Tribological Performance of Microcrystalline Diamond (MCD) and Nanocrystalline Diamond (NCD) Coating in Dry and Seawater Environment
Abstract
:1. Introduction
2. Materials and Methods
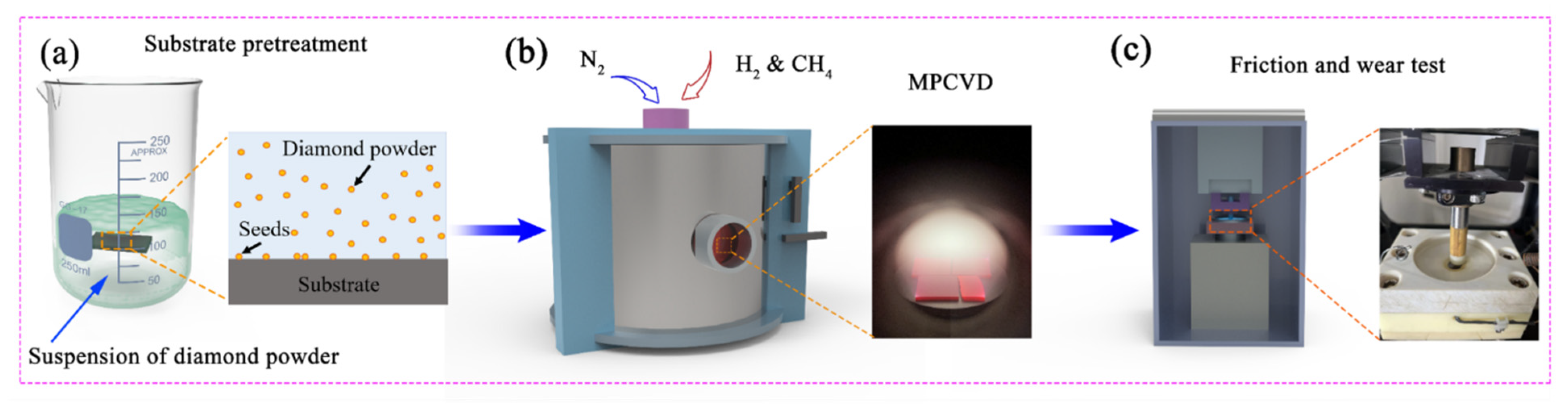
2.1. Preparation of Diamond Coatings
2.2. Friction and Wear Test
2.3. Characterization of Diamond Coatings
3. Results
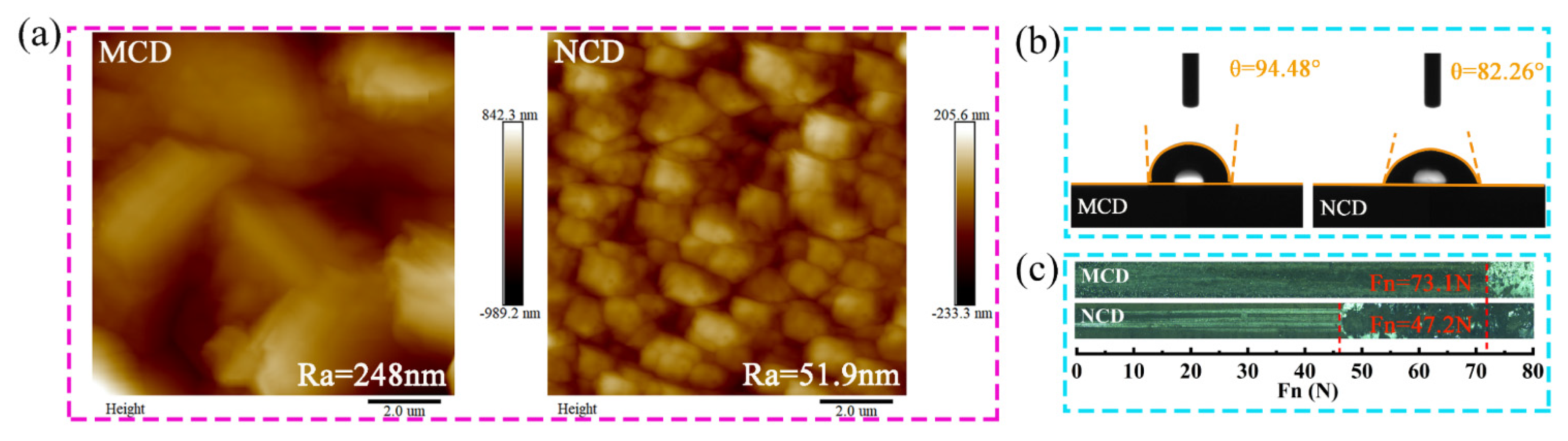
3.1. Characterization of As-Deposited Diamond Coatings
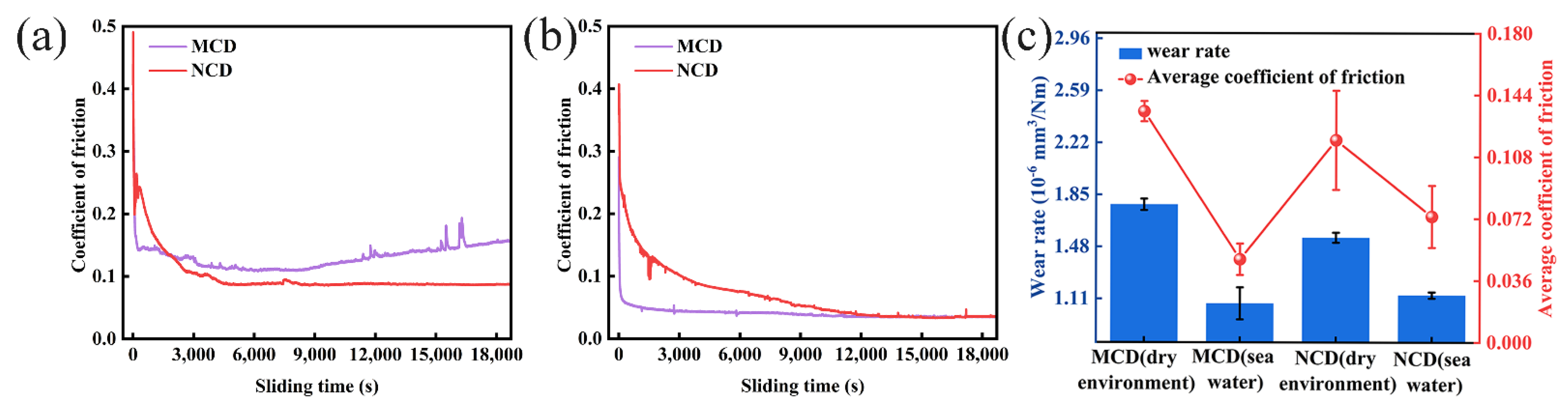
3.2. Tribological Performance of MCD and NCD Coatings
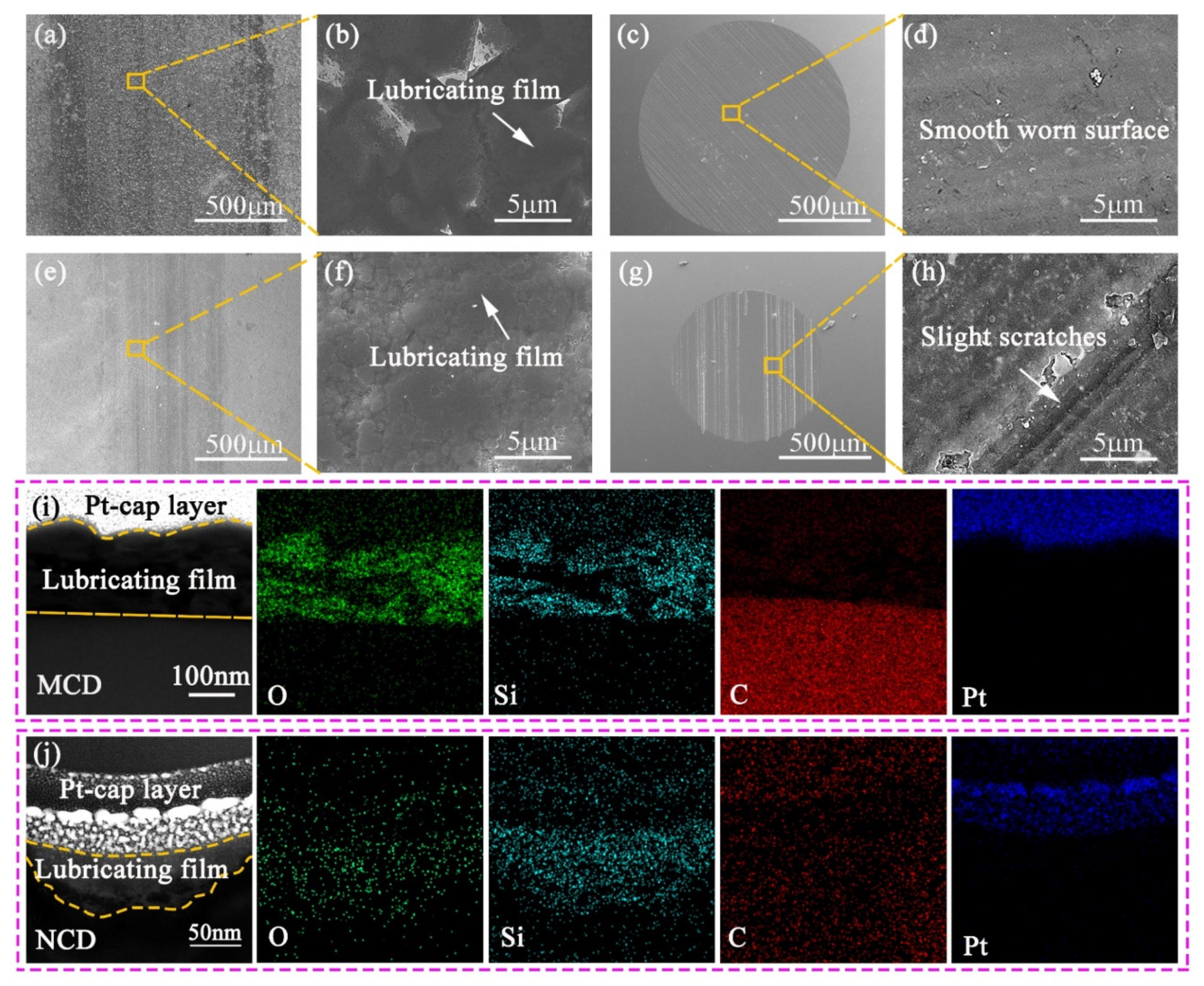
3.3. Friction and Wear Analysis of Diamond Coatings in Dry Environment
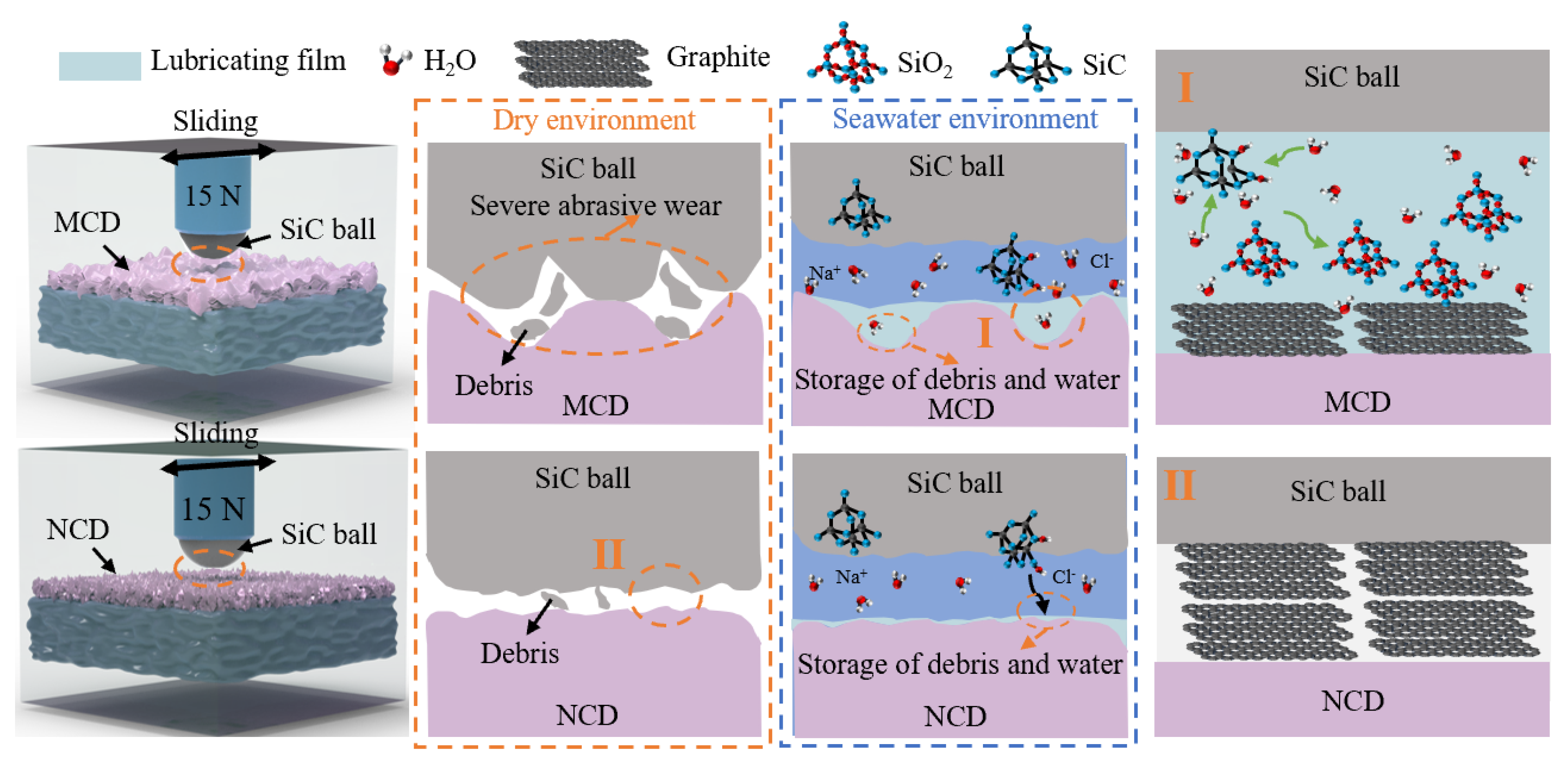
3.4. The Influence of Seawater Environment on Friction Behavior of Diamond Coatings
4. Conclusions
- (1)
- Continuous and similar thicknesses of MCD and NCD coatings were successfully deposited by MPCVD under different deposition parameters, which eliminates the great influence of the thicknesses on the properties of tribological performance. Both MCD and NCD coatings have good bonding properties.
- (2)
- The main wear mechanism of MCD coating and NCD coating in a dry friction environment is abrasive wear. The surface of the wear track of the NCD coating is smooth and the wear debris generated is small. Additionally, more sp2 and graphite phases are generated in the wear track due to frictional heat, which makes the NCD coating have better tribological performance in a dry friction environment.
- (3)
- Compared with the dry friction environment, the average COF of MCD and NCD coatings in the seawater environment are reduced 64.1% and 37.8%, respectively, and the wear rate is reduced by 39.5% and 26.5%, respectively. The main wear mechanism of the diamond coating is the SiO2 lubricating film generated by the tribochemical reaction. Moreover, the MCD coating is a synergistic lubrication effect consisting of graphite phase and SiO2 layer, resulting in a low average coefficient of friction and low wear.
- (4)
- Based on the discussion on the friction mechanism of MCD and NCD coatings on diamond coatings in a dry environment and seawater environment, the diamond coating has good tribological properties and corrosion resistance in a seawater environment, which provides guidance on protective coatings on the surface of sea-related friction parts.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gicquel, A.; Hassouni, K.; Silva, F.; Achard, J. CVD diamond films: From growth to applications. Curr. Appl. Phys. 2001, 1, 479–496. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Sun, F.; Shen, B. Tribological properties of MCD films synthesized using different carbon sources when sliding against stainless steel. Tribol. Lett. 2016, 61, 1–16. [Google Scholar] [CrossRef]
- Gaydaychuk, A.; Linnik, S. Tribological and mechanical properties of diamond films synthesized with high methane concentration. Int. J. Refract. Met. Hard Mater. 2019, 85, 105057. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Sun, F.; Zhang, T.; Shen, B. Investigations on the fabrication and erosion behavior of the composite diamond coated nozzles. Wear 2013, 304, 126–137. [Google Scholar] [CrossRef]
- Chandran, M.; Kumaran, C.R.; Dumpala, R.; Shanmugam, P.; Natarajan, R.; Bhattacharya, S.S.; Ramachandra Rao, M.S. Nanocrystalline diamond coatings on the interior of WC–Co dies for drawing carbon steel tubes: Enhancement of tube properties. Diamond Relat. Mater. 2014, 50, 33–37. [Google Scholar] [CrossRef]
- Perle, M.; Bareiss, C.; Rosiwal, S.M.; Singer, R.F. Generation and oxidation of wear debris in dry running tests of diamond coated SiC bearings. Diamond Relat. Mater. 2006, 15, 749–753. [Google Scholar] [CrossRef]
- Sochr, J.; Švorc, Ľ.; Rievaj, M.; Bustin, D. Electrochemical determination of adrenaline in human urine using a boron-doped diamond film electrode. Diamond Relat. Mater. 2014, 43, 5–11. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Sarangi, S.K.; Chattopadhyay, A.K. Effect of negative dc substrate bias on morphology and adhesion of diamond coating synthesised on carbide turning tools by modified HFCVD method. Appl. Surf. Sci. 2008, 255, 1661–1671. [Google Scholar] [CrossRef]
- Miyake, S.; Shindo, T.; Miyake, M. Friction properties of surface-modified polished chemical-vapor-deposited diamond films under boundary lubrication with water and poly-alpha olefin. Tribol. Int. 2016, 102, 287–296. [Google Scholar] [CrossRef]
- Ashcheulov, P.; Škoda, R.; Škarohlíd, J.; Taylor, A.; Fekete, L.; Fendrych, F.; Vega, R.; Shao, L.; Kalvoda, L.; Vratislav, S.; et al. Thin polycrystalline diamond films protecting zirconium alloys surfaces: From technology to layer analysis and application in nuclear facilities. Appl. Surf. Sci. 2015, 359, 621–628. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Gao, J.; Wang, X.; Shen, X.; Gao, J.; Sun, F. Consecutive deposition of amorphous SiO2 interlayer and diamond film on graphite by chemical vapor deposition. Carbon 2017, 117, 126–136. [Google Scholar] [CrossRef]
- Din, S.H.; Shah, M.A.; Sheikh, N.A.; Mursaleen Butt, M. CVD Diamond. Trans. Indian Inst. Met. 2019, 72, 1–9. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, S.; Li, Y.S.; Lu, X.; Hirose, A. NEXAFS characterization of nanocrystalline diamond thin films synthesized with high methane concentrations. Diamond Relat. Mater. 2007, 16, 730–734. [Google Scholar] [CrossRef]
- Fabisiak, K.; Torz-Piotrowska, R.; Staryga, E.; Szybowicz, M.; Paprocki, K.; Banaszak, A.; Popielarski, P. The influence of working gas on CVD diamond quality. Mater. Sci. Eng. C 2012, 177, 1352–1357. [Google Scholar] [CrossRef]
- Salgueiredo, E.; Amaral, M.; Neto, M.A.; Fernandes, A.J.S.; Oliveira, F.J.; Silva, R.F. HFCVD diamond deposition parameters optimized by a Taguchi Matrix. Vacuum 2011, 85, 701–704. [Google Scholar] [CrossRef]
- Shabani, M.; Abreu, C.S.; Gomes, J.R.; Silva, R.F.; Oliveira, F.J. Effect of relative humidity and temperature on the tribology of multilayer micro/nanocrystalline CVD diamond coatings. Diamond Relat. Mater. 2017, 73, 190–198. [Google Scholar] [CrossRef]
- Lei, X.; Shen, B.; Chen, S.; Wang, L.; Sun, F. Tribological behavior between micro-and nano-crystalline diamond films under dry sliding and water lubrication. Tribol. Int. 2014, 69, 118–127. [Google Scholar] [CrossRef]
- De Barros Bouchet, M.I.; Zilibotti, G.; Matta, C.; Clelia Righi, M.; Vandenbulcke, L.; Vacher, B.; Martin, J.M. Friction of diamond in the presence of water vapor and hydrogen gas. Coupling gas-phase lubrication and first-principles studies. J. Phys. Chem. C 2012, 116, 6966–6972. [Google Scholar] [CrossRef]
- Cui, Y.; He, Y.; Ji, C.; Lin, B.; Zhang, D. Anti-wear performance of polished microcrystalline diamond films sliding against Si3N4 under water lubrication. Surf. Rev. Lett. 2020, 27, 2050008. [Google Scholar] [CrossRef]
- Feng, Z.; Tzeng, Y.; Field, J.E. Friction of diamond on diamond in ultra-high vacuum and low-pressure environments. J. Phys. D Appl. Phys. 1992, 25, 1418. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, S.; Ji, Z.; Huang, Z.; Zhang, Z.; Shen, B. High-temperature wear behavior of micro-and ultrananocrystalline diamond films against titanium alloy. Surf. Coat. Technol. 2021, 422, 127537. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, H.; Ma, G.; Sun, L.; Guo, P.; Ke, P.; Lee, K.R.; Wang, A. Controllable defect engineering to enhance the corrosion resistance of Cr/GLC multilayered coating for deep-sea applications. Corros. Sci. 2022, 199, 110175. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Li, S.; Wang, R.; Guo, P.; Wang, A.; Ke, P. Accelerated deterioration mechanism of 316L stainless steel in NaCl solution under the intermittent tribocorrosion process. J. Mater. Sci. Technol. 2022, 121, 67–79. [Google Scholar] [CrossRef]
- Xu, X.; Guo, P.; Zuo, X.; Sun, L.; Li, X.; Lee, K.R.; Wang, A. Understanding the effect of Al/Ti ratio on the tribocorrosion performance of Al/Ti co-doped diamond-like carbon films for marine applications. Surf. Coat. Technol. 2020, 402, 126347. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, H.; Hua, Q.; Liao, B.; Ouyang, X. Tribocorrosion behaviors of superhard yet tough Ti-CN ceramic coatings. Surf. Coat. Technol. 2022, 439, 128448. [Google Scholar] [CrossRef]
- Dong, M.; Zhu, Y.; Xu, L.; Ren, X.; Ma, F.; Mao, F.; Li, J.; Wang, L. Tribocorrosion performance of nano-layered coating in artificial seawater. Appl. Surf. Sci. 2019, 487, 647–654. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.; Ye, C.; Du, C. Probing the tribocorrosion behaviors of three nickel-based superalloys in sodium chloride solution. Tribol. Int. 2022, 172, 107581. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Wang, X.; Sun, F. Effects of carbon concentration and gas pressure with hydrogen-rich gas chemistry on synthesis and characterizations of HFCVD diamond films on WC-Co substrates. Surf. Coat. Technol. 2021, 409, 126839. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Wang, H.; Song, X.; Wang, X.; Sun, F. Fabrication, tribological properties and cutting performances of high-quality multilayer graded MCD/NCD/UNCD coated PCB end mills. Diamond Relat. Mater. 2021, 118, 108505. [Google Scholar] [CrossRef]
- Klauser, F.; Steinmüller-Nethl, D.; Kaindl, R.; Bertel, E.; Memmel, N. Raman studies of nano-and ultra-nanocrystalline diamond films grown by hot-filament CVD. Chem. Vap. Depos. 2010, 16, 127–135. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, N.; Dhara, S.; Dash, S.; Bahuguna, A.; Kamruddin, M.; Tyagi, A.K.; Raj, B. Tribological properties of ultra nanocrystalline diamond film-effect of sliding counterbodies. Tribol. Int. 2012, 53, 167–178. [Google Scholar] [CrossRef]
- Kumar, N.; Kozakov, A.T.; Sankaran, K.J.; Sidashov, A.V.; Lin, I.N. Controlled atmosphere dependent tribological properties of thermally annealed ultrananocrystalline diamond films. Diamond Relat. Mater. 2019, 97, 107437. [Google Scholar] [CrossRef]
- Law, K.Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Song, H.; Ji, L.; Li, H.; Liu, X.; Zhou, H.; Liu, L.; Chen, J. Interface design for aC: H film with super long wear life in high vacuum environment. Tribol. Int. 2016, 95, 298–305. [Google Scholar] [CrossRef]
- Yan, G.; Wu, Y.; Cristea, D.; Liu, L.; Tierean, M.; Wang, Y.; Lu, F.; Wang, H.; Yuan, Z.; Munteanu, D.; et al. Mechanical properties and wear behavior of multi-layer diamond films deposited by hot-filament chemical vapor deposition. Appl. Surf. Sci. 2019, 494, 401–411. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Shankar, P.; Van Enckevort, W.J.P.; Schermer, J.J.; Ter Meulen, J.J. Adhesion analysis of polycrystalline diamond films on molybdenum by means of scratch, indentation and sand abrasion testing. Thin Solid Films 2005, 474, 186–196. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Y.; Zhang, X.; Luo, B. Effects of Superhydrophobic Surface on Tribological Properties:Mechanism, Status and Prospects. Prog. Chem. 2020, 32, 320. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Sun, F. Tribological behavior and cutting performance of monolayer, bilayer and multilayer diamond coated milling tools in machining of zirconia ceramics. Surf. Coat. Technol. 2018, 353, 49–57. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Phelps, A.W.; Zabinski, J.S.; Donley, M.S. Friction induced phase transfor-mation of pulsed laser deposited diamond-like carbon. Diamond Relat. Mater. 1996, 5, 1264–1269. [Google Scholar] [CrossRef]
- Kumar, N.; Sankaran, K.J.; Kozakov, A.T.; Sidashov, A.V.; Nicolskii, A.V.; Haenen, K.; Kolesnikov, V.I. Surface and bulk phase analysis of the tribolayer of nanocrystalline diamond films sliding against steel balls. Diamond Relat. Mater. 2019, 97, 107472. [Google Scholar] [CrossRef]
- Huang, K.; Hu, X.; Xu, H.; Shen, Y.; Khomich, A. The oxidization behavior and mechanical properties of ultrananocrystalline diamond films at high temperature annealing. Appl. Surf. Sci. 2014, 317, 11–18. [Google Scholar] [CrossRef]
- Erdemir, A.; Fenske, G.R.; Krauss, A.R.; Gruen, D.M.; McCauley, T.; Csencsits, R.T. Tribological properties of nanocrystalline diamond films. Surf. Coat. Technol. 1999, 120, 565–572. [Google Scholar] [CrossRef]
- Abreu, C.S.; Oliveira, F.J.; Belmonte, M.; Fernandes, A.J.S.; Gomes, J.R.; Silva, R.F. CVD diamond coated silicon nitride self-mated systems: Tribological behaviour under high loads. Tribol. Lett. 2006, 21, 141–151. [Google Scholar] [CrossRef]
- Kumar, N.; Panda, K.; Dash, S.; Popov, C.; Reithmaier, J.P.; Panigrahi, B.K.; Tyagi, A.K. Baldev Raj. Tribological properties of nanocrystalline diamond films deposited by hot filament chemical vapor deposition. AIP Adv. 2012, 2, 032164. [Google Scholar] [CrossRef]
- Chen, C.; Fan, D.; Xu, H.; Jiang, M.; Li, X.; Lu, S.; Ke, C.; Hu, X. Monoatomic tantalum induces ordinary-pressure phase transition from graphite to n-type diamond. Carbon 2022, 196, 466–473. [Google Scholar] [CrossRef]
- Shen, B.; Ji, Z.; Lin, Q.; Gong, P.; Xuan, N.; Chen, S.; Liu, H.; Huang, Z.; Xiao, T.; Sun, Z. Graphenization of Diamond. Chem. Mater. 2022, 34, 3941–3947. [Google Scholar] [CrossRef]
- Lu, Y.; Man, W.; Wang, B.; Rosenkranz, A.; Yang, M.; Yang, K.; Yi, J.; Song, H.; Li, H.; Jiang, N. (100) oriented diamond film prepared on amorphous carbon buffer layer containing nano-crystalline diamond grains. Surf. Coat. Technol. 2020, 385, 125368. [Google Scholar] [CrossRef]
- Gates, R.S.; Hsu, S.M. Tribochemistry between water and Si3N4 and SiC: Induction time analysis. Tribol. Lett. 2004, 17, 399–407. [Google Scholar] [CrossRef]
- Ootani, Y.; Xu, J.; Adachi, K.; Kubo, M. First-principles molecular dynamics study of silicon-based ceramics: Different tribochemical reaction mechanisms during the running-in period of silicon nitride and silicon carbide. J. Phys. Chem. C 2020, 124, 20079–20089. [Google Scholar] [CrossRef]
- Wang, Y.; Yukinori, K.; Koike, R.; Ootani, Y.; Adachi, K.; Kubo, M. Selective Wear Behaviors of a Water-Lubricating SiC Surface under Rotating-Contact Conditions Revealed by Large-Scale Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2021, 125, 14957–14964. [Google Scholar] [CrossRef]
- Kasuya, M.; Hino, M.; Yamada, H.; Mizukami, M.; Mori, H.; Kajita, S.; Ohmori, T.; Suzuki, A.; Kurihara, K. Characterization of Water Confined between Silica Surfaces Using the Resonance Shear Measurement. J. Phys. Chem. C 2013, 117, 13540–13546. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Zhang, Z.; Zhao, Z. Exploring a diamond film to improve wear resistance of the hydraulic drilling impactor. Surf. Coat. Technol. 2019, 360, 297–306. [Google Scholar] [CrossRef]
- Ootani, Y.; Xu, J.; Nakamura, F.; Kawaura, M.; Uehara, S.; Kanda, K.; Wang, Y.; Ozawa, N.; Adachi, K.; Kubo, M. Three Tribolayers Self-Generated from SiC Individually Work for Reducing Friction in Different Contact Pressures. J. Phys. Chem. C 2022, 126, 2728–2736. [Google Scholar] [CrossRef]
- Ponthiaux, P.; Wenger, F.; Drees, D.; Celis, J.P. Electrochemical techniques for studying tribocorrosion processes. Wear 2004, 256, 459–468. [Google Scholar] [CrossRef]
- Namus, R.; Rainforth, W.M. The influence of cathodic potentials on the surface oxide layer status and tribocorrosion behaviour of Ti6Al4V and CoCrMo alloys in simulated body fluid. Biotribology 2022, 30, 100212. [Google Scholar] [CrossRef]





| CH4/sccm | H2/sccm | N2/sccm | Pressure/KPa | Power/KW | Substrate Temperature/℃ | |
|---|---|---|---|---|---|---|
| MCD | 12 | 400 | -- | 12 | 4.4 | 900 ± 20 |
| NCD | 20 | 400 | 20 | 9 | 3 | 670 ± 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Song, H.; Pang, M.; Yang, G.; Ji, F.; Jiang, N.; Nishimura, K. Tribological Performance of Microcrystalline Diamond (MCD) and Nanocrystalline Diamond (NCD) Coating in Dry and Seawater Environment. Crystals 2022, 12, 1345. https://doi.org/10.3390/cryst12101345
Zhang H, Song H, Pang M, Yang G, Ji F, Jiang N, Nishimura K. Tribological Performance of Microcrystalline Diamond (MCD) and Nanocrystalline Diamond (NCD) Coating in Dry and Seawater Environment. Crystals. 2022; 12(10):1345. https://doi.org/10.3390/cryst12101345
Chicago/Turabian StyleZhang, Hui, Hui Song, Ming Pang, Guoyong Yang, Fengqin Ji, Nan Jiang, and Kazuhito Nishimura. 2022. "Tribological Performance of Microcrystalline Diamond (MCD) and Nanocrystalline Diamond (NCD) Coating in Dry and Seawater Environment" Crystals 12, no. 10: 1345. https://doi.org/10.3390/cryst12101345
APA StyleZhang, H., Song, H., Pang, M., Yang, G., Ji, F., Jiang, N., & Nishimura, K. (2022). Tribological Performance of Microcrystalline Diamond (MCD) and Nanocrystalline Diamond (NCD) Coating in Dry and Seawater Environment. Crystals, 12(10), 1345. https://doi.org/10.3390/cryst12101345





