Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study
Abstract
1. Introduction
2. Methods
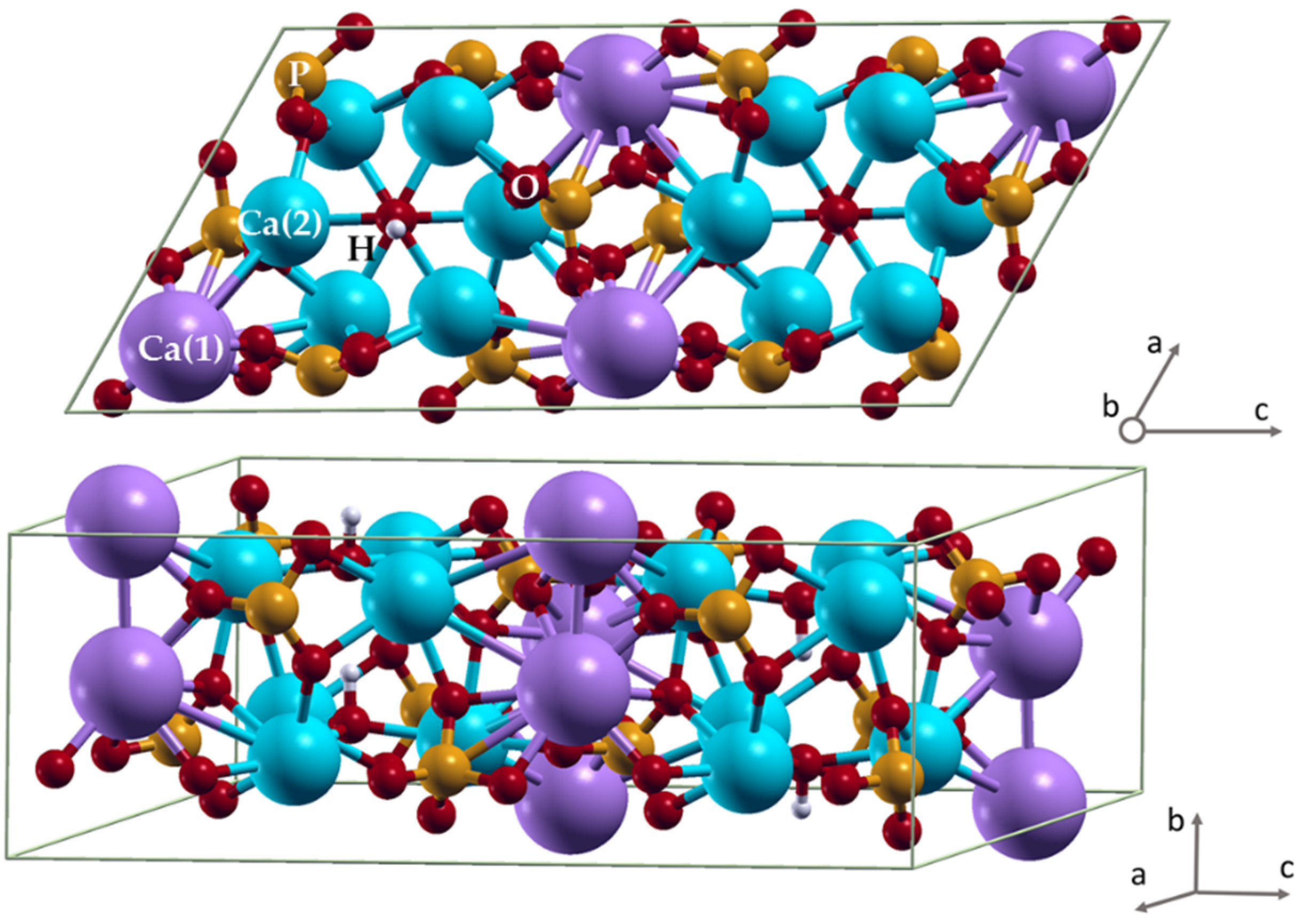
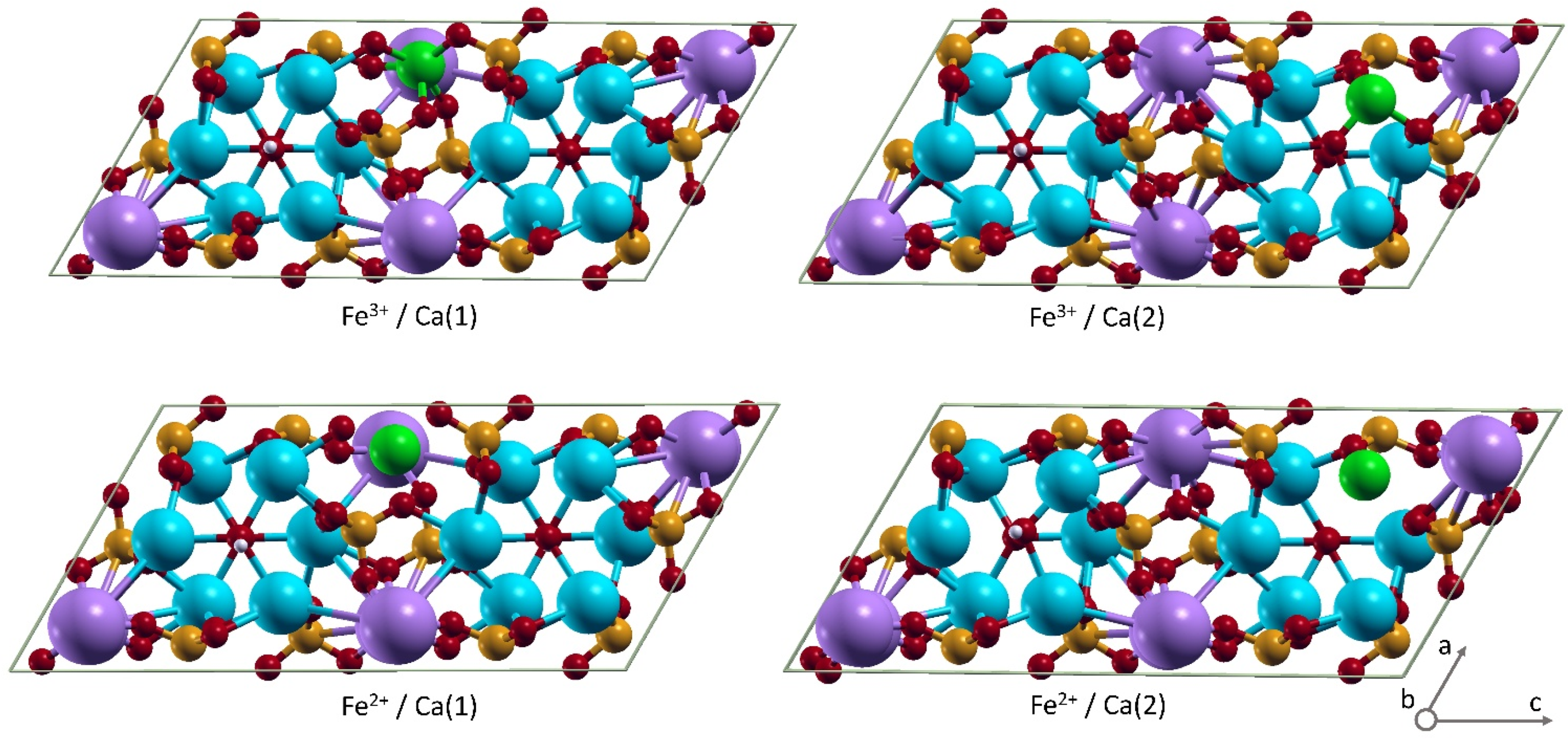
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zahmakıran, M.; Román-Leshkov, Y.; Zhang, Y. Rhodium (0) nanoparticles supported on nanocrystalline hydroxyapatite: Highly effective catalytic system for the solvent-free hydrogenation of aromatics at room temperature. Langmuir 2012, 28, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Vakh, C.; Kuzmin, A.; Sadetskaya, A.; Bogdanova, P.; Voznesenskiy, M.; Osmolovskaya, O.; Bulatov, A. Cobalt-doped hydroxyapatite nanoparticles as a new eco-friendly catalyst of luminol–H2O2 based chemiluminescence reaction: Study of key factors, improvement the activity and analytical application. Spectrochim. Acta Part A Mol. BioMol. Spectrosc. 2020, 237, 118382. [Google Scholar] [CrossRef]
- Gadipelly, C.; Deshmukh, G.; Mannepalli, L.K. Transition Metal Exchanged Hydroxyapatite/Fluorapatite Catalysts for C− C and C− N Bond Forming Reactions. Chem. Rec. 2021, 21, 1398–1416. [Google Scholar]
- Salavati-Niasari, M.; Hasanalian, J.; Najafian, H. Alumina-supported FeCl3, MnCl2, CoCl2, NiCl2, CuCl2, and ZnCl2 as catalysts for the benzylation of benzene by benzyl chloride. J. Mol. Catal. A Chem. 2004, 209, 209–214. [Google Scholar] [CrossRef]
- Benaichouba, B.; Bussiere, P.; Vedrine, J.C. In-situ Mössbauer spectroscopic study of iron site evolution in iron and cobalt molybdates catalysts in propene oxidation reaction conditions. Appl. Catal. A Gen. 1995, 130, 31–45. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Rostovshchikova, T.N.; Smirnov, V.V.; Kiseleva, O.I.; Maksimov, Y.V.; Suzdalev, I.P.; Ikorskii, V.N. Structure and size effects in Catal. by immobilized nanoclusters of iron oxides. Catal. Today 2005, 105, 634–640. [Google Scholar]
- Hayashi, H.; Chen, L.Z.; Tago, T.; Kishida, M.; Wakabayashi, K. Catalytic properties of Fe/SiO2 catalysts prepared using microemulsion for CO hydrogenation. Appl. Catal. A Gen. 2002, 231, 81–89. [Google Scholar] [CrossRef]
- Kuhrs, C.; Arita, Y.; Weiss, W.; Ranke, W.; Schlögl, R. Understanding heterogeneous Catal. on an atomic scale: A combined surface science and reactivity investigation for the dehydrogenation of ethylbenzene over iron oxide catalysts. Top. Catal. 2000, 14, 111–123. [Google Scholar]
- Derbyshire, F.; Hager, T. Coal liquefaction and catalysis. Fuel 1994, 73, 1087–1092. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Lakhova, A.; Petrov, S.; Ibragimova, D.; Kayukova, G.; Safiulina, A.; Shinkarev, A.; Okekwe, R. Aquathermolysis of Heavy Oil Using Nano Oxides of Metals. J. Pet. Sci. Eng. 2017, 153, 385–390. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.M.; Selvaraj, R.; Pugazhendhi, A. A review on the synthesis of hydroxyapatite, its composites and adsorptive removal of pollutants from wastewater. J. Water Process Eng. 2020, 38, 101574. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Sun, W.; Xia, C. A Magnetically Recyclable Heterogeneous Catalyst: Cobalt Nano-Oxide Supported on Hydroxyapatite-Encapsulated γ-Fe2O3 Nanocrystallites for Highly Efficient Olefin Oxidation with H2O2. Catal. Commun. 2008, 10, 237–242. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and anionic substitutions in hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Bucharest, Romania, 2016; pp. 145–211. [Google Scholar] [CrossRef]
- Zilm, M.E.; Chen, L.; Sharma, V.; McDannald, A.; Jain, M.; Ramprasad, R.; Wei, M. Hydroxyapatite substituted by transition metals: Experiment and theory. Phys. Chem. Chem. Phys. 2016, 18, 16457–16465. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Kaneda, K.; Mizugaki, T. Development of concerto metal catalysts using apatite compounds for green organic syntheses. Energy Environ. Sci. 2009, 2, 655–673. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Dardir, F.M.; Shaban, M.; Ahmed, E.A.; Soliman, M.F. Spongy Ni/Fe carbonate-fluorapatite catalyst for efficient conversion of cooking oil waste into biodiesel. Environ. Chem. Lett. 2018, 16, 665–670. [Google Scholar] [CrossRef]
- Valizadeh, S.; Rasoulifard, M.H.; Dorraji, M.S. Modified Fe3O4-hydroxyapatite nanocomposites as heterogeneous catalysts in three UV, Vis and Fenton like degradation systems. Appl. Surface Sci. 2014, 319, 358–366. [Google Scholar] [CrossRef]
- Moriguchi, T.; Sakamoto, Y.; Nakagawa, S. Heterogeneous photo-Fenton reaction of a herbicide atrazine by using Fe (III)-treated hydroxyapatites. Phosphorus Res. Bull. 2013, 28, 10–23. [Google Scholar] [CrossRef][Green Version]
- Campisi, S.; Galloni, M.; Marchetti, S.G.; Auroux, A.; Postole, G.; Gervasini, A. Functionalized Iron Hydroxyapatite as Eco-friendly Catalyst for NH3-SCR Reaction: Activity and Role of Iron Speciation on the Surface. ChemCatChem 2020, 12, 1676–1690. [Google Scholar] [CrossRef]
- Galloni, M.G.; Campisi, S.; Marchetti, S.G.; Gervasini, A. Environmental Reactions of Air-Quality Protection on Eco-Friendly Iron-Based Catalysts. Catalysts 2020, 10, 1415. [Google Scholar] [CrossRef]
- Gibert, O.; Valderrama, C.; Martínez, M.M.; Darbra, R.M.; Moncunill, J.O.; Martí, V. Hydroxyapatite Coatings on Calcite Powder for the Removal of Heavy Metals from Contaminated Water. Water 2021, 13, 1493. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Gafurov, M.R.; Murzakhanov, F.F.; Fomin, A.S.; Antonova, O.S.; Khairutdinova, D.R.; Pyataev, A.V.; Makshakova, O.N.; Konovalov, A.A.; Leonov, A.V.; et al. Mesoporous Iron (III)-Doped Hydroxyapatite Nanopowders Obtained via Iron Oxalate. Nanomaterials 2021, 11, 811. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Akopyan, A.V.; Gafurov, M.R.; Makshakova, O.N.; Donskaya, N.O.; Fomin, A.S.; Polikarpova, P.P.; Anisimov, A.V.; Murzakhanov, F.F.; Leonov, A.V.; et al. Iron-Doped Mesoporous Powders of Hydroxyapatite as Molybdenum-Impregnated Catalysts for Deep Oxidative Desulfurization of Model Fuel: Synthesis and Experimental and Theoretical Studies. J. Phys. Chem. C 2021, 125, 11604–11619. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Monteiro, F.J.; Laranjeira, M.S. Duality of iron (III) doped nano hydroxyapatite in triple negative breast cancer monitoring and as a drug-free therapeutic agent. Ceram. Int. 2020, 46, 16590–16597. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Moço, A.; Ferreira, J.; Coimbra, S.; Costa, E.; Santos-Silva, A.; Monteiro, F.J. Different hydroxyapatite magnetic nanoparticles for medical imaging: Its effects on hemostatic, hemolytic activity and cellular cytotoxicity. Colloids Surfaces B Biointerfaces 2016, 146, 363–374. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Gamal, G.A.; Al-Mufadi, F.A.; Said, A.H. Effect of iron additives on the microstructure of hydroxyapatite. Eng. Technol. Appl. Sci. Res. 2013, 3, 532–539. [Google Scholar] [CrossRef]
- Sarath Chandra, V.; Baskar, G.; Suganthi, R.V.; Elayaraja, K.; Ahymah Joshy, M.I.; Sofi Beaula, W.; Mythili, R.; Venkatraman, G.; Narayana Kalkura, S. Blood Compatibility of Iron-Doped Nanosize Hydroxyapatite and Its Drug Release. Acs Appl. Mater. Interfaces 2012, 4, 1200–1210. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Intrinsically Superparamagnetic Fe-Hydroxyapatite Nanoparticles Positively Influence Osteoblast-like Cell Behaviour. J. Nanobiotechnol. 2012, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, R.; Rodriguez-Lorenzo, L.M.; Gross, K.A. Influence of ferrous iron incorporation on the structure of hydroxyapatite. J. Mater. Sci. Mater. Med. 2005, 16, 387–392. [Google Scholar] [CrossRef]
- Nogueira, R.E.F.Q.; Graca, M.P.F.; Valente, M.A.; Sombra, A.S.B.; Silva, C.C. Structural and mechanical study of the sintering effect in hydroxyapatite doped with iron oxide. Phys. B Condens. Matter 2008, 403, 3826–3829. [Google Scholar]
- Li, Y.; Nam, C.T.; Ooi, C.P. Iron (III) and manganese (II) substituted hydroxyapatite nanoparticles: Characterization and cytotoxicity analysis. J. Phys. Conf. Ser. 2009, 187, 012024. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Goldberg, M.; Gafurov, M.; Makshakova, O.; Smirnov, V.; Komlev, V.; Barinov, S.; Kudryavtsev, E.; Sergeeva, N.; Achmedova, S.; Mamin, G.; et al. Influence of Al on the structure and in vitro behavior of hydroxyapatite nanopowders. J. Phys. Chem. B 2019, 123, 9143–9154. [Google Scholar] [CrossRef]
- Yashima, M.; Yonehara, Y.; Fujimori, H. Experimental visualization of chemical bonding and structural disorder in hydroxyapatite through charge and nuclear-density analysis. J. Phys. Chem. C 2011, 115, 25077–25087. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Combination of EPR measurements and DFT calculations to study nitrate impurities in the carbonated nanohydroxyapatite. J. Phys. Chem. A 2014, 118, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Jiang, M.; Terra, J.; Rossi, A.M.; Morales, M.A.; Saitovitch, E.B.; Ellis, D.E. Fe2+/Fe3+ substitution in hydroxyapatite: Theory and experiment. Phys. Rev. B 2002, 66, 224107. [Google Scholar] [CrossRef]
| Iron | Substitution | Energy, Ry | Cell Volume, Å3 | Fe–O, Å | Ca–O, Å |
|---|---|---|---|---|---|
| Fe2+ | Ca(1) | −3514.79383 | 1060.7 | 2.347 | 2.386 |
| 2.360 | 2.417 | ||||
| 2.389 | 2.444 | ||||
| 2.459 | 2.467 | ||||
| 2.523 | 2.499 | ||||
| 2.743 | 2.595 | ||||
| Fe2+ | Ca(2) | −3514.80879 | 1067.1 | 1.994 | 2.329 |
| 2.042 | 2.354 | ||||
| 2.051 | 2.373 | ||||
| 2.216 | 2.405 | ||||
| 2.814 | 2.595 | ||||
| 2.689 | |||||
| Fe3+ | Ca(1) | −3513.64081 | 1067.1 | 2.056 | 2.386 |
| 2.058 | 2.417 | ||||
| 2.111 | 2.444 | ||||
| 2.124 | 2.467 | ||||
| 2.132 | 2.499 | ||||
| 2.595 | |||||
| Fe3+ | Ca(2) | −3513.7161776 | 1064.7 | 1.803 | 2.373 |
| 2.107 | 2.595 | ||||
| 2.068 | 2.689 | ||||
| 2.240 | 2.329 | ||||
| 2.259 | 2.354 | ||||
| 2.405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makshakova, O.N.; Shurtakova, D.V.; Vakhin, A.V.; Grishin, P.O.; Gafurov, M.R. Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study. Crystals 2021, 11, 1219. https://doi.org/10.3390/cryst11101219
Makshakova ON, Shurtakova DV, Vakhin AV, Grishin PO, Gafurov MR. Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study. Crystals. 2021; 11(10):1219. https://doi.org/10.3390/cryst11101219
Chicago/Turabian StyleMakshakova, Olga Nikolaevna, Daria Vladimirovna Shurtakova, Alexey Vladimirovich Vakhin, Peter Olegovich Grishin, and Marat Revgerovich Gafurov. 2021. "Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study" Crystals 11, no. 10: 1219. https://doi.org/10.3390/cryst11101219
APA StyleMakshakova, O. N., Shurtakova, D. V., Vakhin, A. V., Grishin, P. O., & Gafurov, M. R. (2021). Incorporation of Iron(II) and (III) in Hydroxyapatite—A Theoretical Study. Crystals, 11(10), 1219. https://doi.org/10.3390/cryst11101219








