.; Wu, T.; Lu, H.; Tu, Y.; Zhao, S.; Xie, S.; Han, F.; Xu, S. Structure, luminescence and temperature sensing in rare earth doped glass ceramics containing NaY(WO4)2 nanocrystals. RSC Adv. 2018, 8, 7679–7686. [Google Scholar] [CrossRef]
- Laidler, J.; Battles, J.; Miller, W.; Ackerman, J.; Carls, E. Development of pyroprocessing technology. Prog. Nucl. Energy 1997, 31, 131–140. [Google Scholar] [CrossRef]
- Preinfalk, C.; Morteani, G. The Industrial Applications of Rare Earth Element: Lanthanides, Tantalum and Niobium; Springer: Berlin/Heidelberg, Germany, 1989; pp. 359–370. [Google Scholar]
- Jha, A.R. Rare Earth Materials: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ramana, C.V.; Vemuri, V.R.; Kaichev, V.; Kochubey, V.A.; Saraev, A.; Atuchin, V.V. X-ray photoelectron spectroscopy depth profiling of La2O3/Si thin films deposited by reactive magnetron sputtering. ACS Appl. Mater. Interfaces 2011, 3, 4370–4373. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, Y.; Molokeev, M.S.; Atuchin, V.V. Structural and luminescence properties of yellow-emitting NaScSi2O6:Eu2+ phosphors: Eu2+ site preference analysis and generation of red emission by cooping Mn2+ for white-light-emitting diode applications. J. Phys. Chem. C 2013, 117, 20847–20854. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Microwave sol-gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4:Er3+/Yb3+ phosphors with incommensurately modulated structure. J. Solid State Chem. 2015, 228, 160–166. [Google Scholar] [CrossRef]
- Ho, F.H.; Abdul-Rashid, S.H.; Ghazilla, R.A.R. Analytic hierarchy process-based analysis to determine the barriers to implementing a material efficiency strategy: Electrical and electronics’ companies in the Malaysian context. Sustainability 2016, 8, 1035. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Zhong, S.-J. Whisker growth on SnAgCu-xPr solders in electronic packaging. J. Mater. Sci. Mater. Electron. 2016, 27, 5618–5621. [Google Scholar] [CrossRef]
- Riba, J.-R.; Torres, C.L.; Romeral, L.; Garcia, A. Rare-earth-free propulsion motors for electric vehicles: A technology review. Renew. Sustain. Energy Rev. 2016, 57, 367–379. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Yue, J.; Hou, W.; Wu, J. Multifunctional rare-earth-doped tin oxide compact layers for improving performances of photovoltaic devices. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Dorzhieva, S.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; et al. Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv. Powder Technol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Li, H.; Sheng, T.; Xue, Z.; Zhu, X.; Hu, S.; Wen, Y.; Fu, R.; Zhuo, C.; Wu, X. Synthesis, structure, characterization, and multifunctional properties of a family of rare earth organic frameworks. CrystEngComm 2017, 19, 2106–2112. [Google Scholar] [CrossRef]
- Verma, S.; Amritphale, S.S.; Das, S. Multifunctional application of cytosine for the synthesis of hybrid homogenized nano-sized rare earth oxide (RE2O3) and rare earth oxycarbonate (RE2O2CO3) (RE = Nd, Sm) advance material via microwave irradiation. Prot. Met. Phys. Chem. Surf. 2017, 53, 444–451. [Google Scholar] [CrossRef]
- Ahmad, T.; Lone, I.H. Development of multifunctional lutetium ferrite nanoparticles: Structural characterization and properties. Mater. Chem. Phys. 2017, 202, 50–55. [Google Scholar] [CrossRef]
- Ying, Z.; Jingqin, W.; Huiling, K. Study on electrical properties of AgSnO2 contact materials doped with rare-earth La, Ce, and Y. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 9, 864–870. [Google Scholar] [CrossRef]
- Azarapin, N.O.; Atuchin, V.V.; Maximov, N.G.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; Krylov, A.S.; Burkhanova, T.M.; Mukherjee, S.; et al. Synthesis, structure, melting and optical properties of three complex orthorhombic sulfides BaDyCuS3, BaHoCuS3 and BaYbCuS3. Mater. Res. Bull. 2021, 140, 111314. [Google Scholar] [CrossRef]
- Kaplyanskii, A.A.; Macfarlane, R. Spectroscopy of Solids Containing Rare Earth Ions; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Chatterjee, A.; Singh, A.K.; Jayaraman, A. Pressure-induced electronic collapse and structural changes in rare-earth mono-chalcogenides. Phys. Rev. B 1972, 6, 2285. [Google Scholar] [CrossRef]
- Greenwood, N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Yu, D.; Tret’yakov, L.I.; Martynenko, A.N.; Grigor’ev, A.; Tsivadze, Y. Inorganic Chemistry: Chemistry of the Elements; Students Book; Butterworth-Heinemann: Oxford, UK, 2001; pp. 131–204. (In Russian) [Google Scholar]
- Cooper, B.R. Magnetic properties of rare earth metals. In Solid State Physics; Seitz, F., Turnbull, D., Ehrenreich, H., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 21, pp. 393–490. [Google Scholar]
- Wakeshima, M.; Nishimine, H.; Hinatsu, Y. Crystal structures and magnetic properties of rare earth tantalates RE3TaO7(RE = rare earths). J. Phys. Condens. Matter 2004, 16, 4103–4120. [Google Scholar] [CrossRef]
- Gupta, S.; Suresh, K.G. Review on magnetic and related properties of RTX compounds. J. Alloys Compd. 2015, 618, 562–606. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Behpour, M.; Sobhani-Nasab, A.; Hosseinpour-Mashkani, S.M. ZnFe2−xLaxO4 nanostructure: Synthesis, characterization, and its magnetic properties. J. Mater. Sci. Mater. Electr. 2015, 26, 9776–9781. [Google Scholar] [CrossRef]
- Hinatsu, Y.; Doi, Y. Structures and magnetic properties of new fluorite-related quaternary rare earth oxides LnY2TaO7 and LaLn2RuO7 (Ln = rare earths). J. Solid State Chem. 2016, 233, 37–43. [Google Scholar] [CrossRef]
- Nishiyama, A.; Doi, Y.; Hinatsu, Y. Magnetic interactions in rhenium-containing rare earth double perovskites Sr2LnReO6 (Ln = rare earths). J. Solid State Chem. 2017, 248, 134–141. [Google Scholar] [CrossRef]
- Shi, P.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V. Crystal chemistry and luminescence properties of red-emitting CsGd1−xEux(MoO4)2 solid-solution phosphors. Dalton Trans. 2014, 43, 9669–9676. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3 and Sr3Eu(PO4)3. Dalton Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef]
- Behrendt, M.; Mahlik, S.; Grinberg, M.; Stefańska, D.; Dereń, P.J. Influence of charge transfer state on Eu3+ luminescence in LaAlO3, by high pressure spectroscopy. Opt. Mater. 2017, 63, 158–166. [Google Scholar] [CrossRef]
- Puchalska, M. High enhancement of Eu3+ luminescence in SrAl4O7 phosphor by means of charge compensation with Na+ ions. Opt. Mater. 2017, 72, 452–458. [Google Scholar] [CrossRef]
- Laishram, R.; Maitra, U. Bile salt-derived Eu3+ organogel and hydrogel: Water-enhanced luminescence of Eu3+ in a gel matrix. ChemistrySelect 2018, 3, 519–523. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Tang, D.; Cho, Y.; Liu, X.; Zhou, X.; Lu, L.; Zhang, L.; Takeda, T.; Hirosaki, N.; et al. Achieving high quantum efficiency narrow-band β-Sialon:Eu2+ phosphors for high-brightness LCD backlights by reducing the Eu3+ luminescence killer. Chem. Mater. 2017, 30, 494–505. [Google Scholar] [CrossRef]
- Van De Haar, M.A.; Werner, J.; Kratz, N.; Hilgerink, T.; Tachikirt, M.; Honold, J.; Krames, M. Increasing the effective absorption of Eu3+-doped luminescent materials towards practical light emitting diodes for illumination applications. Appl. Phys. Lett. 2018, 112, 132101. [Google Scholar] [CrossRef]
- Baur, F.; Jüstel, T. Uranyl sensitized Eu3+ luminescence in Ln(UO2)3(PO4)2O(OH)·6H2O phosphors (Ln = Y, Eu, La) for warm-white light emitting diodes. J. Lumin. 2018, 196, 431–436. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Z.; Takei, T.; Wang, X.; Zhu, Q.; Li, X.; Kim, B.-N.; Sun, X.; Li, J.-G. Selective crystallization of four tungstates (La2W3O12, La2W2O9, La14W8O45, and La6W2O15) via hydrothermal reaction and comparative study of Eu3+ luminescence. Inorg. Chem. 2018, 57, 6632–6640. [Google Scholar] [CrossRef]
- Li, C.; Fan, X.; Jiang, P.; Jin, X. Delamination-indicating of atmosphere-plasma-sprayed thermal barrier coating system using Eu3+ luminescence mapping. Mater. Lett. 2018, 222, 41–44. [Google Scholar] [CrossRef]
- Paama, L.; Pitkänen, I.; Valkonen, J.; Pärnoja, E.; Kola, H.; Perämäki, P. Thermal and spectroscopic investigation of europium and samarium sulphates hydrates by TG-FTIR and ICP-MS techniques. Talanta 2005, 67, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, S.; Zheng, X. Hydrothermal synthesis, crystal structure and properties of 2-D and 3-D lanthanide sulfates. J. Solid State Chem. 2007, 180, 2020–2025. [Google Scholar] [CrossRef]
- Choi, M.-H.; Kim, M.-K.; Jo, V.; Lee, D.-W.; Shim, I.-W.; Ok, K.M. Hydrothermal syntheses, structures, and characterizations of two lanthanide sulfate hydrates materials, La2(SO4)3·H2O and Eu2(SO4)3·4H2O. Bull. Korean Chem. Soc. 2010, 31, 1077–1080. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Zhao, H.; Jiang, C.; Sun, Y.; Xu, Y. Synthesis, characterization and very strong luminescence of a new 3D europium sulfate Eu2(H2O)4(SO4)3. J. Struct. Chem. 2011, 52, 954–958. [Google Scholar] [CrossRef]
- Wang, X.J.; Molokeev, M.S.; Zhu, Q.; Li, J.G. Controlled hydrothermal crystallization of anhydrous Ln2(OH)4SO4 (Ln = Eu-Lu, Y) as a new family of layered rare earth metal hydrooxides. Chem. Eur. J. 2017, 23, 16034–16043. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krulov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S.Y. Structural and spectroscopic properties of noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater. Des. 2018, 140, 488–494. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Volkova, S.S.; Andreev, O.V. Structure, thermal stability, and spectroscopic properties of triclinic double sulfate AgEu(SO4)2 with isolated SO4 groups. Inorg. Chem. 2018, 57, 13279–13288. [Google Scholar] [CrossRef]
- Lal, H.B.; Lundgren, L. Magnetic susceptibility, electrical conductivity and dielectric constant of Eu2(WO4)3 single crystals. J. Phys. Soc. Jpn. 1976, 41, 1216–1223. [Google Scholar] [CrossRef]
- Lal, H.; Dar, N. Magnetic susceptibility of Eu2(WO4)3 single crystals. Phys. B + C 1976, 84, 254–258. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, J.Z.; Li, W. Preparation of tetragonal defect scheelite-type RE2(MoO4)3 (RE = La to Ho) by precipitation method. Solid State Ion. 1989, 32, 244–249. [Google Scholar] [CrossRef]
- Imanaka, N.; Hiraiwa, M.; Tamura, S.; Adachi, G. A new realization route of Al2(WO4)4-Ln2(WO4)3 (Ln = Lu, Eu) solid solution single crystals by electrochemical ion doping. Electrochem. Solid-State Lett. 1999, 2, 570–571. [Google Scholar] [CrossRef]
- Dmitriev, V.; Sinitsyn, V.; Dilanian, R.; Machon, D.; Kuznetsov, A.; Ponyatovsky, E.; Lucazeau, G.; Weber, H.P. In situ pressure-induced solid-state amorphization in Sm2(MoO4)3, Eu2(MoO4)3 and Gd2(MoO4)3 crystals: Chemical decomposition scenario. J. Phys. Chem. Solids 2003, 64, 307–312. [Google Scholar] [CrossRef]
- Kodaira, C.; Brito, H.; Malta, O.; Serra, O. Luminescence and energy transfer of the europium (III) tungstate obtained via the Pechini method. J. Lumin. 2003, 101, 11–21. [Google Scholar] [CrossRef]
- Machon, D.; Dmitriev, V.P.; Sinitsyn, V.V.; Lucazeau, G. Eu2(MoO4)3 single crystal at high pressure: Structural phase transitions and amorphization probed by fluorescence spectroscopy. Phys. Rev. B 2004, 70, 094117. [Google Scholar] [CrossRef]
- Shmyt’ko, I.M.; Kudrenko, E.A.; Sinitsyn, V.V.; Red’kin, B.S.; Ponyatovsky, E.G. Features of the pressure-induced phase transi-tions in Eu2(MoO4)3 single crystals. J. Exp. Theor. Phys. Lett. 2005, 82, 409–412. [Google Scholar] [CrossRef]
- Park, K.-C.; Ahn, H.-C.; Nguyen, H.-D.; Jang, H.-Y.; Mho, S.-I. Optical properties of Eu2(WO4)3 and Tb2(WO4)3 and of CaWO4 doped with Eu3+ or Tb3+—Revisited. J. Korean Phys. Soc. 2008, 53, 2220–2223. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.; Arakcheeva, A.; Pattison, P.; Morozov, V.; Chapuis, G. Validating the model of a (3 + 1)-dimensional in-commensurately modulated structure as generator of a family of compounds for the Eu2(MoO4)3 scheelite structure. Philos. Mag. Lett. 2009, 89, 257–266. [Google Scholar] [CrossRef]
- Wang, Y.; Honma, T.; Doi, Y.; Hinatsu, Y.; Komatsu, T. Magnetism of β′-Gd2(MoO4)3 and photo-luminescence of β′-Eu2(MoO4)3 crystallized in rare-earth molybdenum borate glasses. J. Ceram. Soc. Jpn. 2013, 121, 230–235. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.; Chimitova, O.D.; Gavrilova, T.A.; Krylov, A.; Molokeev, M.; Oreshonkov, A.S.; Bazarov, B.G.; Bazarova, J.G. Synthesis and spectroscopic properties of monoclinic α-Eu2(MoO4)3. J. Phys. Chem. C 2014, 118, 15404–15411. [Google Scholar] [CrossRef]
- Lahoz, F.; Sabalisck, N.P.; Cerdeiras, E.; Mestres, L. Nano-to millisecond lifetime luminescence properties in Ln2(WO4)3 (Ln = La, Ho, Tm and Eu) microcrystalline powders with different crystal structures. J. Alloys Compd. 2015, 649, 1253–1259. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Aleksandrovsky, A.; Atuchin, V.; Krylov, A.; Molokeev, M.; Oreshonkov, A.; Shestakov, N. Exploration of structural, thermal and spectroscopic properties of self-activated sulfate Eu2(SO4)3 with isolated SO4 groups. J. Ind. Eng. Chem. 2018, 68, 109–116. [Google Scholar] [CrossRef]
- Sirotinkin, S.P.; Efremov, V.A.; Kovba, L.M.; Pokrovskii, A.N. Crystal structure of lithium-europium double sulfate. Kristallografiya 1977, 22, 966–970. [Google Scholar]
- Iyer, P.N.; Kulkarni, N.K. Preparation and characterization of TIMIII(SO4)2 4H2O (M (III) ≡ Pu, Sm to Dy). J. Alloys Compd. 1995, 217, 253–257. [Google Scholar] [CrossRef]
- Iyer, P.N.; Mudher, K.D.S.; Kulkarni, N.K. Preparation and characterisation of TlLn(SO4)2·H2O (Ln = Sm to Lu, Y). J. Alloys Compd. 1997, 252, 71–75. [Google Scholar] [CrossRef]
- Kazmierczak, K.; Höppe, H.A. Syntheses, crystal structures and vibrational spectra of KLn(SO4)2·H2O (Ln = La, Nd, Sm, Eu, Gd, Dy). J. Solid State Chem. 2010, 183, 2087–2094. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Chimitova, O.D.; Denisenko, Y.G.; Gavrilova, T.A.; Krylov, A.S.; Maximovskiy, E.A.; Molokeev, M.S.; et al. Exploration of structural, vibrational and spectroscopic properties of self-activated orthorhombic double molybdate RbEu(MoO4)2 with isolated MoO4 units. J. Alloys Compd. 2019, 785, 692–697. [Google Scholar] [CrossRef]
- Yamamoto, H.; Seki, S.; Ishiba, T. The Eu site symmetry in AEu(MoO4)2 (A= Cs or Rb) generating saturated red luminescence. J. Solid State Chem. 1991, 94, 396–403. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Gong, M.; Su, Q. The red phosphor NaEu(MoO4)2 prepared by the combustion method. Mater. Lett. 2008, 62, 619–622. [Google Scholar] [CrossRef]
- Guo, C.; Wang, S.; Chen, T.; Luan, L.; Xu, Y. Preparation of phosphors AEu(MoO4)2 (A = Li, Na, K and Ag) by sol-gel method. Appl. Phys. A 2009, 94, 365–371. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Luo, H.; Yu, X.; Li, Y. Effect of alkali-metal ions on the local structure and luminescence for double tungstate compounds AEu(WO4)2 (A = Li, Na, K). Inorg. Chem. 2011, 50, 11487–11492. [Google Scholar] [CrossRef]
- Perles, J.; Fortes-Revilla, C.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, M.A.; Ruiz-Valero, A.C.; Snejko, N. Synthesis, structure, and catalytic properties of rare-earth ternary sulfates. Chem. Mater. 2005, 17, 2701–2706. [Google Scholar] [CrossRef]
- Deng, Z.; Bai, F.; Xing, Y.; Xing, N.; Xu, L. Reaction in situ found in the synthesis of a series of lanthanide sulfate complexes and investigation on their structure, spectra and catalytic activity. Open J. Inorg. Chem. 2013, 3, 76–99. [Google Scholar] [CrossRef][Green Version]
- Denisenko, Y.G.; Sedykh, A.E.; Molokeev, M.S.; Oreshonkov, A.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Nikolay, A.; Khritokhin, E.; Sal’nikova, I.; Andreev, O.V.; et al. Crystal and electronic structure, thermochemical and photophysical properties of europium-silver sulfate monohydrate AgEu(SO4)2⋅H2O. J. Solid State Chem. 2021, 294, 121898. [Google Scholar] [CrossRef]
- Jasty, S.; Malhotra, V.M.; Robinson, P.D. Effect of the lanthanide ion on the structure and low-temperature phase transitions in RbL(SO4)2⋅4H2O (L ≡ La−Er) crystals. J. Phys. Condens. Matter 1992, 4, 4769–4778. [Google Scholar] [CrossRef]
- Eriksson, B.; Larsson, L.O.; Niinisto, L.; Skoglund, U. Crystal structure of ammonium samarium sulfate tetrahydrate. Inorg. Chem. 1974, 13, 290–295. [Google Scholar] [CrossRef]
- Chadha, A.; Sampath, S.; Chackraburtty, D. X-ray powder diffraction and thermal studies on some uranium (III) compounds. Inorg. Chim. Acta 1980, 42, 163–167. [Google Scholar] [CrossRef]
- Safianov, I.N.; Kuz’min, E.A.; Iskhakova, L.D.; Ilyukhin, V.V.; Belov, N.V. Crystal structure of double Cs,La-sulphate, Cs2SO4·La2(SO4)3·8H2O. Dokl. Akad. Nauk. SSSR 1975, 220, 346–348. [Google Scholar]
- Bukovec, P.; Golic, L. The salts and double salts of rare earths. Crystal structure of cesium bi-sulfato tri-aquopraseodimate (III) monohydrate. Vestn. Slov. Kem. Drus. 1975, 22, 19–25. [Google Scholar]
- Bukovec, N.; Golic, L.; Siftar, J. The salts and double salts of rare earths. Structural study of dehydratation differences between Cs[Pr(SO4)2(H2O)3]·H2O and Cs[Lu(SO4)2(H2O)3]·H2O. Vestn. Slov. Kem. Drus. 1979, 26, 377–385. [Google Scholar]
- Boujelben, M.; Toumi, M.; Mhiri, T. NH4Ce(SO4)2·4H2O. Acta Cryst. E 2007, 63, i144–i145. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Gavrilova, T.A.; Kuratieva, N.V.; Okotrub, K.A.; Pervukhina, N.V.; Surovtsev, N.V. Sublimation growth and vibrational microspectrometry of α-MoO3 single crystals. J. Cryst. Growth 2011, 318, 987–990. [Google Scholar] [CrossRef]
- Alekseev, E.V.; Felbinger, O.; Wu, S.; Malcherek, T.; Depmeier, W.; Modolo, G.; Gesing, T.M.; Krivovichev, S.V.; Suleimanov, E.V.; Gavrilova, T.A.; et al. K[AsW2O9], the first member of the arsenate tungsten bronze family: Synthesis, structure, spec-troscopic and non-linear optical properties. J. Solid State Chem. 2013, 204, 59–63. [Google Scholar] [CrossRef]
- Kokh, K.; Atuchin, V.; Gavrilova, T.; Kuratieva, N.; Pervukhina, N.; Surovtsev, N. Microstructural and vibrational properties of PVT grown Sb2Te3 crystals. Solid State Commun. 2014, 177, 16–19. [Google Scholar] [CrossRef]
- Troitskaia, I.B.; Gavrilova, T.A.; Gromilov, S.A.; Sheglov, D.V.; Atuchin, V.V.; Vemuri, R.S.; Ramana, C.V. Growth and structural properties of α-MoO3 (010) microplates with atomically flat surface. Mater. Sci. Eng. B 2010, 174, 159–163. [Google Scholar] [CrossRef]
- Denisenko, Y.; Khritokhin, N.; Andreev, O.; Basova, S.; Sal’Nikova, E.; Polkovnikov, A. Thermal decomposition of europium sulfates Eu2(SO4)3·8H2O and EuSO4. J. Solid State Chem. 2017, 255, 219–224. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON—A Multipurpose Crystallographic Tool. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2008. [Google Scholar]
- Brandenburg, K.; Berndt, M. DIAMOND—Visual Crystal Structure Information System CRYSTAL IMPACT. J. Appl. Crystallogr. 1999, 32, 1028–1029. [Google Scholar]
- Bruker. TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual. 2008. Available online: http://algol.fis.uc.pt/jap/TOPAS%204-2%20Users%20Manual.pdf (accessed on 8 August 2021).
- Sarukhanyan, N.L.; Iskhakova, L.D.; Trunov, V.K. Crystal structure of RbEu(SO4)2. Kristallografiya 1983, 28, 452–456. [Google Scholar]
- Clark, S.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef]
- Ceperley, D.; Alder, B. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 1980. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley and Sons: New York, NY, USA, 2009. [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Hinuma, Y.; Pizzi, G.; Kumagai, Y.; Oba, F.; Tanaka, I. Band structure diagram paths based on crystallography. Comput. Mater. Sci. 2017, 128, 140–184. [Google Scholar] [CrossRef]
- Kolesnikov, I.; Kolokolov, D.; Kurochkin, M.; Voznesenskiy, M.; Osmolowsky, M.; Lähderanta, E.; Osmolovskaya, O. Morphology and doping concentration effect on the luminescence properties of SnO2:Eu3+ nanoparticles. J. Alloy Compd. 2020, 822, 153640. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).