Exploring High-Pressure Transformations in Low-Z (H2, Ne) Hydrates at Low Temperatures
Abstract
:1. Introduction
2. Experimental Details and Methods of Analysis
3. Results
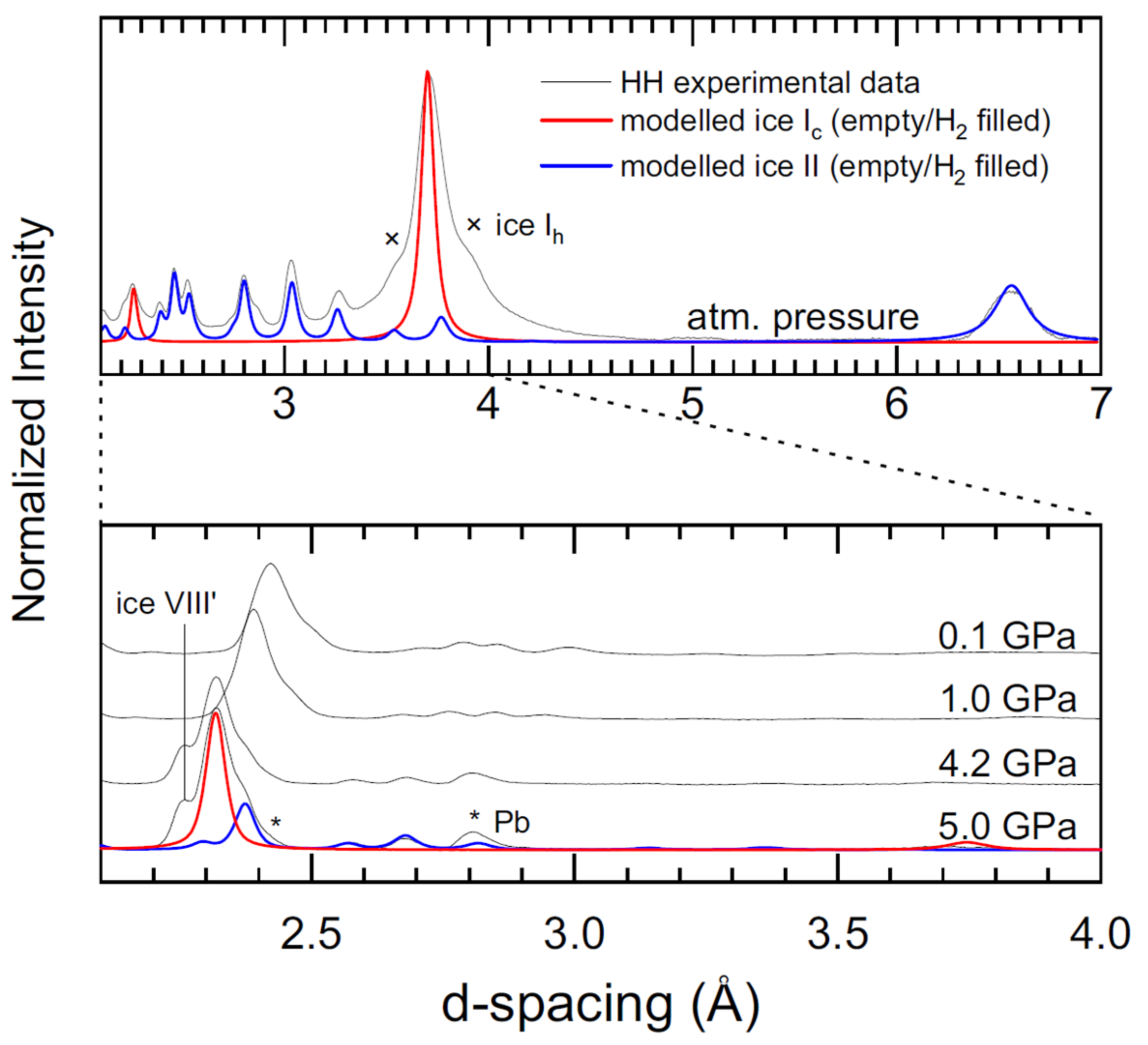
3.1. CS-II HH and NeH Starting Materials
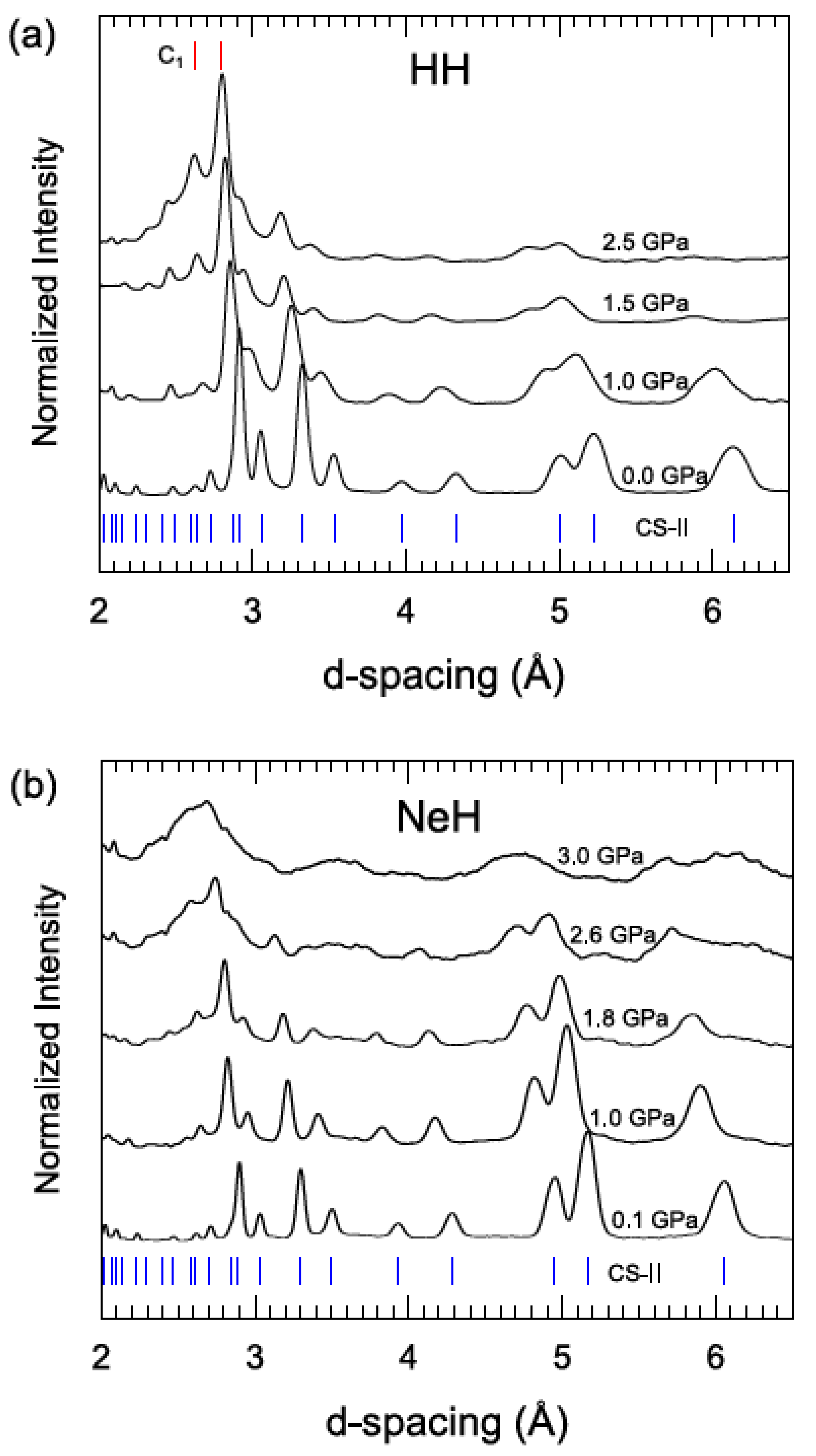
3.2. Compression of CS-II HH and NeH at ~95 K
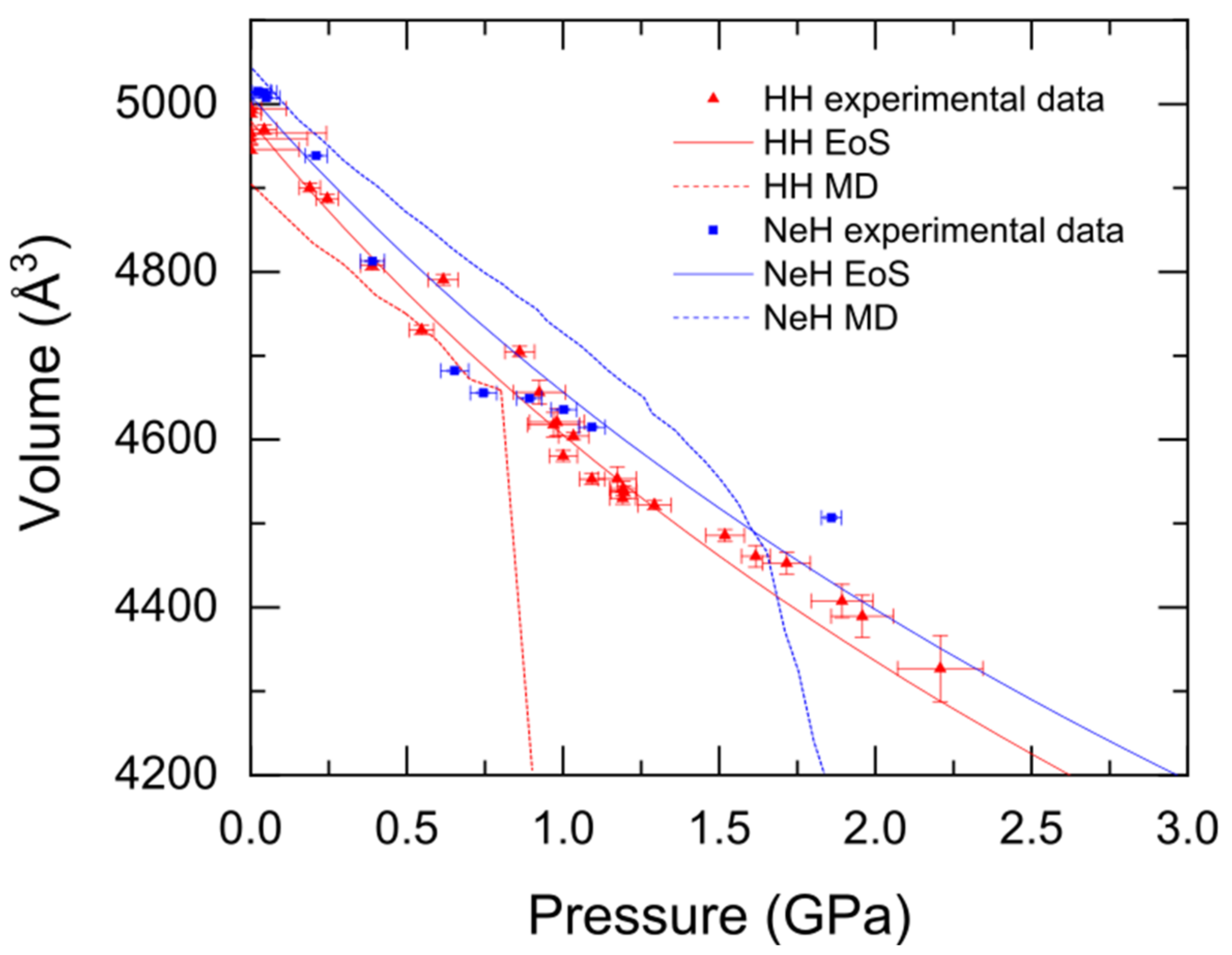
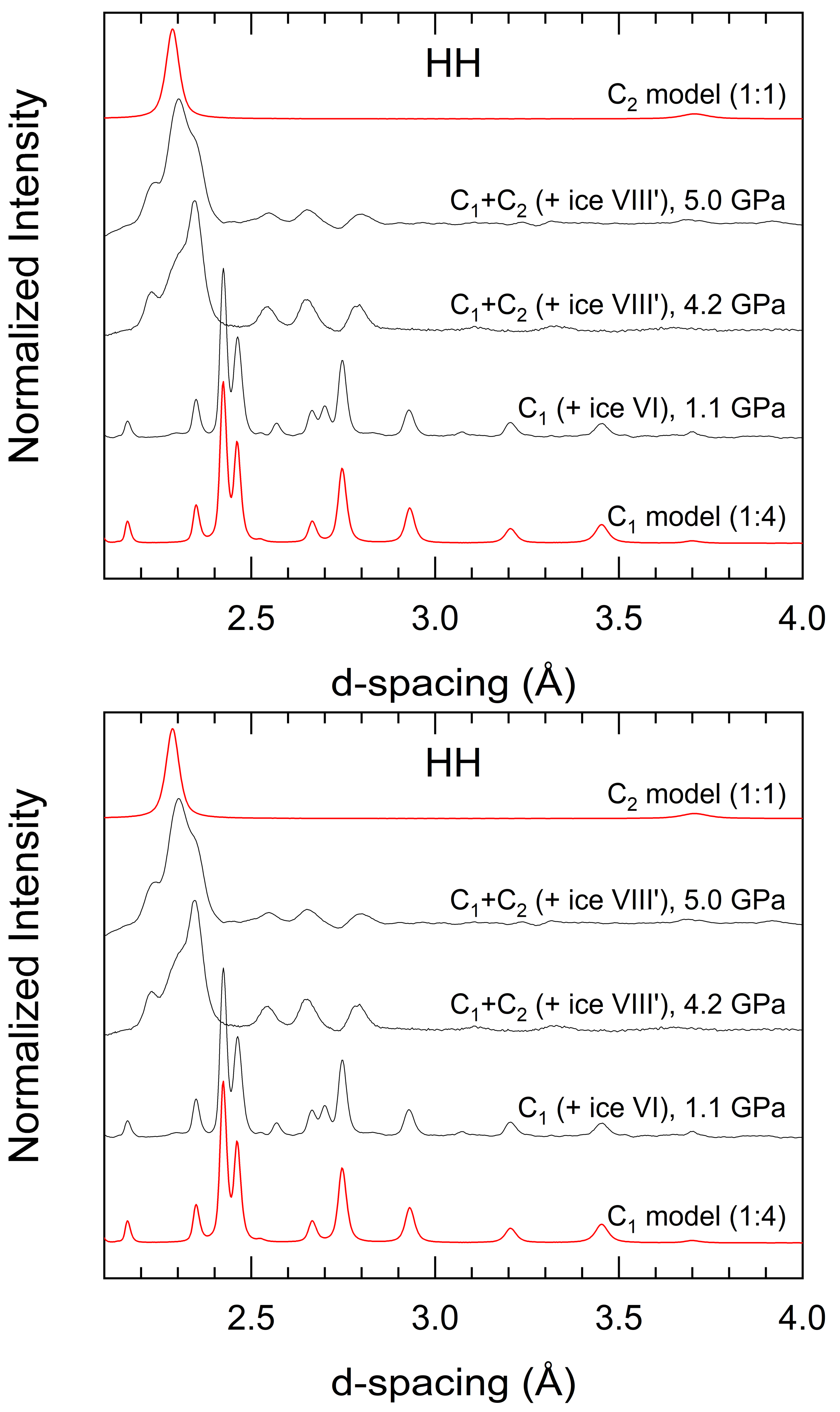
3.3. Formation, Compressibility and Decompression Behavior of C1 and C2 HH Phases
3.4. Synthesis of C2 NeH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sloan, E.D., Jr.; Koh, C.A.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429129148. [Google Scholar]
- Van der Waals, J.H.; Platteeuw, J.C. Clathrate Solutions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–57. [Google Scholar]
- Loveday, J.S.; Nelmes, R.J. High-pressure gas hydrates. Phys. Chem. Chem. Phys. 2008, 10, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Dyadin, Y.A.; Larionov, E.G.; Manakov, A.Y.; Zhurko, F.V.; Aladko, E.Y.; Mikina, T.V.; Komarov, V.Y. Clathrate hydrates of hydrogen and neon. Mendeleev Commun. 1999, 9, 209–210. [Google Scholar] [CrossRef]
- Lokshin, K.A.; Zhao, Y. Fast synthesis method and phase diagram of hydrogen clathrate hydrate. Appl. Phys. Lett. 2006, 88, 131909. [Google Scholar] [CrossRef]
- Lokshin, K.A.; Zhao, Y.; He, D.; Mao, W.L.; Mao, H.-K.K.; Hemley, R.J.; Lobanov, M.V.; Greenblatt, M. Structure and dynamics of hydrogen molecules in the novel clathrate hydrate by high pressure neutron diffraction. Phys. Rev. Lett. 2004, 93, 125503. [Google Scholar] [CrossRef]
- Falenty, A.; Hansen, T.C.; Kuhs, W.F. Formation and properties of ice XVI obtained by emptying a type sII clathrate hydrate. Nature 2014, 516, 231–233. [Google Scholar] [CrossRef]
- Bozhko, Y.Y.; Subbotin, O.S.; Fomin, V.M.; Belosludov, V.R.; Kawazoe, Y. Theoretical investigation of structures, compositions, and phase transitions of neon hydrates based on ices Ih and II. J. Eng. Thermophys. 2014, 23, 20–26. [Google Scholar] [CrossRef]
- Trinh, T.T.; Waage, M.H.; Van Erp, T.S.; Kjelstrup, S. Low barriers for hydrogen diffusion in sII clathrate. Phys. Chem. Chem. Phys. 2015, 17, 13808–13812. [Google Scholar] [CrossRef] [Green Version]
- Burnham, C.J.; English, N.J. Free-energy calculations of the intercage hopping barriers of hydrogen molecules in clathrate hydrates. J. Phys. Chem. C 2016, 120, 16561–16567. [Google Scholar] [CrossRef]
- Burnham, C.J.; Futera, Z.; English, N.J. Quantum and classical inter-cage hopping of hydrogen molecules in clathrate hydrate: Temperature and cage-occupation effects. Phys. Chem. Chem. Phys. 2017, 19, 717–728. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Hansen, T.C.; Falenty, A. Filling Ices with Helium and the Formation of Helium Clathrate Hydrate. J. Phys. Chem. Lett. 2018, 9, 3194–3198. [Google Scholar] [CrossRef]
- Londono, D.; Finney, J.L.; Kuhs, W.F. Formation, stabilty, and structure of helium hydrate at high pressure. J. Chem. Phys. 1992, 97, 547–552. [Google Scholar] [CrossRef]
- Londono, D.; Kuhs, W.F.; Finney, J.L. Enclathration of helium in ice II: The first helium hydrate. Nature 1988, 332, 141–142. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, J.; Du, S.; Xu, H.; Vogel, S.C.; Han, J.; Germann, T.C.; Zhang, J.; Jin, C.; Francisco, J.S.; et al. Crystal structure and encapsulation dynamics of ice II-structured neon hydrate. Proc. Natl. Acad. Sci. USA 2014, 111, 10456–10461. [Google Scholar] [CrossRef] [Green Version]
- Vos, W.L.; Finger, L.W.; Hemley, R.J.; Mao, H. Novel H2-H2O Clathrates at High Pressures. Phys. Rev. Lett. 1993, 71, 3150–3153. [Google Scholar] [CrossRef]
- Machida, S.; Hirai, H.; Kawamura, T.; Yamamoto, Y.; Yagi, T. Structural changes of filled ice Ic structure for hydrogen hydrate under high pressure. J. Chem. Phys. 2008, 129, 224505. [Google Scholar] [CrossRef]
- Brant Carvalho, P.H.B.; Mace, A.; Bull, C.L.; Funnell, N.P.; Tulk, C.A.; Andersson, O.; Häussermann, U. Elucidation of the pressure induced amorphization of tetrahydrofuran clathrate hydrate. J. Chem. Phys. 2019, 150, 204506. [Google Scholar] [CrossRef] [Green Version]
- Brant Carvalho, P.H.B.; Mace, A.; Andersson, O.; Tulk, C.A.; Molaison, J.; Lyubartsev, A.P.; Nangoi, I.M.; Leitão, A.A.; Häussermann, U. Pressure-induced amorphization of noble gas clathrate hydrates. Phys. Rev. B 2021, 103, 064205. [Google Scholar] [CrossRef]
- Amann-Winkel, K.; Böhmer, R.; Fujara, F.; Gainaru, C.; Geil, B.; Loerting, T. Colloquium: Water’s controversial glass transitions. Rev. Mod. Phys. 2016, 88, 011002. [Google Scholar] [CrossRef]
- Besson, J.M.; Nelmes, R.J.; Hamel, G.; Loveday, J.S.; Weill, G.; Hull, S. Neutron powder diffraction above 10 GPa. Phys. B Condens. Matter 1992, 180–181, 907–910. [Google Scholar] [CrossRef]
- Strässle, T.; Klotz, S.; Kunc, K.; Pomjakushin, V.; White, J.S. Equation of state of lead from high-pressure neutron diffraction up to 8.9 GPa and its implication for the NaCl pressure scale. Phys. Rev. B 2014, 90, 014101. [Google Scholar] [CrossRef] [Green Version]
- Arnold, O.; Bilheux, J.C.; Borreguero, J.M.; Buts, A.; Campbell, S.I.; Chapon, L.; Doucet, M.; Draper, N.; Ferraz Leal, R.; Gigg, M.A.; et al. Mantid—Data analysis and visualization package for neutron scattering and μ SR experiments. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2014, 764, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Matsumoto, M.; Yagasaki, T.; Tanaka, H. GenIce: Hydrogen-Disordered Ice Generator. J. Comput. Chem. 2018, 39, 61–64. [Google Scholar] [CrossRef]
- Alavi, S.; Ripmeester, J.A.; Klug, D.D. Molecular-dynamics study of structure II hydrogen clathrates. J. Chem. Phys. 2005, 123, 24507. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. Available online: http://lammps.sandia.gov for informatio (accessed on 15 November 2021). [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Harada, A.; Arman, Y.; Miura, S. Molecular dynamics study on fast diffusion of hydrogen molecules in filled ice II. J. Mol. Liq. 2019, 292, 111316. [Google Scholar] [CrossRef]
- Melchionna, S.; Ciccotti, G.; Holian, B.L. Hoover npt dynamics for systems varying in shape and size. Mol. Phys. 1993, 78, 533–544. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Hockney, R.; Eastwood, J. Computer Simulation Using Particles, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9780367806934. [Google Scholar]
- Abascal, J.L.F.; Sanz, E.; Fernández, R.G.; Vega, C. A potential model for the study of ices and amorphous water: TIP4P/Ice. J. Chem. Phys. 2005, 122, 234511. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Economou, I.G.; Stubos, A.K. Influence of combining rules on the cavity occupancy of clathrate hydrates by Monte Carlo simulations. Mol. Phys. 2014, 112, 2258–2274. [Google Scholar] [CrossRef]
- Strobel, T.A.; Somayazulu, M.; Hemley, R.J. Phase behavior of H2 + H2O at high pressures and low temperatures. J. Phys. Chem. C 2011, 115, 4898–4903. [Google Scholar] [CrossRef]
- Salzmann, C.G. Advances in the experimental exploration of water’s phase diagram. J. Chem. Phys. 2019, 150, 190901. [Google Scholar] [CrossRef]
- Ranieri, U.; Koza, M.M.; Kuhs, W.F.; Gaal, R.; Klotz, S.; Falenty, A.; Wallacher, D.; Ollivier, J.; Gillet, P.; Bove, L.E. Quantum Dynamics of H2 and D2 Confined in Hydrate Structures as a Function of Pressure and Temperature. J. Phys. Chem. C 2019, 123, 1888–1903. [Google Scholar] [CrossRef]
- MacDonald, J.R. Review of some experimental and analytical equations of state. Rev. Mod. Phys. 1969, 41, 316–349. [Google Scholar] [CrossRef]
- Manakov, A.Y.; Ogienko, A.G.; Tkacz, M.; Lipkowski, J.; Stoporev, A.S.; Kutaev, N.V. High-pressure gas hydrates of argon: Compositions and equations of state. J. Phys. Chem. B 2011, 115, 9564–9569. [Google Scholar] [CrossRef]
- Katsumasa, K.; Koga, K.; Tanaka, H. On the thermodynamic stability of hydrogen clathrate hydrates. J. Chem. Phys. 2007, 127, 044509. [Google Scholar] [CrossRef] [Green Version]
- Kuzovnikov, M.A.; Tkacz, M. T-P-X Phase Diagram of the Water-Hydrogen System at Pressures up to 10 kbar. J. Phys. Chem. C 2019, 123, 3696–3702. [Google Scholar] [CrossRef]
- Kamb, B.; Hamilton, W.C.; LaPlaca, S.J.; Prakash, A. Ordered Proton Configuration in Ice II, from Single-Crystal Neutron Diffraction. J. Chem. Phys. 1971, 55, 1934–1945. [Google Scholar] [CrossRef]
- Komatsu, K.; Machida, S.; Noritake, F.; Hattori, T.; Sano-Furukawa, A.; Yamane, R.; Yamashita, K.; Kagi, H. Ice Ic without stacking disorder by evacuating hydrogen from hydrogen hydrate. Nat. Commun. 2020, 11, 464. [Google Scholar] [CrossRef] [Green Version]
- Fortes, A.D.; Wood, I.G.; Alfredsson, M.; Vočadlo, L.; Knight, K.S. The incompressibility and thermal expansivity of D2O ice II determined by powder neutron diffraction. J. Appl. Crystallogr. 2005, 38, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Bertie, J.E.; Calvert, L.D.; Whalley, E. Transformations of Ice II, Ice III, and Ice V at Atmospheric Pressure. J. Chem. Phys. 1963, 38, 840–846. [Google Scholar] [CrossRef]
- Fuentes-Landete, V.; Rasti, S.; Schlögl, R.; Meyer, J.; Loerting, T. Calorimetric Signature of Deuterated Ice II: Turning an Endotherm to an Exotherm. J. Phys. Chem. Lett. 2020, 11, 8268–8274. [Google Scholar] [CrossRef]
- Brant Carvalho, P.H.B.; Moraes, P.I.R.; Leitão, A.A.; Andersson, O.; Tulk, C.A.; Molaison, J.; Lyubartsev, A.P.; Häussermann, U. Structural investigation of three distinct amorphous forms of Ar hydrate. RSC Adv. 2021, 11, 30744–30754. [Google Scholar] [CrossRef]
- Hakim, L.; Koga, K.; Tanaka, H. Novel neon-hydrate of cubic ice structure. Phys. A Stat. Mech. Appl. 2010, 389, 1834–1838. [Google Scholar] [CrossRef]
- Jorgensen, J.D.; Worlton, T.G. Disordered structure of D2O ice VII from in situ neutron powder diffraction. J. Chem. Phys. 1985, 83, 329–333. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brant Carvalho, P.H.B.; Mace, A.; Nangoi, I.M.; Leitão, A.A.; Tulk, C.A.; Molaison, J.J.; Andersson, O.; Lyubartsev, A.P.; Häussermann, U. Exploring High-Pressure Transformations in Low-Z (H2, Ne) Hydrates at Low Temperatures. Crystals 2022, 12, 9. https://doi.org/10.3390/cryst12010009
Brant Carvalho PHB, Mace A, Nangoi IM, Leitão AA, Tulk CA, Molaison JJ, Andersson O, Lyubartsev AP, Häussermann U. Exploring High-Pressure Transformations in Low-Z (H2, Ne) Hydrates at Low Temperatures. Crystals. 2022; 12(1):9. https://doi.org/10.3390/cryst12010009
Chicago/Turabian StyleBrant Carvalho, Paulo H. B., Amber Mace, Inna Martha Nangoi, Alexandre A. Leitão, Chris A. Tulk, Jamie J. Molaison, Ove Andersson, Alexander P. Lyubartsev, and Ulrich Häussermann. 2022. "Exploring High-Pressure Transformations in Low-Z (H2, Ne) Hydrates at Low Temperatures" Crystals 12, no. 1: 9. https://doi.org/10.3390/cryst12010009





