Hydroxyapatite and Silicon-Modified Hydroxyapatite as Drug Carriers for 4-Aminopyridine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Preparation of HAP and Si–HAP
2.3. Adsorption of 4AP on HAP and Si–HAP Carriers
2.4. Electrochemical Measurements
2.5. Desorption of 4AP from HAP Composites
3. Results and Discussion
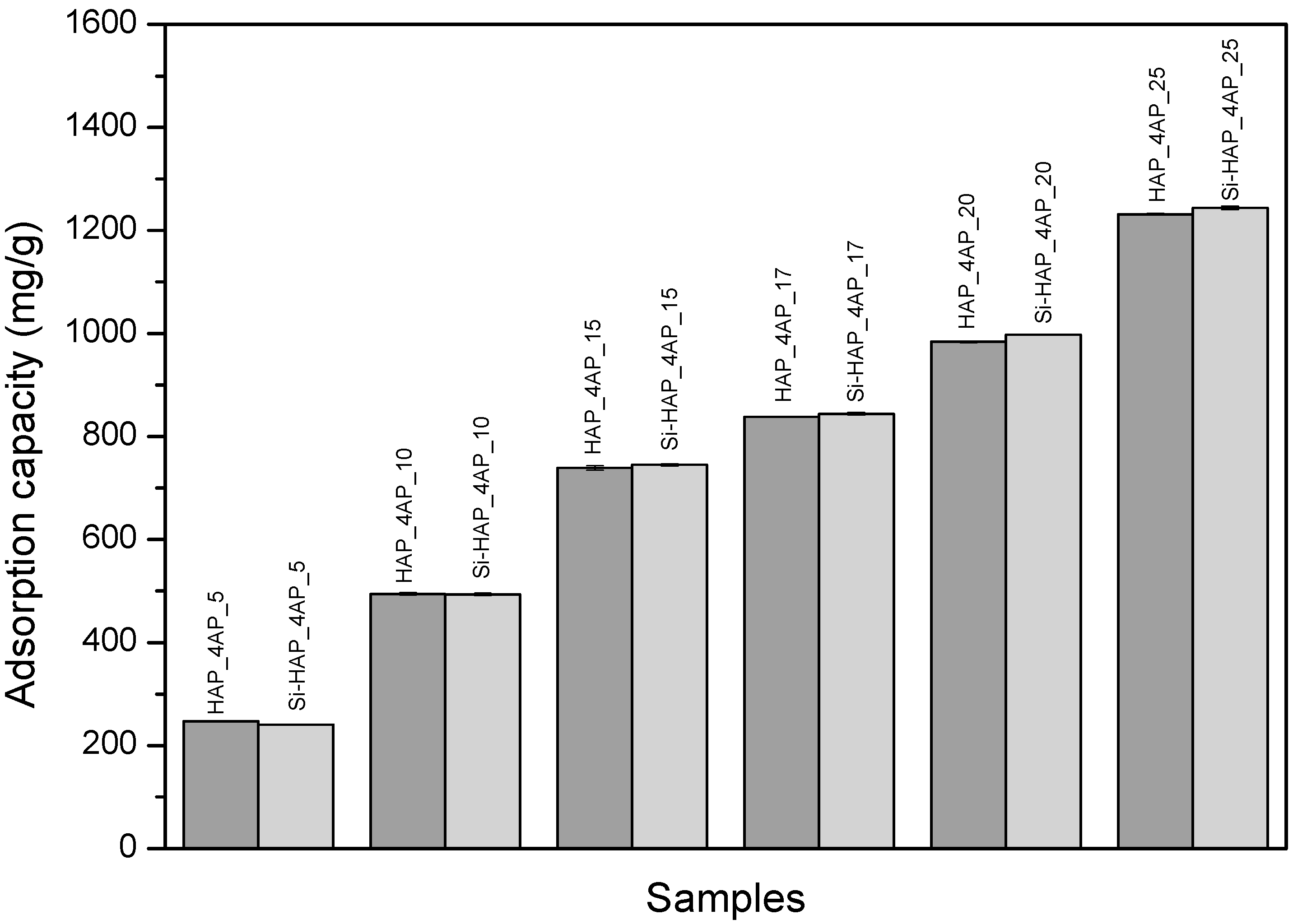
3.1. UV-VIS Spectrophotometric Study of Adsorption
3.2. Thermal Analyses
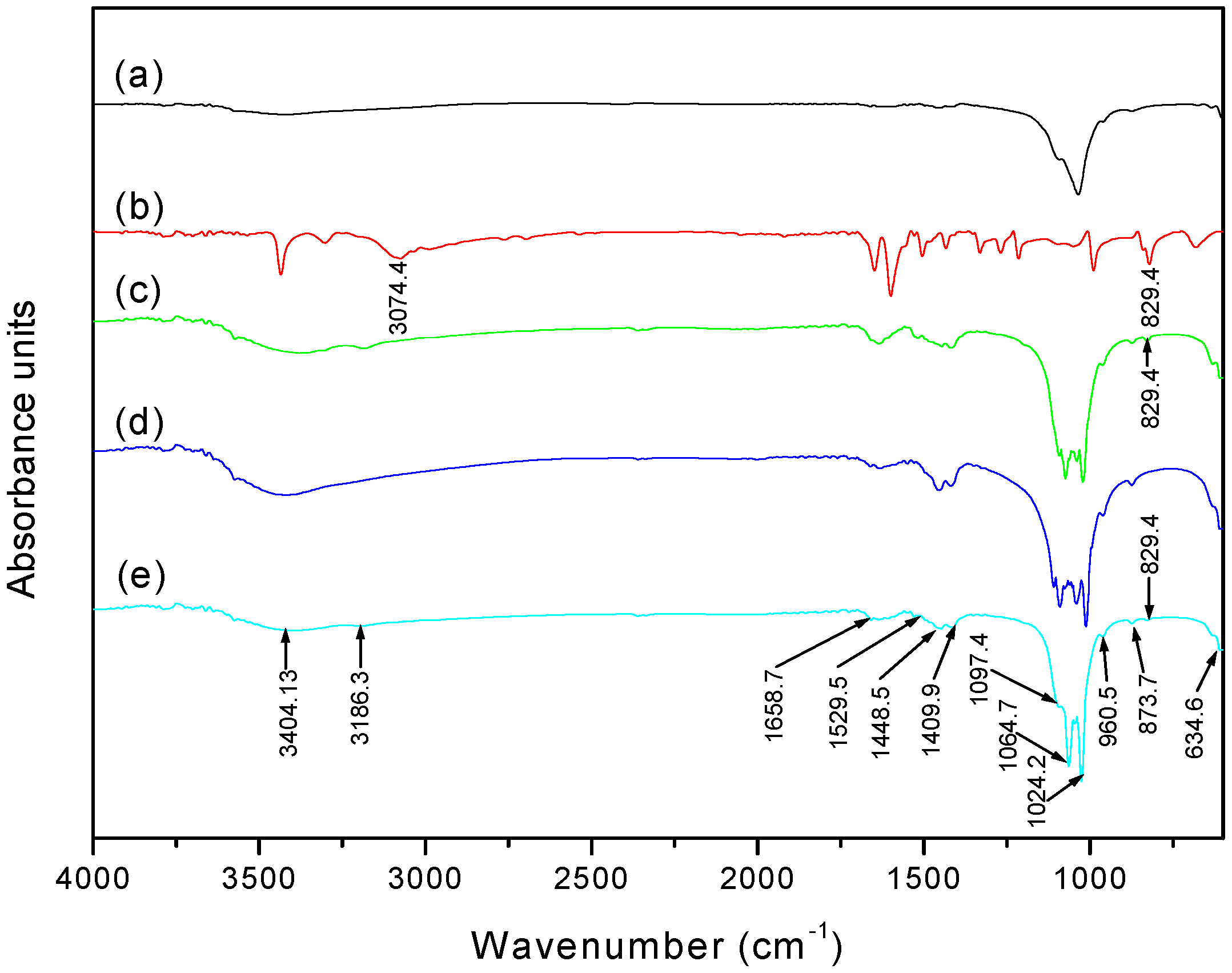
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.4. Scanning Electron Microscopy (SEM)/Energy Dispersive X-ray (EDX)
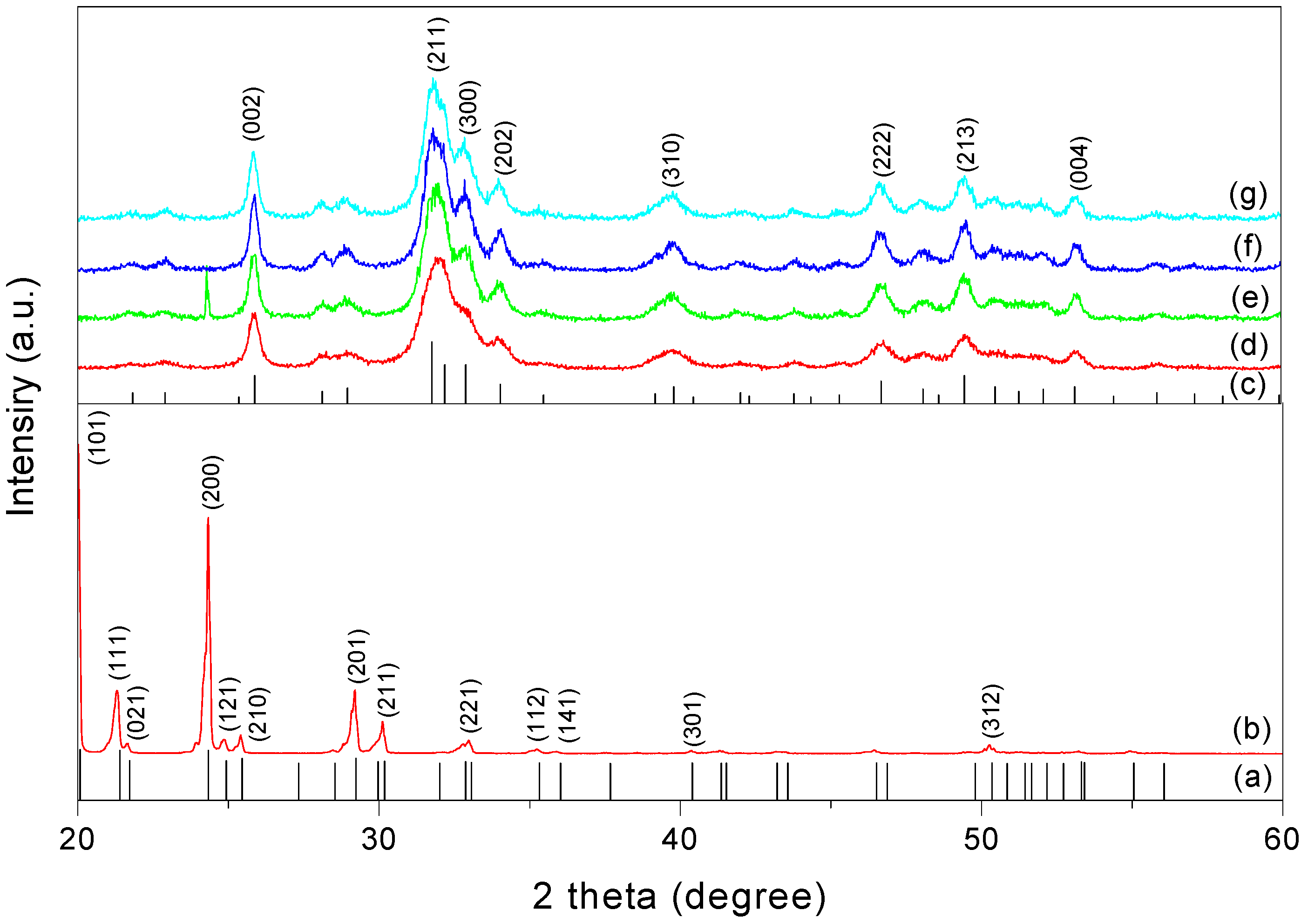
3.5. X-ray Powder Diffraction (XRPD)
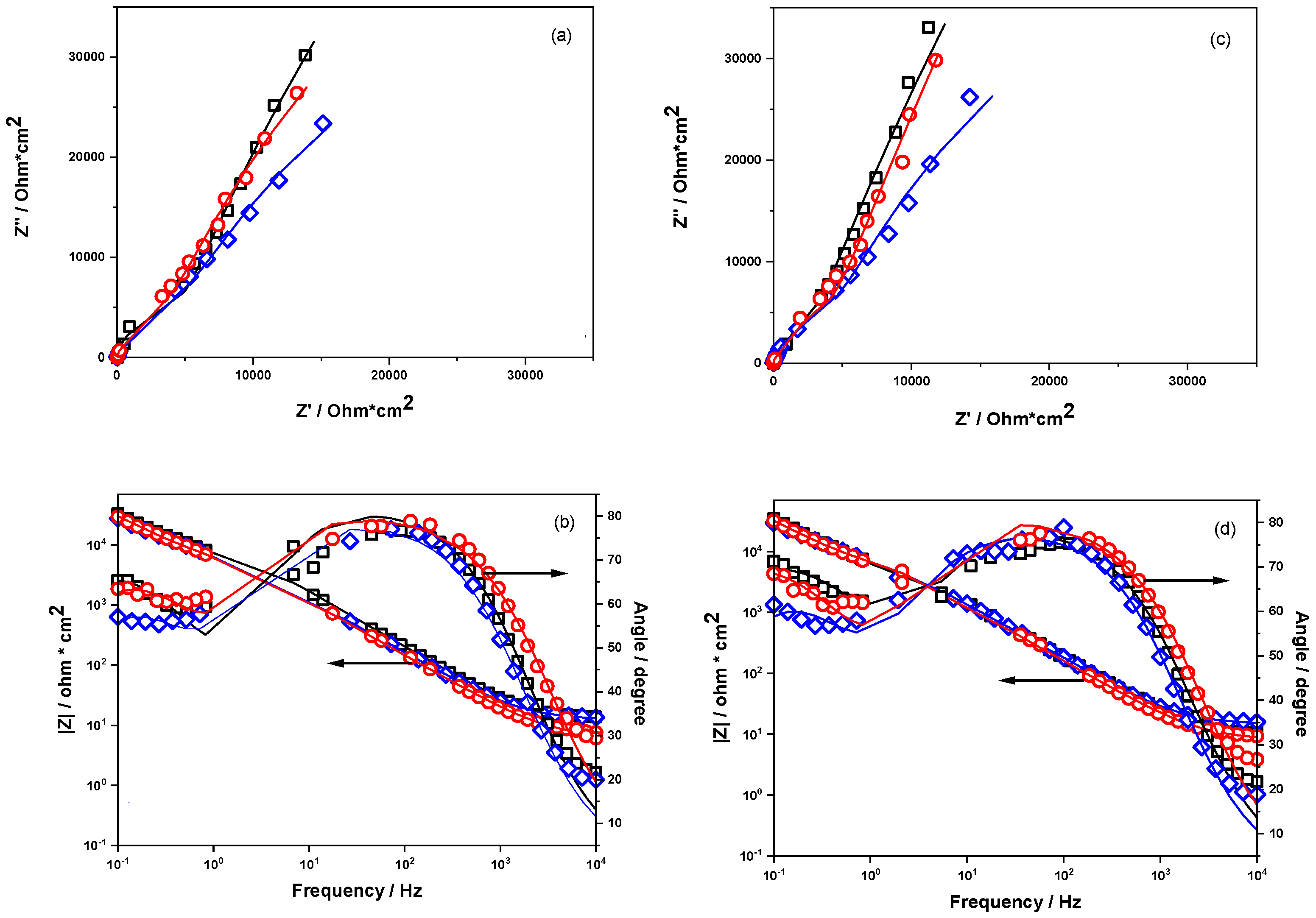
3.6. Electrochemical Impedance Spectroscopy Measurements
3.7. Investigation of Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Maleki, S. Biomedicine & Pharmacotherapy Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including den-tistry, cancer treatment and bone repair. Compos. Part B 2021, 215, 108790. [Google Scholar] [CrossRef]
- Taha, M.A.; Youness, R.A.; Ibrahim, M. Biocompatibility, physico-chemical and mechanical properties of hydroxyapatite-based silicon dioxide nanocomposites for biomedical applications. Ceram. Int. 2020, 46, 23599–23610. [Google Scholar] [CrossRef]
- Gan, C.-D.; Jia, Y.-B.; Yang, J.-Y. Remediation of fluoride contaminated soil with nano-hydroxyapatite amendment: Response of soil fluoride bioavailability and microbial communities. J. Hazard. Mater. 2021, 405, 124694. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, H.; Li, J.; Zhao, H.; Wang, L. Polydopamine modified ultrathin hydroxyapatite nanosheets for anti-corrosion reinforcement in polymeric coatings. Corros. Sci. 2021, 178, 109064. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef] [PubMed]
- Geuli, O.; Metoki, N.; Zada, T.; Reches, M.; Eliaz, N.; Mandler, D. Synthesis, coating, and drug-release of hydroxyapatite nanoparticles loaded with antibiotics. J. Mater. Chem. B 2017, 5, 7819–7830. [Google Scholar] [CrossRef]
- Öner, M.; Yetiz, E.; Ay, E.; Uysal, U. Ibuprofen release from porous hydroxyapatite tablets. Ceram. Int. 2011, 37, 2117–2125. [Google Scholar] [CrossRef]
- Barabás, R.; Farkas, N.-I.; Nagy, C.L.; Cadar, O.; Moisa, C.; Bizo, L. Adsorption and desorption behavior of natural and synthetic active compounds on hydroxyapatite-based nanocomposites. Ceram. Int. 2021, 47, 8584–8592. [Google Scholar] [CrossRef]
- Barabás, R.; de Sousa Ávila, E.; Ladeira, L.O.; Antônio, L.M.; Tötös, R.; Simedru, D.; Bizo, L.; Cadar, O. Graphene Oxides/Carbon Nanotubes-Hydroxyapatite Nanocomposites for Biomedical Applications. Arab. J. Sci. Eng. 2020, 45, 219–227. [Google Scholar] [CrossRef]
- Bogya, E.S.; Barabas, R.; Bizo, L.; Dejeu, V.R. Preparation and characterization of silicate hydroxyapatites used for copper sorption. In Proceedings of the 11th ECERS Conference, Krakow, Poland, 21–25 June 2009; pp. 1109–1113. [Google Scholar]
- Czikó, M.; Bogya, E.S.; Paizs, C.; Katona, G.; Konya, Z.; Kukovecz, Á.; Barabás, R. Albumin adsorption study onto hydroxyap-atite-multiwall carbon nanotube based composites. Mater. Chem. Phys. 2016, 180, 314–325. [Google Scholar] [CrossRef]
- Barabas, R.; Rigo, M.; Sarkozi, M.; Hoaghia, M.-A.; Cadar, O. Hydroxyapatite–Carbon Nanotube Composites for Drug Delivery Applications. Braz. J. Chem. Eng. 2019, 36, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubbu, A.P.S.P.G.D. Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 2018, 7, 289–296. [Google Scholar]
- Barabás, R.; Muntean, N.; Szabó, G.; Maurer, K.; Bizo, L. Preparation and characterizations of new biomaterials by anthocyanins adsorption on hydroxyapatite-based materials. Studia UBB Chem. 2017, 62, 253–268. [Google Scholar] [CrossRef]
- Jani, A.T.; Haghighi, N.B.; Pour, M.S.H.; Aminian, M.; Molzemi, S. Hydroxyapatite incorporation into MCM-41 and study of ibuprofen drug release. J. Aust. Ceram. Soc. 2019, 56, 653–661. [Google Scholar] [CrossRef]
- Bogya, E.S. Kinetic and Equilibrium Studies of Retention Processes on Apatitic Materials. Ph.D. Thesis, Babeș-Bolyai University of Cluj-Napoca, Cluj-Napoca, Romania, 2010. [Google Scholar]
- Mostafa, N.Y.; Hassan, H.M.; Elkader, O.A. Preparation and Characterization of Na+, SiO44−, and CO32− Co-Substituted Hydroxyapatite. J. Am. Ceram. Soc. 2010, 94, 1584–1590. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Prestidge, C.A. Enhancing oral bioavailability of poorly soluble drugs with mesoporous silica based systems: Opportunities and challenges. Drug Dev. Ind. Pharm. 2018, 45, 349–358. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.J.; Cao, S.W.; Chen, F. Hierachically nanostructured mesoporous spheres of calcium silicate hydrate: Surfactant-free sonochemical synthesis and drug-delivery system with ultrahigh drug-loading capacity. Adv. Mater. 2010, 22, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, H.; Nourbakhsh, A.A.; Karbasi, S.; Javadkalbasi, R.; Rafienia, M.; Nourbakhsh, N.; Bonakdar, S.; MacKenzie, K.J.D. In-vestigation on bioactivity and cytotoxicity of mesoporous nano-composite MCM-48/hydroxyapatite for ibuprofen drug delivery. Ceram. Int. 2014, 40, 7355–7362. [Google Scholar] [CrossRef]
- Dos Apostolos, R.C.R.; Andrade, G.F.; Silva, W.; Gomes, D.D.A.; Miranda, M.; De Sousa, E.M.B. Hybrid polymeric systems of mesoporous silica/hydroxyapatite nanoparticles applied as antitumor drug delivery platform. Int. J. Appl. Ceram. Technol. 2019, 16, 1836–1849. [Google Scholar] [CrossRef]
- Sousa, A.; Souza, K.; Sousa, E. Mesoporous silica/apatite nanocomposite: Special synthesis route to control local drug delivery. Acta Biomater. 2008, 4, 671–679. [Google Scholar] [CrossRef]
- Albrecht, P.; Bjørnå, I.K.; Brassat, D.; Farrell, R.; Feys, P.; Hobart, J.; Hupperts, R.; Linnebank, M.; Magdič, J.; Oreja-Guevara, C.; et al. Prolonged-release fampridine in multiple sclerosis: Clinical data and real-world experience. Report of an expert meeting. Ther. Adv. Neurol. Disord. 2018, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rommer, P.S.; Weber, M.S.; Illes, Z.; Zettl, U.K. Editorial: Multiple Sclerosis—From Bench to Bedside: Currents Insights Into Pathophysiological Concepts and Their Potential Impact on Patients. Front. Immunol. 2020, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Hospira, Z.A. EMA Assessment report Fampyra. Report ARZ 2012, 44, 1–91. [Google Scholar]
- Department of Health and Ageing of Australian Government. Australian Public Assessment Report for Fampridine (Fampyra); Department of Health and Ageing of Australian Government: Canberra, Australia, 2011.
- Dunn, J.; Blight, A. Dalfampridine: A brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Curr. Med. Res. Opin. 2011, 27, 1415–1423. [Google Scholar] [CrossRef]
- Malhotra, M.; Ghai, P.; Narasimhan, B.; Deep, A. Dalfampridine: Review on its recent development for symptomatic improvement in patients with multiple sclerosis. Arab. J. Chem. 2016, 9, S1443–S1449. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Shandilya, S.; Bharti, A.; Agarwal, A. A stability indicating simultaneous dual wavelength UV–HPLC method for the determination of potential impurities in fampridine active pharmaceutical ingredient. J. Pharm. Biomed. Anal. 2012, 58, 136–140. [Google Scholar] [CrossRef]
- Zope, M.V.; Patel, R.M.; Patel, A.; Patel, S.G. Development and validation of a stability indicating rp-hplc method for the determination of potential degradation products of difluprednate in ophthalmic emulsion. Int. J. Pharm. Pharm. Sci. 2018, 10, 79–86. [Google Scholar] [CrossRef]
- Dharani, N.; Padmini, K.; Sumakala, S. Stability indicating RP-HPLC method development and validation for estimation of dalfampridine in its bulk and formulation. Int. J. Adv. Res. 2016, 4, 184–191. [Google Scholar] [CrossRef] [Green Version]
- U.S.P. Convention. United States Pharmacopeia/National Formulary (USP 42-NF 37); U.S.P. Convention: Rockville, MD, USA, 2017. [Google Scholar]
- Kovács, B.; Boda, F.; Fülöp, I.; Székely-Szentmiklósi, I.; Kelemen, É.K.; Kovács-Deák, B.; Székely-Szentmiklósi, B. HPLC method development for fampridine using Analytical Quality by Design approach. Acta Pharm. 2020, 70, 465–482. [Google Scholar] [CrossRef]
- Smith, W.; Swan, S.; Marbury, T.; Henney, H. Single-Dose Pharmacokinetics of Sustained-Release Fampridine (Fampridine-SR) in Healthy Volunteers and Adults with Renal Impairment. J. Clin. Pharmacol. 2010, 50, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Achanti, S.; Katta, R.R. High-throughput liquid chromatography tandem mass spectrometry method for simultaneous determination of fampridine, paroxetine, and quinidine in rat plasma: Application to in vivo perfusion study. J. Food Drug Anal. 2016, 24, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Suneetha, A.; Raja, R.K. Comparison of LC-UV and LC–MS methods for simultaneous determination of teriflunomide, dimethyl fumarate and fampridine in human plasma: Application to rat pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Redasani, V.K.; Shaikh, G.L.; Surana, S.S. Development and validation of spectroscopic methods for the estimation of dalfampridine in bulk and in tablet formulation. Anal. Chem. Indian J. 2014, 14, 37–41. [Google Scholar]
- Jain, V.; Dehariya, R.; Parkhe, G. Method development and validation for estimation of dalfampridine in synthetic mixture by using UV spectrophotometry and RP-HPLC. J. Pharm. Pharmacol. 2019, 1, 84–87. [Google Scholar]
- U.S. Food and Drug Administration. Dissolution Methods, (n.d.). Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/dissolution-methods-database (accessed on 8 August 2021).
- Padash, R.; Sobhani, A.; Rahimi-Nasrabadi, M.; Mirmotahari, M.; Ehrlich, H.; Rad, A.S.; Peyravi, M. Is it possible to use X12Y12 (X = Al, B, and Y = N, P) nanocages for drug-delivery systems? A DFT study on the adsorption property of 4-aminopyridine drug. Appl. Phys. A 2018, 124, 582. [Google Scholar] [CrossRef]
- Akyuz, S.; Akyuz, T. Adsorption of 3- and 4-aminopyridine on loughlinite—An IR spectroscopic study. Vib. Spectrosc. 2010, 53, 136–139. [Google Scholar] [CrossRef]
- Szcześ, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, S.; Nanda, S.S.; Yi, D.K. Hydroxyapatite-alginate Based Matrices for Drug Delivery. Curr. Pharm. Des. 2019, 25, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Romero, L.D.; Oropeza-Guzman, M.T.; López-Maldonado, E.A.; Iglesias, A.L.; Paz-González, J.A.; Ng, T.; Serena-Gómez, E.; Villarreal-Gómez, L.J. Synthetic hydroxyapatite and its use in bioactive coatings. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018817463. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.-R.; Li, X.; Zhou, Y.; Huang, Y.; Chen, W.; Wang, H.; Li, J. Synthesis of Spongy-Like Mesoporous Hydroxyapatite from Raw Waste Eggshells for Enhanced Dissolution of Ibuprofen Loaded via Supercritical CO2. Int. J. Mol. Sci. 2015, 16, 7960–7975. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Wang, T.; Wang, J.; Zheng, L.; Jiang, T.; Cheng, G.; Wang, S. Template-directed hydrothermal synthesis of hydroxyapatite as a drug delivery system for the poorly water-soluble drug carvedilol. Appl. Surf. Sci. 2011, 257, 10126–10133. [Google Scholar] [CrossRef]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine Towards Cancer: 111In-Labeled Nanoparticles. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Chacón, C.; Bruno-Colmenárez, J.; Jamalis, J.; Delgado, G.E. Synthesis, thermal studies and crystal structure of 4-aminopyridinium semi-oxalate hemihydrate, Scientific Study & Research. Chem. Chem. Eng. 2017, 18, 111–119. [Google Scholar]
- Barabás, R.; Deemter, D.; Katona, G.; Batin, G.; Barabás, L.; Bizo, L.; Cadar, O. Comparative study on physicochemical and me-chanical characterization of new nanocarbon-based hydroxyapatite nanocomposites. Turk. J. Chem. 2019, 43, 809–824. [Google Scholar] [CrossRef]
- Skwarek, E.; Janusz, W.; Sternik, D. Adsorption of citrate ions on hydroxyapatite synthetized by various methods. J. Radioanal. Nucl. Chem. 2014, 299, 2027–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Kim, H.; Mondal, S.; Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Oh, J. Optimized Zn-doped hydroxyapatite/doxorubicin bioceramics system for efficient drug delivery and tissue engineering application. Ceram. Int. 2018, 44, 6062–6071. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Brantley, W.A.; Choe, H.C. AC impedance behavior of silicon-hydroxyapatite doped film on the Ti-35Nb-xZr alloy by EB-PVD method. Surf. Coat. Technol. 2013, 228, S505–S510. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Choe, H.-C. AC impedance behaviors of electrochemically deposited Si-hydroxyapatite films on nanotube-formed Ti-Nb-Zr alloys. J. Nanosci. Nanotechnol. 2014, 14, 9014–9029. [Google Scholar] [CrossRef]
- Turdean, G.L.; Craciun, A.; Popa, D.; Constantiniuc, M. Study of electrochemical corrosion of biocompatible Co–Cr and Ni–Cr dental alloys in artificial saliva. Influence of pH of the solution. Mater. Chem. Phys. 2019, 233, 390–398. [Google Scholar] [CrossRef]
- Souto, R.M.; Laz, M.M.; Reis, R.L. Degradation characteristics of hydroxyapatite coatings on orthopaedic TiAlV in simulated physiological media investigated by electrochemical impedance spectroscopy. Biomaterials 2003, 24, 4213–4221. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.T.H.; Fort, I.C.; Pham, T.H.; Turdean, G.L. Ordered Mesoporous Silica Incorporating Platinum Nanoparticles as Electrode Material for Paracetamol Detection. Electroanalytics 2021, 33, 323–335. [Google Scholar]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Shanaghi, A.; Mehrjou, B.; Ahmadian, Z.; Souri, A.R.; Chu, P.K. Enhanced corrosion resistance, antibacterial properties, and biocompatibility by hierarchical hydroxyapatite/ciprofloxacin-calcium phosphate coating on nitrided NiTi alloy. Mater. Sci. Eng. C 2021, 118, 111524. [Google Scholar] [CrossRef]



| Processes | HAP | 4AP | HAP_4AP_25 | |||
|---|---|---|---|---|---|---|
| T (°C) | Weight Loss (wt %) | T (°C) | Weight Loss (wt %) | T (°C) | Weight Loss (wt %) | |
| Dehydration and dehydroxylation | 30–190 | 3.51 | - | - | 30–150 | 2.78 |
| Melting of 4AP | - | - | 120–180 | 11.79 | - | - |
| Elimination of 4AP | - | - | 180–225 | 87.85 | - | - |
| CO32− elimination | 200–400 | 2.1 | - | - | 150–315 | 5.11 |
| Elimination of H2O from the pores | 390–500 | 0.55 | - | - | 315–540 | 3.14 |
| Decomposition of Cax(PO4)y(OH)z Reaction of P2O74− and OH− | 500–1000 | 1.47 | - | - | 540–1000 | 1.39 |
| Circuit Parameters | 5 mM K3[Fe(CN)]6/K4[Fe(CN)]6 + 0.1 M KCl Solution, pH 7 | SBF Solution, pH 7 | ||||
|---|---|---|---|---|---|---|
| HAP | HAP_ 4AP_25 | Si–HAP_ 4AP_25 | HAP | HAP_ 4AP_25 | Si–HAP_ 4AP_25 | |
| Rsol/ ohm·m2 | 12.54 ±5.15 | 12.38 ±4.05 | 6.26 ±4.92 | 11.83 ±2.93 | 14.68 ±4.01 | 8.07 ±5.58 |
| Qcoat/ S·sn/cm2 | 4.36 × 10−5 ±5.02 | 3.01 × 10−5 ±21.62 | 3.17 × 10−5 ±31.3 | 4.2 × 10−5 ±2.44 | 2.81 × 10−5 ±9.97 | 2.52 × 10−5 ±33.79 |
| n | 0.8430 | 0.9177 | 0.9745 | 0.8405 | 0.9065 | 1 |
| Rcoat/ ohm·cm2 | 3.99 × 105 ±10.41 | 4534 ±7.17 | 3003 ±32.8 | 7.79 ×105 ±10.39 | 4186 ±6.42 | 3130 ±30.31 |
| Qct/ S·sn/cm2 | 1.55 × 10−5 ±23.44 | 5.38 × 10−5 ±13.24 | 4.88 × 10−5 ±5.26 | 2.64 × 10−5 ±15.17 | 4.89 × 10−5 ±8.69 | 4.60 × 10−5 ±6.37 |
| n | 1 | 0.8631 | 0.8483 | 0.9673 | 0.8719 | 0.8421 |
| Rct/ ohm·cm2 | 3.8 × 103 ±19.8 | 1.04 × 105 ±5.64 | 1.88 × 105 ±4.45 | 2.16 × 103 ±23.56 | 1.26 × 105 ±6.36 | 6.78 × 105 ±20.02 |
| Ccoat/F·cm−2 | 7.41 × 10−5 | 2.51 × 10−5 | 2.98 × 10−5 | 8.13 × 10−5 | 2.25 × 10−5 | 2.53 × 10−5 |
| χ2 | 9.25 × 10−3 | 4.14 × 10−3 | 5.22 × 10−3 | 2.87 × 10−3 | 5.71 × 10−3 | 8.06 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marincaș, L.; Turdean, G.L.; Toșa, M.; Kovács, Z.; Kovács, B.; Barabás, R.; Farkas, N.-I.; Bizo, L. Hydroxyapatite and Silicon-Modified Hydroxyapatite as Drug Carriers for 4-Aminopyridine. Crystals 2021, 11, 1124. https://doi.org/10.3390/cryst11091124
Marincaș L, Turdean GL, Toșa M, Kovács Z, Kovács B, Barabás R, Farkas N-I, Bizo L. Hydroxyapatite and Silicon-Modified Hydroxyapatite as Drug Carriers for 4-Aminopyridine. Crystals. 2021; 11(9):1124. https://doi.org/10.3390/cryst11091124
Chicago/Turabian StyleMarincaș, Laura, Graziella Liana Turdean, Monica Toșa, Zsolt Kovács, Béla Kovács, Réka Barabás, Noémi-Izabella Farkas, and Liliana Bizo. 2021. "Hydroxyapatite and Silicon-Modified Hydroxyapatite as Drug Carriers for 4-Aminopyridine" Crystals 11, no. 9: 1124. https://doi.org/10.3390/cryst11091124
APA StyleMarincaș, L., Turdean, G. L., Toșa, M., Kovács, Z., Kovács, B., Barabás, R., Farkas, N.-I., & Bizo, L. (2021). Hydroxyapatite and Silicon-Modified Hydroxyapatite as Drug Carriers for 4-Aminopyridine. Crystals, 11(9), 1124. https://doi.org/10.3390/cryst11091124









