Orientation Selection of Supported Au Nanoparticles on (111)- and (001)-Terminated SrTiO3 Substrates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Criterion of Orientation Selection
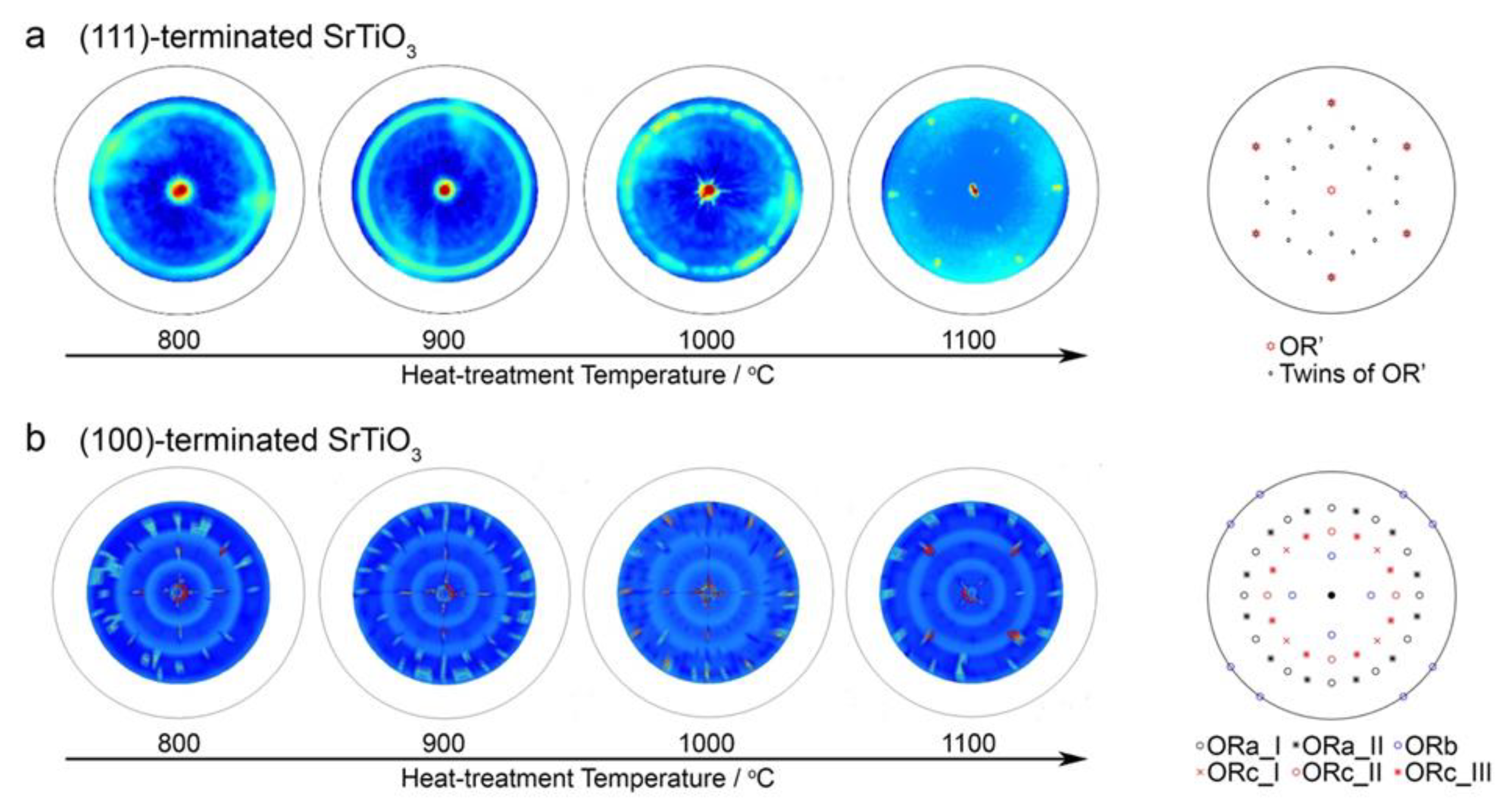
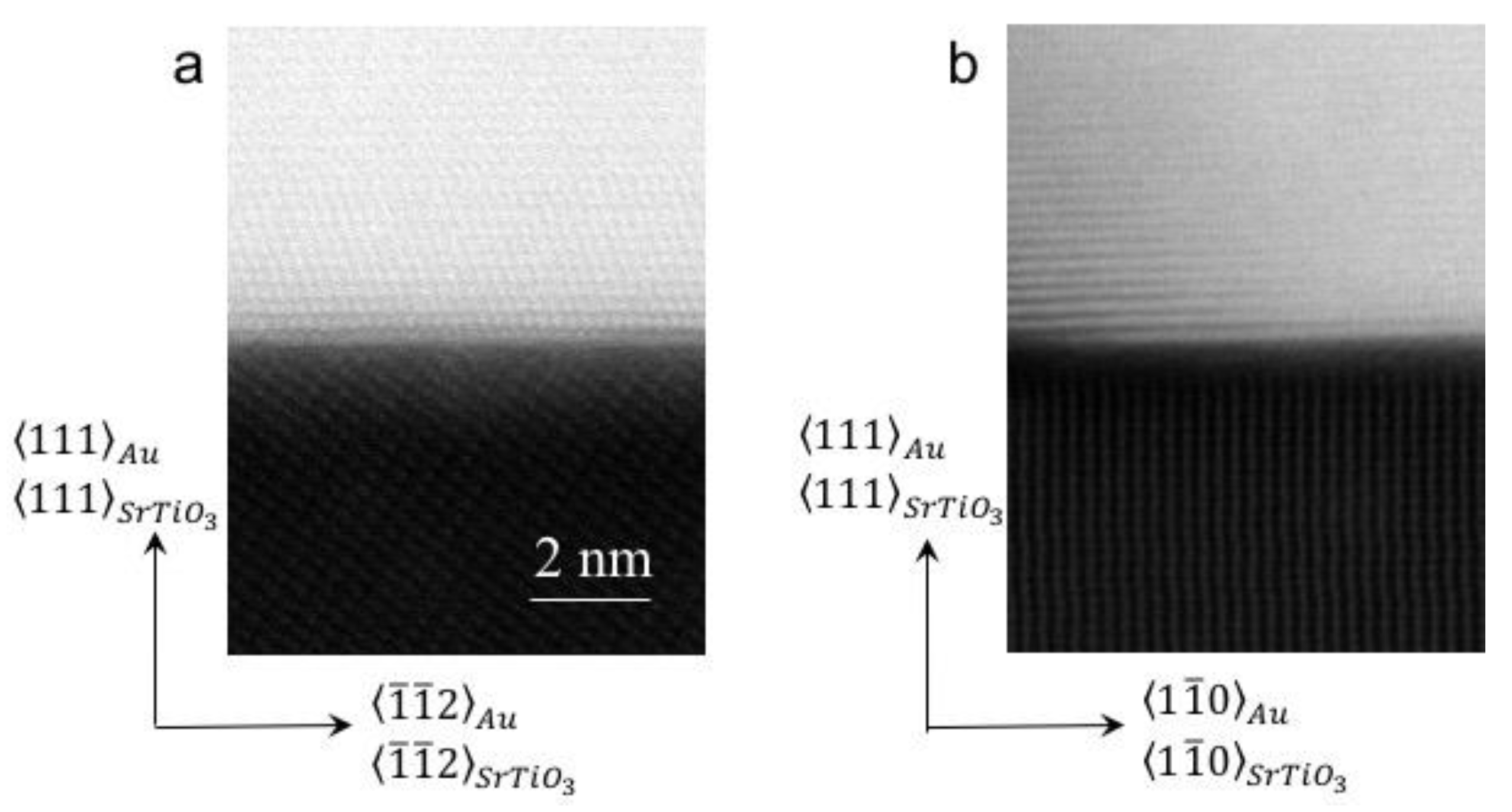
3.2. Experimental Validation Using Dewetted Au Nanoparticles on (111)- and (001)-Terminated SrTiO3 Substrates
- ORa-I:
- ORa-II:
- ORb:
- ORc-I:
- ORc-II:
- ORc-III:.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enterkin, J.A.; Setthapun, W.; Elam, J.W.; Christensen, S.T.; Rabuffetti, F.A.; Marks, L.D.; Stair, P.C.; Poeppelmeier, K.R.; Marshall, C.L. Propane oxidation over Pt/SrTiO3 nanocuboids. ACS Catal. 2011, 1, 629–635. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-based resistive switching memories—Nanoionic mechanisms, prospects, and challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Qin, W.; Hou, J.C.; Bonnell, D.A. Effect of interface atomic structure on the electronic properties of nano-sized metal−oxide interfaces. Nano Lett. 2015, 15, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hagglund, C.; Zach, M.; Petersson, G.; Kasemo, B. Electromagnetic coupling of light into a silicon solar cell by nanodisk plasmons. Appl. Phys. Lett. 2008, 92, 053110. [Google Scholar] [CrossRef]
- Brown, M.D.; Suteewong, T.; Kumar, R.S.S.; D’Innocenzo, V.; Petrozza, A.; Lee, M.M.; Wiesner, U.; Snaith, H.J. Plasmonic dye-sensitized solar cells using core-shell metalinsulator nanoparticle. Nano Lett. 2011, 11, 438–445. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Z.; Liu, Q.; Xu, Y.; Chen, M.; Lin, H.; Li, Y.; Gross, E.; Zhang, Q. The synergy between metal facet and oxide support facet for enhanced catalytic performance: The case of Pd−TiO2. Nano Lett. 2016, 16, 5298–5302. [Google Scholar] [CrossRef]
- Li, X.R.; Ge, Z.C.; Xue, F.; Liu, H.; Lyu, B.; Liu, M.C. Lattice-oriented contact in Pd/SrTiO3 heterojunction for rapid electron transfer during photocatalytic H2 production. Mater. Res. Bull. 2020, 123, 110722. [Google Scholar] [CrossRef]
- Kraya, R.; Kraya, L.Y.; Bonnell, D.A. Orientation controlled schottky barrier formation at Au nanoparticle−SrTiO3 interfaces. Nano Lett. 2010, 10, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, X.W.; Toda, T.; Oshikiri, T.; Ueno, K.; Suzuki, K.; Murakoshi, K.; Misawa, H. Interfacial structure-modulated plasmon-induced water oxidation on strontium titanate. Appl. Energy Mater. 2020, 3, 5675–5683. [Google Scholar] [CrossRef]
- Barmparis, G.D.; Lodziana, Z.; Lopez, N.; Remediakis, L.N. Nanoparticle shapes by using Wulff constructions and first-principles calculations. Beilstein J. Nanotechnol. 2015, 6, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Honkala, K.; Hellman, A.; Remediakis, I.N.; Logadottir, A.; Carlsson, A.; Dahl, S.; Christensen, C.H.; Nørskov, J.K. Ammonia synthesis from first-principles calculations. Science 2005, 307, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Hellman, A.; Honkala, K.; Remediakis, I.N.; Logadóttir, Á.; Carlsson, A.; Dahl, S.; Christensen, C.H.; Nørskov, J.K. Insights into ammonia synthesis from first-principles. Surf. Sci. 2006, 600, 4264–4268. [Google Scholar] [CrossRef]
- Kaishew, R. Sur la thermodynamique des germes cristallins. Bull. Acad. Sci. Bulg. Ser. Phys. 1951, 2, 191. [Google Scholar]
- Winterbottom, W.L. Equilibrium shape of a small particle in contact with a foreign substrate. Acta Metall. 1967, 15, 303–310. [Google Scholar] [CrossRef]
- Müller, P.; Kern, R. Equilibrium nano-shape changes induced by epitaxial stress (generalised Wulf–Kaishew theorem). Surf. Sci. 2000, 457, 229–253. [Google Scholar] [CrossRef]
- Zhu, G.Z.; Majdi, T.; Shao, Y.; Bugnet, M.; Preston, J.S.; Botton, G. Atomic structure and bonding of the interfacial bilayer between Au nanoparticles and epitaxially regrown MgAl2O4 substrates. Appl. Phys. Lett. 2014, 105, 231607. [Google Scholar] [CrossRef]
- Majdi, T.; Zhu, G.Z.; Carvalho, J.; Jarvis, V.; Meinander, K.; Britten, J.F.; Botton, G.; Preston, J.S. Evidence for an equilibrium epitaxial complexion at the Au-MgAl2O4 interface. Appl. Phys. Lett. 2015, 107, 241601. [Google Scholar] [CrossRef]
- Lin, M.; Zhou, W.; Gu, X.; Zhu, G. Gold-rutile interfaces with irrational crystallographic orientations. Mater. Charact. 2021, 176, 11116. [Google Scholar] [CrossRef]
- Chatterjee, D.; Kamalnath, A.R.K.; Ahmad, R.; Singh, A.K.; Ravishankar, N. Orientation selection during heterogeneous nucleation: Implications for heterogeneous catalysis. J. Phys. Chem. C 2017, 121, 10027–10037. [Google Scholar] [CrossRef]
- Chen, P.; Murugappan, K.; Castell, M.R. Shapes of epitaxial gold nanocrystals on SrTiO3 Substrates. Phys. Chem. Chem. Phys. 2020, 22, 4416–4428. [Google Scholar] [CrossRef] [PubMed]
- Yi, A.C.; Liu, F.; Xie, D.Y.; Wen, M.; Zhu, G.Z. Gold-assisted growth of oxide bases underneath dewetted gold nanoparticles. Mater. Charact. 2019, 151, 237–241. [Google Scholar] [CrossRef]
- Yao, S.Y.; Wen, M.; Zhu, G.Z. Bimodal size distribution of dewetted gold nanoparticles with regrown oxide bases. Appl. Surf. Sci. 2020, 501, 144227. [Google Scholar] [CrossRef]
- Vitos, L.; Ruban, A.V.; Skriver, H.L.; Kollar, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202. [Google Scholar] [CrossRef]
- Hou, J.; Nonnenmann, S.S.; Qin, W.; Bonnell, D.A. Size dependence of resistive switching at nanoscale metal-oxide interfaces. Adv. Funct. Mater. 2014, 24, 4113–4118. [Google Scholar] [CrossRef]
- Liu, F.; Xie, D.Y.; Bugnet, M.; Majdi, T.; Preston, J.S.; Wang, J.; Zhu, G.Z. Temperature induced atomic reconstruction at Au/MgAl2O4 interfaces. Adv. Mater. Interfaces 2018, 5, 1701664. [Google Scholar] [CrossRef]
- Liu, F.; Xie, D.Y.; Majdi, T.; Zhu, G.Z. Twin-assisted growth of nominally stable substrates underneath dewetted Au nanoparticles. Mater. Charact. 2016, 113, 67–70. [Google Scholar] [CrossRef]
- Silly, F.; Castell, M.R. Bimodal growth of Au on SrTiO3 (001). Phy. Rev. Lett. 2006, 96, 086104. [Google Scholar] [CrossRef] [PubMed]
- Cheula, R.; Soon, A.; Maestri, M. Prediction of morphological changes of catalyst materials under reaction conditions by combined ab initio thermodynamics and microkinetic modelling. Catal. Sci. Technol. 2018, 8, 3493–3503. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, W.; Zhu, G. Orientation Selection of Supported Au Nanoparticles on (111)- and (001)-Terminated SrTiO3 Substrates. Crystals 2022, 12, 1414. https://doi.org/10.3390/cryst12101414
Kuang W, Zhu G. Orientation Selection of Supported Au Nanoparticles on (111)- and (001)-Terminated SrTiO3 Substrates. Crystals. 2022; 12(10):1414. https://doi.org/10.3390/cryst12101414
Chicago/Turabian StyleKuang, Wangwang, and Guozhen Zhu. 2022. "Orientation Selection of Supported Au Nanoparticles on (111)- and (001)-Terminated SrTiO3 Substrates" Crystals 12, no. 10: 1414. https://doi.org/10.3390/cryst12101414
APA StyleKuang, W., & Zhu, G. (2022). Orientation Selection of Supported Au Nanoparticles on (111)- and (001)-Terminated SrTiO3 Substrates. Crystals, 12(10), 1414. https://doi.org/10.3390/cryst12101414






