Calcium Carbonate Crystallization on a Microalgal Matrix: The Effects of Heavy Metal Presence
Abstract
:1. Introduction
2. Materials and Methods
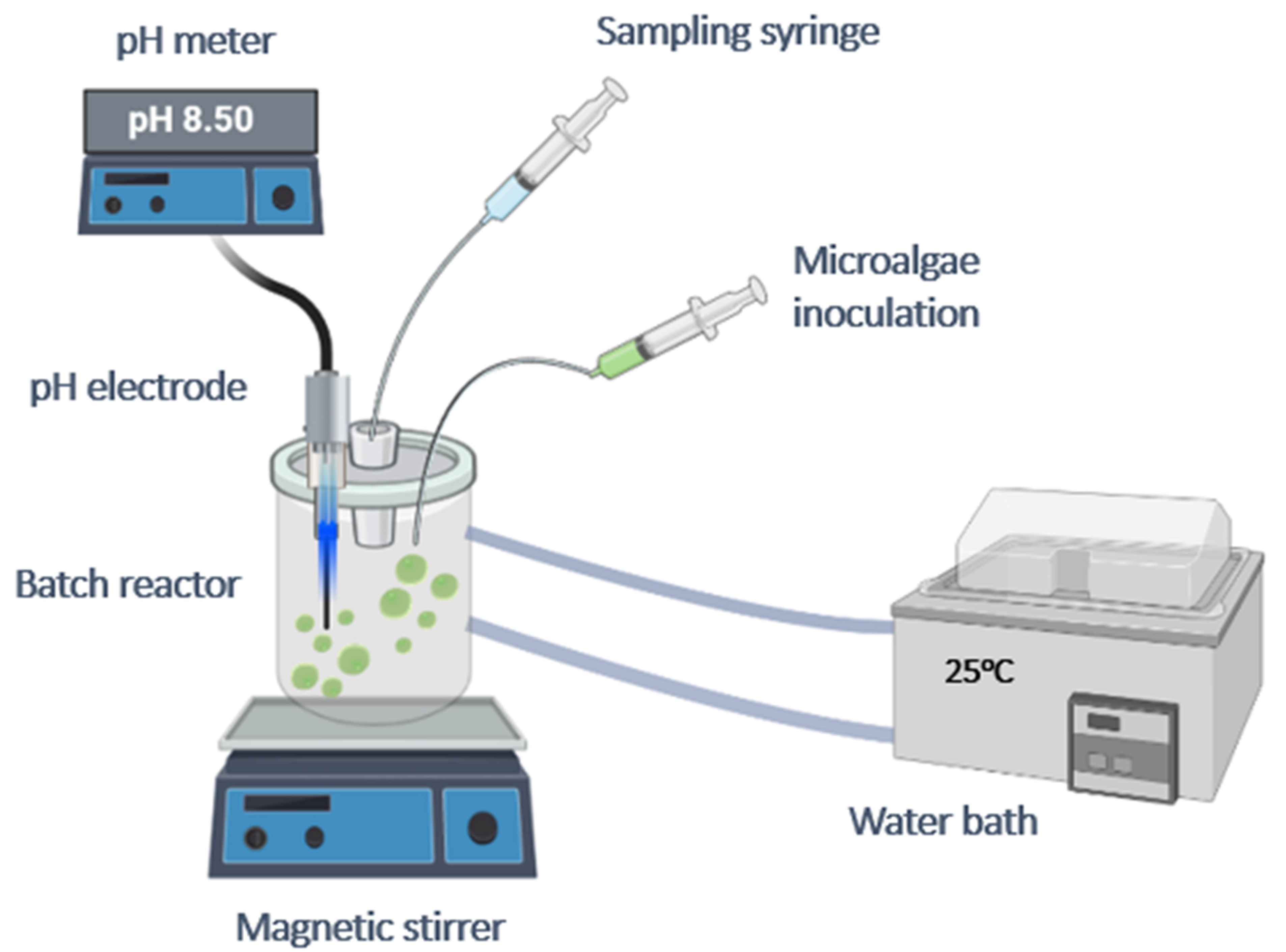
2.1. Mineralization Experiments in the Presence of Heavy Metals (HM) and A. obliquus
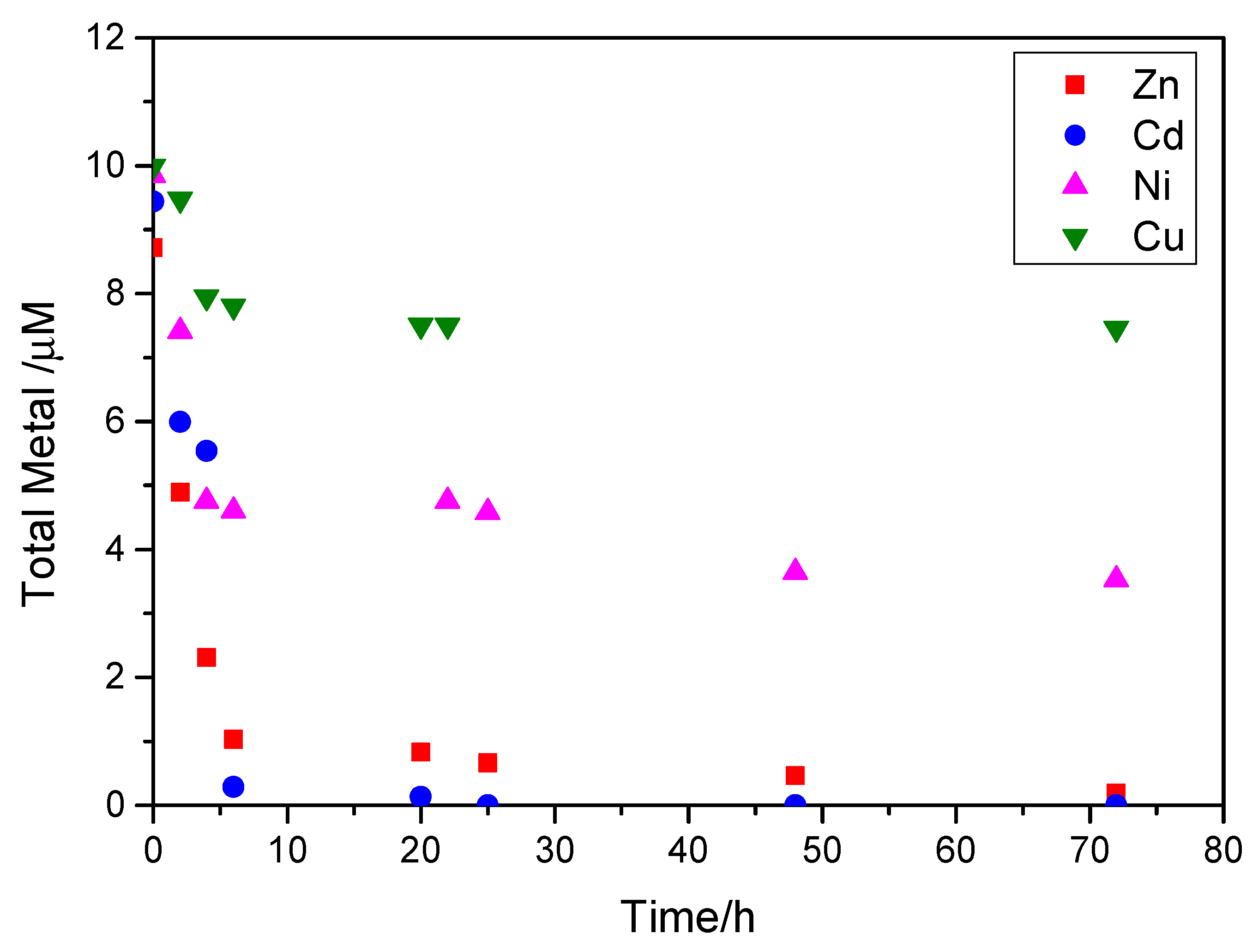
2.2. Measurements of the Metals Uptake
3. Results and Discussion
3.1. Mineralization Experiments in the Presence of Heavy Metals and of A. obliquus Culture
3.1.1. Precipitation of Calcium Carbonate in the Presence of A. obliquus Including the Corresponding Nutrient Growth Medium in the Presence of Zn
3.1.2. Precipitation of Calcium Carbonate in the Presence of A. obliquus Culture and the Corresponding Nutrient Growth Medium in the Presence of Ni
3.1.3. Precipitation of Calcium Carbonate in Supersaturated Solutions in the Presence of Metabolically Active A. obliquus Cultures and 10 μM of Zn, Cd, Ni, Cu
3.2. Adsorption
3.2.1. Uptake of Zn, Cd, Ni and Cu by Calcite
3.2.2. Adsorption of Zinc on Calcite and Microalgae
Adsorption of Zinc on Calcite in Presence of Metabolically Active A. obliquus Culture
Adsorption of Zinc on Calcite and A. obliquus Culture
Adsorption of Zinc on Dried (25 °C) A. obliquus Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Estrella, L.R.; Guevara-Garcia, A.A. Heavy Metal Adaptation. eLS Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 1–9. [Google Scholar]
- Gaur, A.; Adholeya, A. Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr. Sci. 2004, 86, 528–534. [Google Scholar]
- Hafeburg, G.; Kohe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2929. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, H.M.; Bae, J.S.; Ha, M.G.; Jin, J.S.; Hong, T.E.; Kim, J.P.; Jeong, E.D. Removal of heavy metal ions by using calcium carbonate extracted from starfish treated by protease and amylase. J. Anal. Sci. Technol. 2011, 2, 75–82. [Google Scholar] [CrossRef]
- Olade, M.A. Heavy Metal Pollution and the Need for Monitoring: Illustrated for Developing Countries in West Africa. In Lead, Mercury, Cadmium and Arsenic in the Environment; Hutchinson, T.C., Meema, K.M., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 1987; pp. 335–341. [Google Scholar]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Freitas, O.M.M.; Martins, R.J.E.; Delerue-Matos, C.M.; Boaventura, R.A.R. Removal of Cd(II), Zn(II) and Pb(II) from aqueous solutions by brown marine macro algae: Kinetic modelling. J. Hazard. Mater. 2008, 153, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wilde, W.E.; Benemann, J.R. Bioremoval of heavy metals by the use of microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The effect of heavy metals on the viability of Tetraselmis marina AC16-MESO and an evaluation of the potential use of this microalga in bioremediation. PeerJ 2018, 6, e5295. [Google Scholar] [CrossRef] [Green Version]
- Santomauro, G.; Baier, J.; Huang, W.; Pezold, S.; Bill, J. Formation of Calcium Carbonate Polymorphs Induced by Living Microalgae. J. Biomater. Nanobiotechnol. 2012, 3, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Chin, Z.W.; Arumugam, K.; Ashari, S.E.; Faizal Wong, F.W.; Tan, J.S.; Ariff, A.B.; Mohamed, M.S. Enhancement of Biomass and Calcium Carbonate Biomineralization of Chlorella vulgaris through Plackett–Burman Screening and Box–Behnken Optimization Approach. Molecules 2020, 25, 3416. [Google Scholar] [CrossRef]
- Mazzone, E.J.; Guentzel, J.L.; Olaizola, M. Carbon removal through algal mediated precipitation of calcium carbonate. Mar. Sci. 2002, 223, 537–538. [Google Scholar]
- Xu, P.; Fan, H.; Leng, L.; Fan, L.; Liu, S.; Chen, P.; Zhou, W. Feasibility of microbially induced carbonate precipitation through a Chlorella-Sporosaricina co-culture system. Algal Res. 2020, 47, 101831. [Google Scholar] [CrossRef]
- Heath, C.R.; Leadbeater, B.C.S.; Callow, M.E. Effect of inhibitors on calcium carbonate deposition mediated by freshwater algae. J. Appl. Phycol. 1995, 7, 367–380. [Google Scholar] [CrossRef]
- Irfan, M.F.; Hossain, S.M.Z.; Khalid, H.; Sadaf, F.; Al-Thawadi, S.; Alshater, A.; Hossain, M.M.; Razzak, S.A. Optimization of bio-cement production from cement kiln dust using microalgae. Biotechnol. Rep. 2019, 23, e00356. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Algal Culturing Techniques. In Physiological Society of America; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Wintermans, J.F.; de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods, Book 6; U.S. Geological Survey: Reston, VA, USA, 2013; Chapter A43; p. 497. [Google Scholar]
- Cavalletti, E.; Romano, G.; Palma Esposito, P.; Barra, L.; Chiaiese, P.; Balzano, S.; Angela, S. Copper Effect on Microalgae: Toxicity and Bioremediation Strategies. Toxics 2022, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, S.D.; Santhanam, P.; Ananth, S.; Shenbaga Devi, A.; Nandakumar, R.; Balaji Prasath, B.; Jeyanthi, S.; Jayalakshmi, T.; Ananth, P. Effect of different dosages of zinc on the growth and biomass in five marine microalgae. Int. J. Fish. Aquac. 2014, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.M.; Fonseca, S.C.; Castro, P.M.L. Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. J. Appl. Phycol. 2011, 23, 97–103. [Google Scholar] [CrossRef]
- Ghizellaoui, S.; Euvrard, M. Assessing the effect of zinc on the crystallization of calcium carbonate. Desalination 2008, 220, 394–402. [Google Scholar] [CrossRef]
- Mugwar, A.J.; Harbottle, M.J. Toxicity effects on metal sequestration by microbially induced carbonate precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Dalas, E.; Kallitsis, J.; Koutsoukos, P.G. The growth of sparingly soluble salts on polymeric substrates. Colloids Surf. 1991, 53, 197–208. [Google Scholar] [CrossRef]
- Giannimaras, E.K.; Koutsoukos, P.G. Precipitation of Calcium Carbonate in Aqueous Solutions in the Presence of Oxalate Anions. Langmuir 1988, 4, 855–861. [Google Scholar] [CrossRef]
- Wada, N.; Yamashita, K.; Umegaki, T. Effects of divalent cations upon nucleation, growth and transformation of calcium carbonate polymorphs under conditions of double diffusion. J. Cryst. Growth 1995, 148, 297–304. [Google Scholar] [CrossRef]
- Kitano, Y.; Kanamori, N.; Yoshioka, S. Adsorption of zinc and copper ions on calcite and aragonite and its influence on the transformation of aragonite to calcite. Geochem. J. 1976, 10, 175–179. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, C.; Jimenez-Lopez, C.; Rodríguez-Navarro, A.; Gonzalez-Munoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralizarion of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wang, W.; Zhang, J.; Wang, Y.; Yu, S.H. Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide type block copolymer as crystal growth modifier in a mixed solution. CrystEngComm 2011, 13, 2054. [Google Scholar] [CrossRef]
- Aizenberg, J.; Muller, D.A.; Grazul, J.L.; Haman, D.R. Direct Fabrication of Large Micropatterned Single Crystals. Science 2003, 299, 1205–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanos, N.; Koutsoukos, P.G. Kinetics of Precipitation of Calcium Carbonate in Alkaline pH at Constant Supersaturation. Spontaneous and Seeded Growth. J. Phys. Chem. B 1998, 102, 6679–6684. [Google Scholar] [CrossRef]
- Zachara, J.M.; Cowan, C.E.; Retsch, C.T. Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 1991, 55, 1549–1562. [Google Scholar] [CrossRef]




 ), Cd(
), Cd( ), Ni (
), Ni ( ), Cu (
), Cu ( ), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd(
), Cd( ), Ni (
), Ni ( ), Cu (
), Cu ( ), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
), active A. obliquus culture and the appropriate illumination. Change in pH value as a function of time (a) SRcalcite = 14.79 and (b) SRcalcite =31.36, 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd (
), Cd ( ), Ni (
), Ni ( ), Cu(
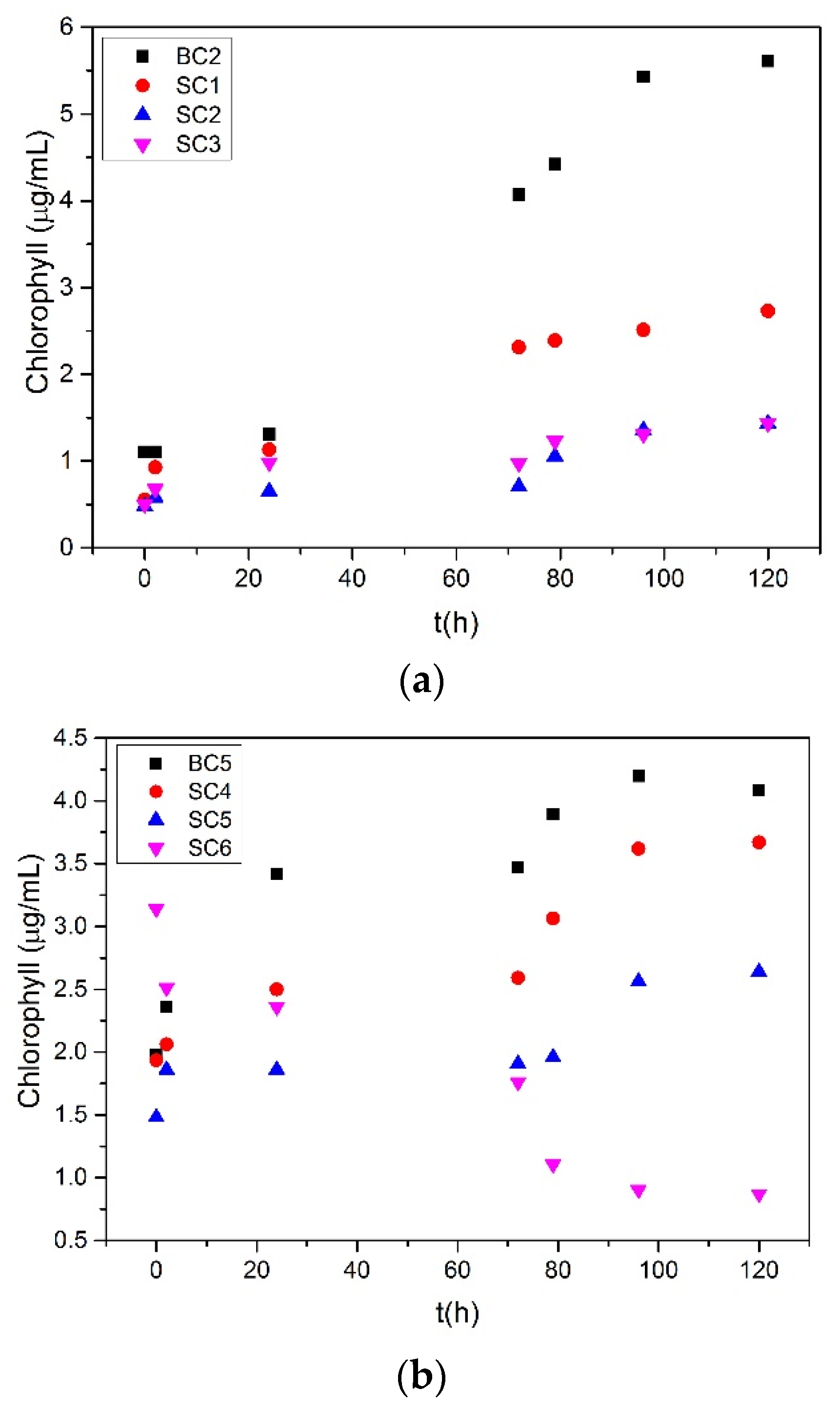
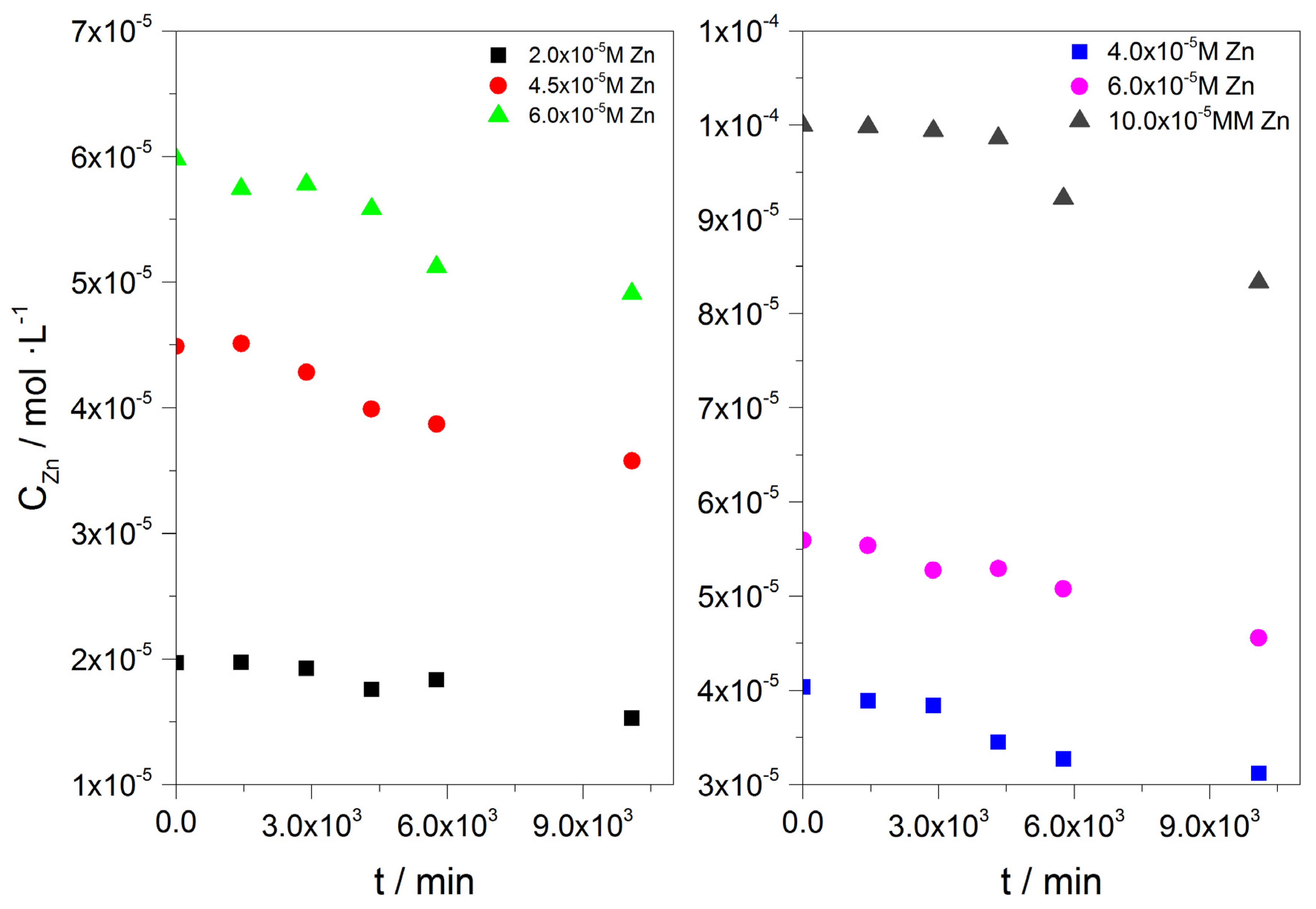
), Cu( ); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd (
), Cd ( ), Ni (
), Ni ( ), Cu(
), Cu( ); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
); calcium concentration as a function of time. A total of 20 mL of active A. obliquus culture with modified culture medium and illumination. (a) SRcalcite = 14.79 and (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.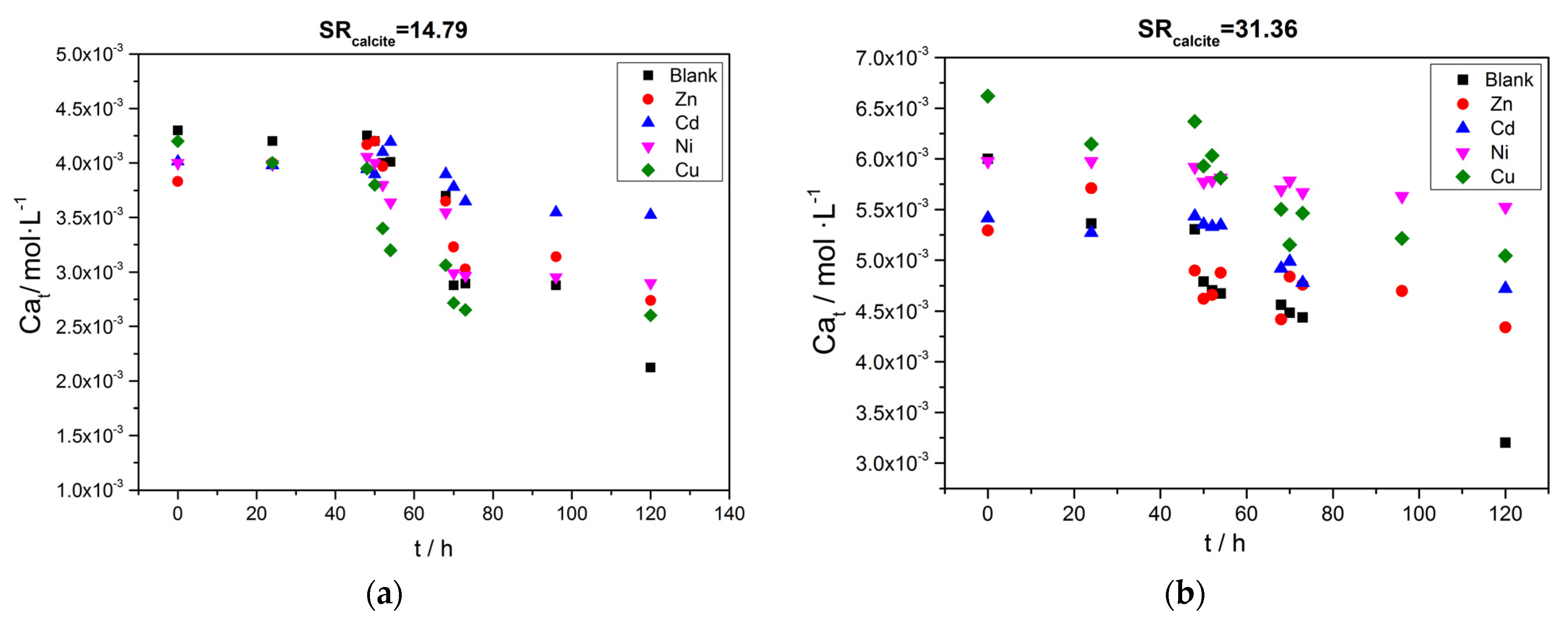
 ), Cd (
), Cd ( ), Ni (
), Ni ( ) and Cu (
) and Cu ( ), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
 ), Cd (
), Cd ( ), Ni (
), Ni ( ) and Cu (
) and Cu ( ), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.
), in the presence of active A. obliquus culture under illumination; (a) SRcalcite = 14.79; (b) SRcalcite = 31.36; 25 °C, pH 8.50, 0.15 M of NaCl.

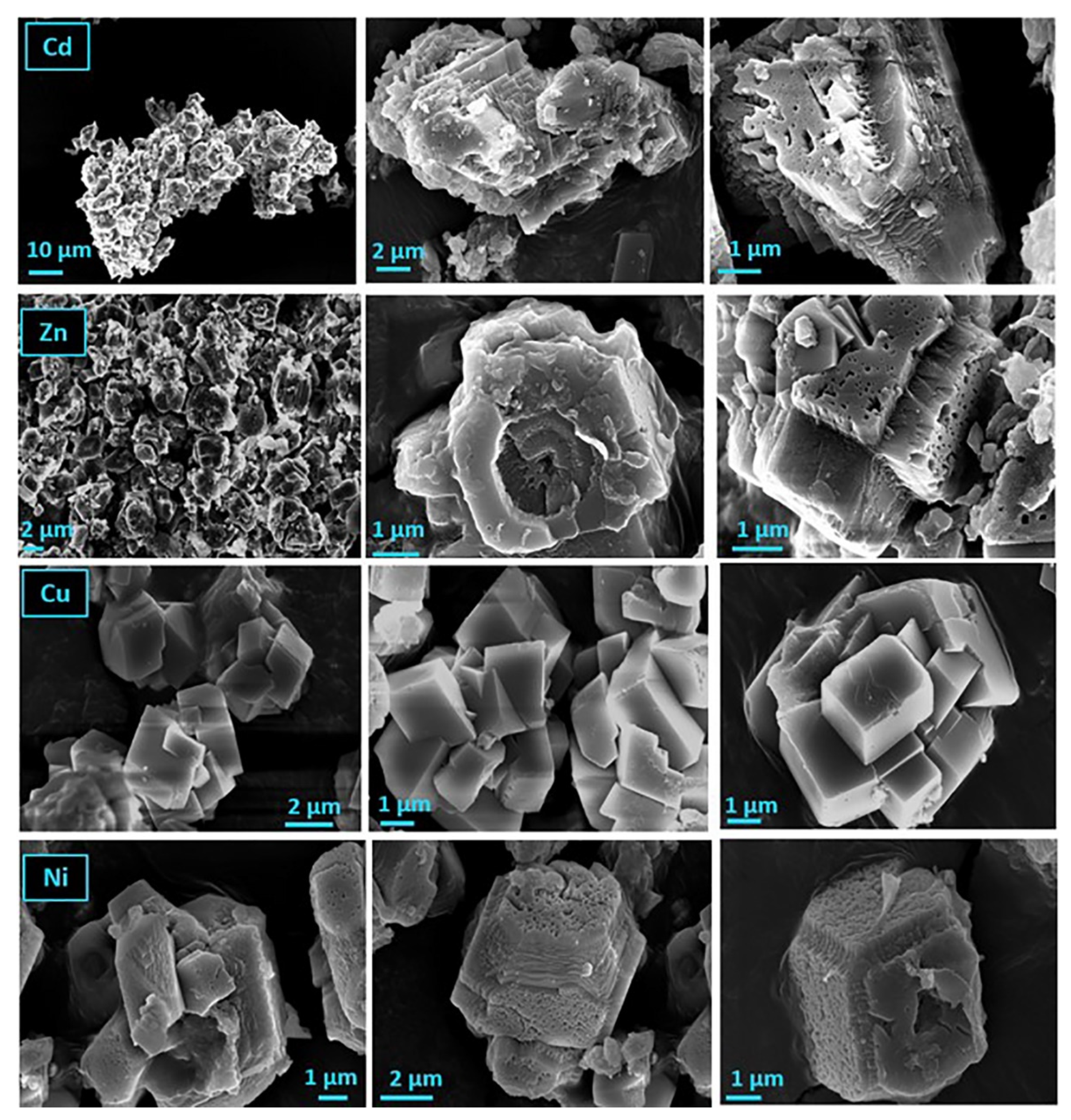
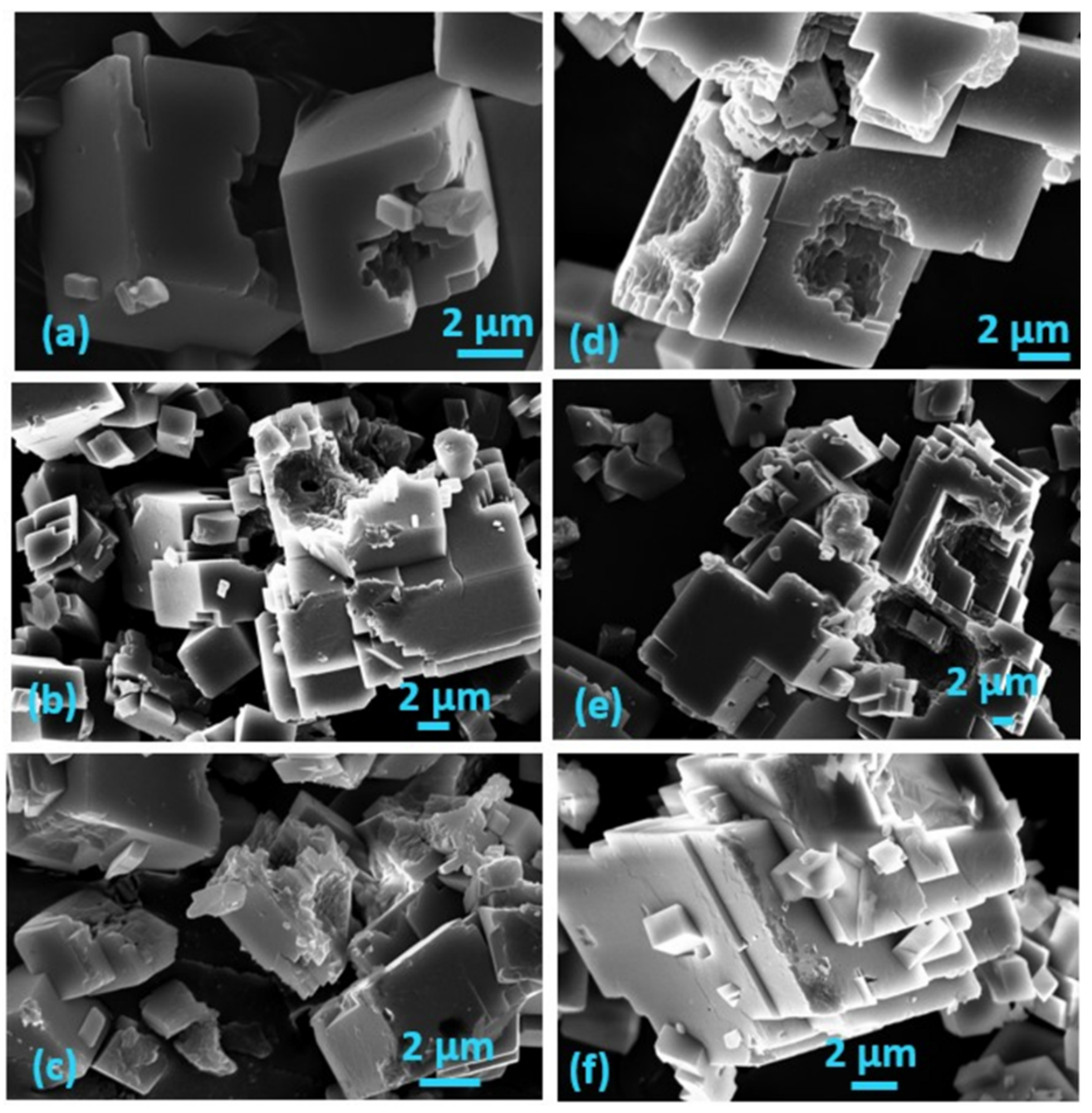
 ) and on air-dried A. obliquus culture (
) and on air-dried A. obliquus culture ( ); pH 8.50, 0.15 M of NaCl, 25 °C.
); pH 8.50, 0.15 M of NaCl, 25 °C.
 ) and on air-dried A. obliquus culture (
) and on air-dried A. obliquus culture ( ); pH 8.50, 0.15 M of NaCl, 25 °C.
); pH 8.50, 0.15 M of NaCl, 25 °C.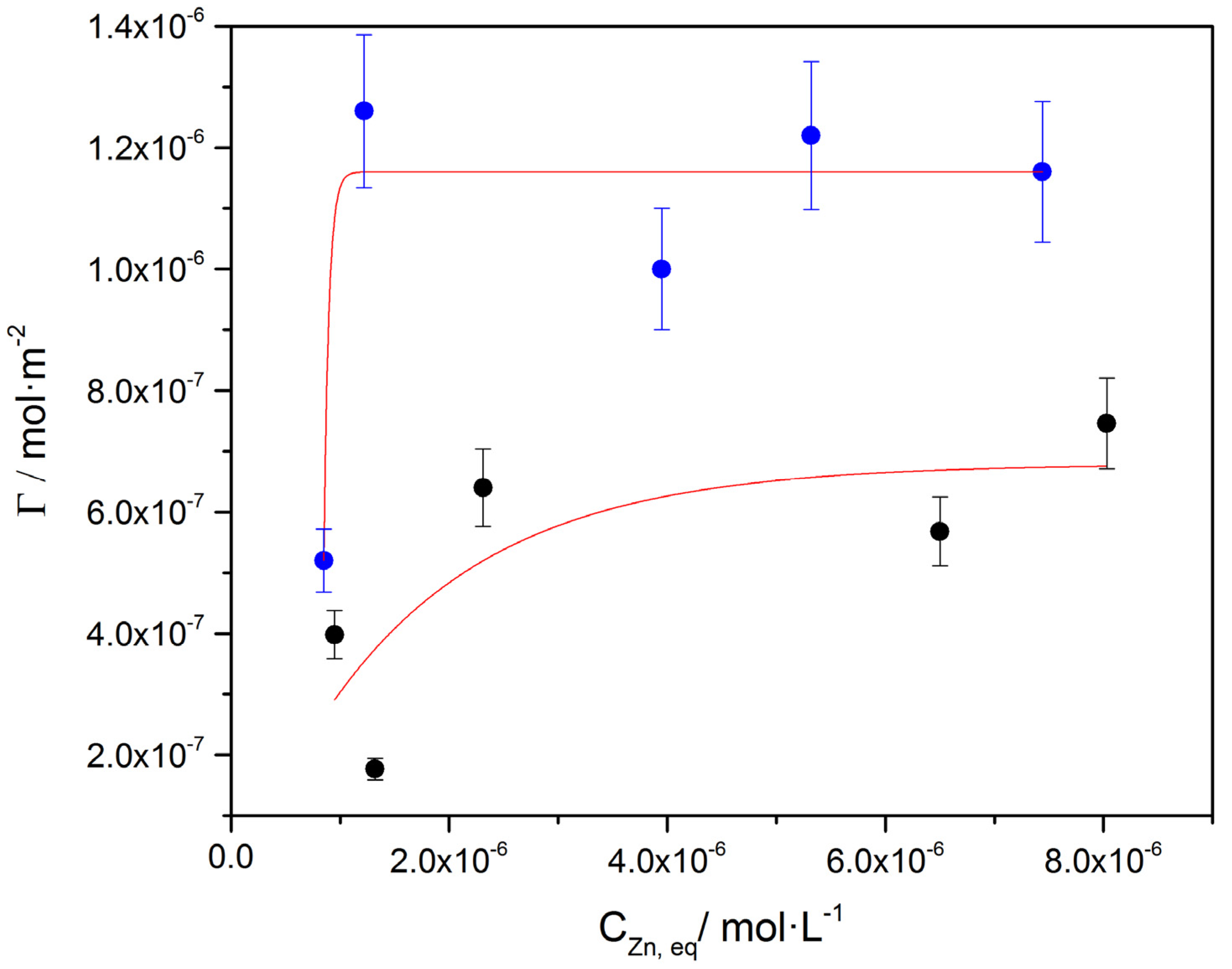
| Component | Concentration/mM |
|---|---|
| MgSO4∙7H2O | 0.400 |
| KNO3 | 0.247 |
| NaNO2 | 0.005 |
| H3BO3 | 0.040 |
| FeCl3∙2H2O | 0.006 |
| Expt. # | (Cat = Ct) /×10−3 M | CZn/×10−6 M | A. obliquus Culture (mL) |
|---|---|---|---|
| BC1 | 1.7 | 0 | 0 |
| BC2 | 1.7 | 0 | 10 |
| BC3 | 1.7 | 5.6 | 0 |
| SC1 | 1.7 | 5.6 | 10 |
| SC2 | 1.7 | 11 | 10 |
| SC3 | 1.7 | 15 | 10 |
| BC4 | 3.4 | 0 | 0 |
| BC5 | 3.4 | 0 | 0 |
| BC6 | 3.4 | 30 | 10 |
| SC4 | 3.4 | 30 | 0 |
| SC5 | 3.4 | 61 | 10 |
| SC6 | 3.4 | 92 | 10 |
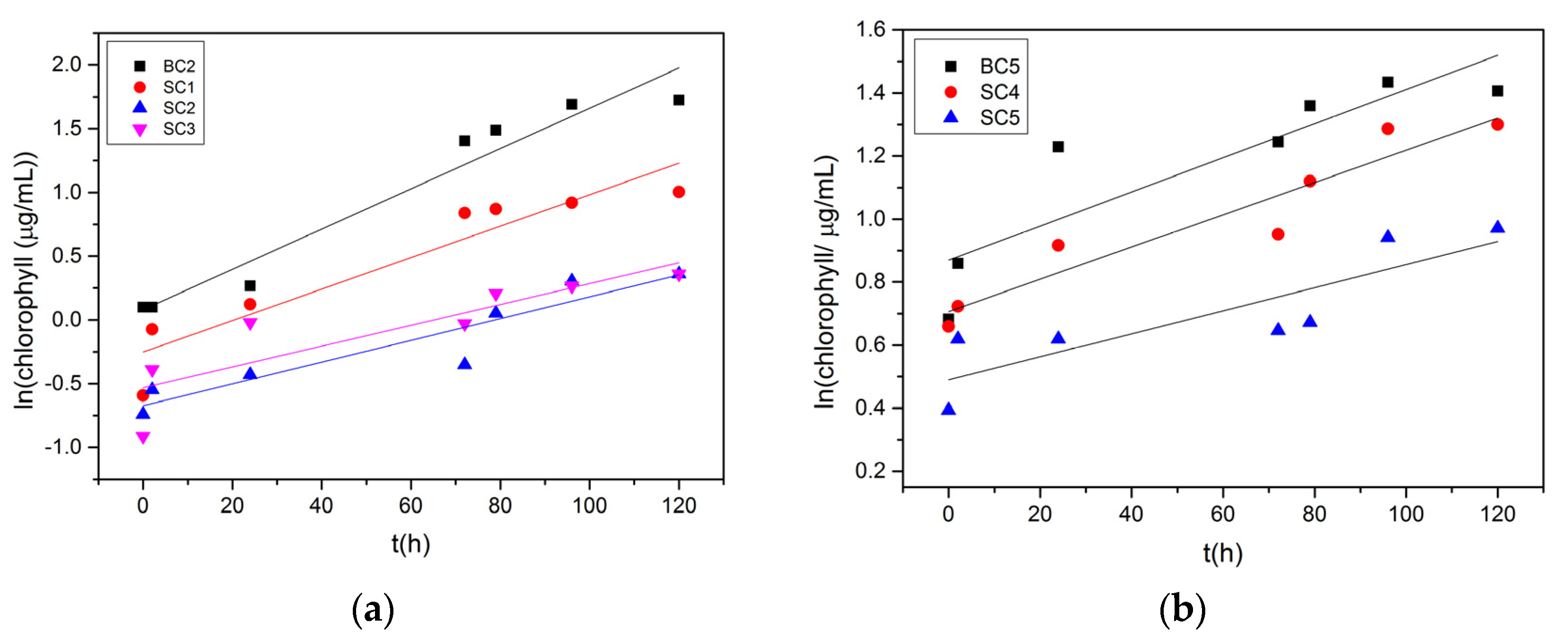
| Expt. # | Slope /×10−3 μg·mL−1·h−1 | Growth Rate of Microalgae/μg·h−1 |
|---|---|---|
| BC2 | 16.0 | 4.00 |
| SC1 | 12.0 | 3.00 |
| SC2 | 8.5 | 2.13 |
| SC3 | 8.2 | 2.04 |
| BC5 | 5.4 | 1.35 |
| SC4 | 5.1 | 1.28 |
| SC5 | 3.7 | 0.91 |
| Cat /×10−3 mol∙L−1 | Supersaturation Ratio, SRcalcite | Relative Supersaturation, σcaclite | Induction Time tind/min | Precipitation Rate, Rp/×10−6 mol·min−1·L−1 |
|---|---|---|---|---|
| 4.5 | 16.0 | 4.00 | 218 | 0.36 |
| 5.0 | 12.0 | 3.00 | 175 | 0.38 |
| 5.5 | 8.5 | 2.13 | 79 | 0.46 |
| 6.0 | 8.2 | 2.04 | 45 | 0.70 |
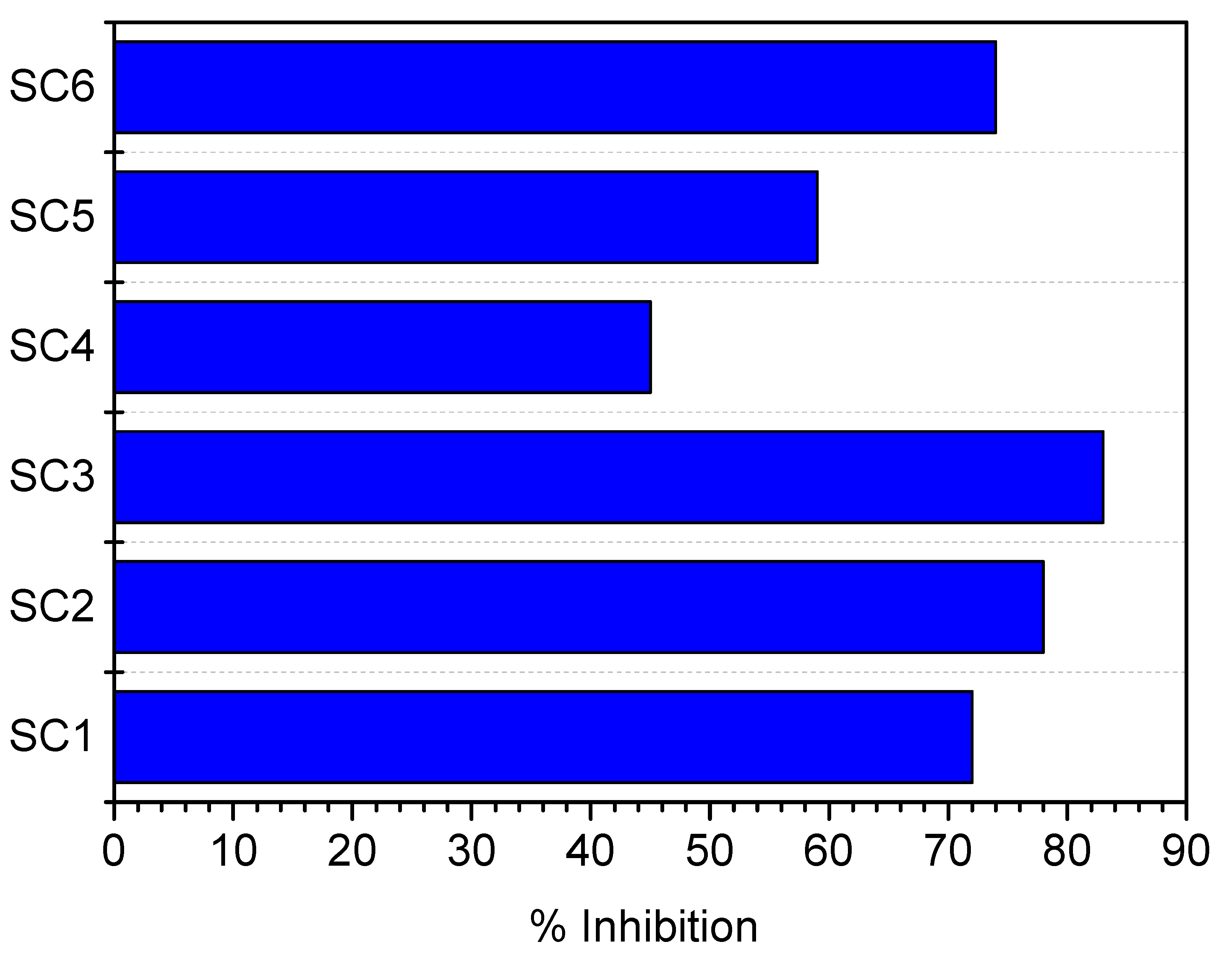
| SRcalcite | % Inhibition |
|---|---|
| 18.19 | 88 |
| 22.39 | 79 |
| 26.91 | 78 |
| 31.36 | 50 |
| Growth Rate of A. obliquus Culture (μg/h) | ||
|---|---|---|
| Metal Present (10 μM) | SRcalcite = 14.79 | SRcalcite = 31.36 |
| None (Blank) | 4.96 | 2.94 |
| Zn | 3.91 | 1.47 |
| Cd | 3.48 | 0.60 |
| Ni | 4.45 | 1.81 |
| Cu | 3.34 | 2.25 |
| SRcalcite | 14.79 | 31.36 | ||
|---|---|---|---|---|
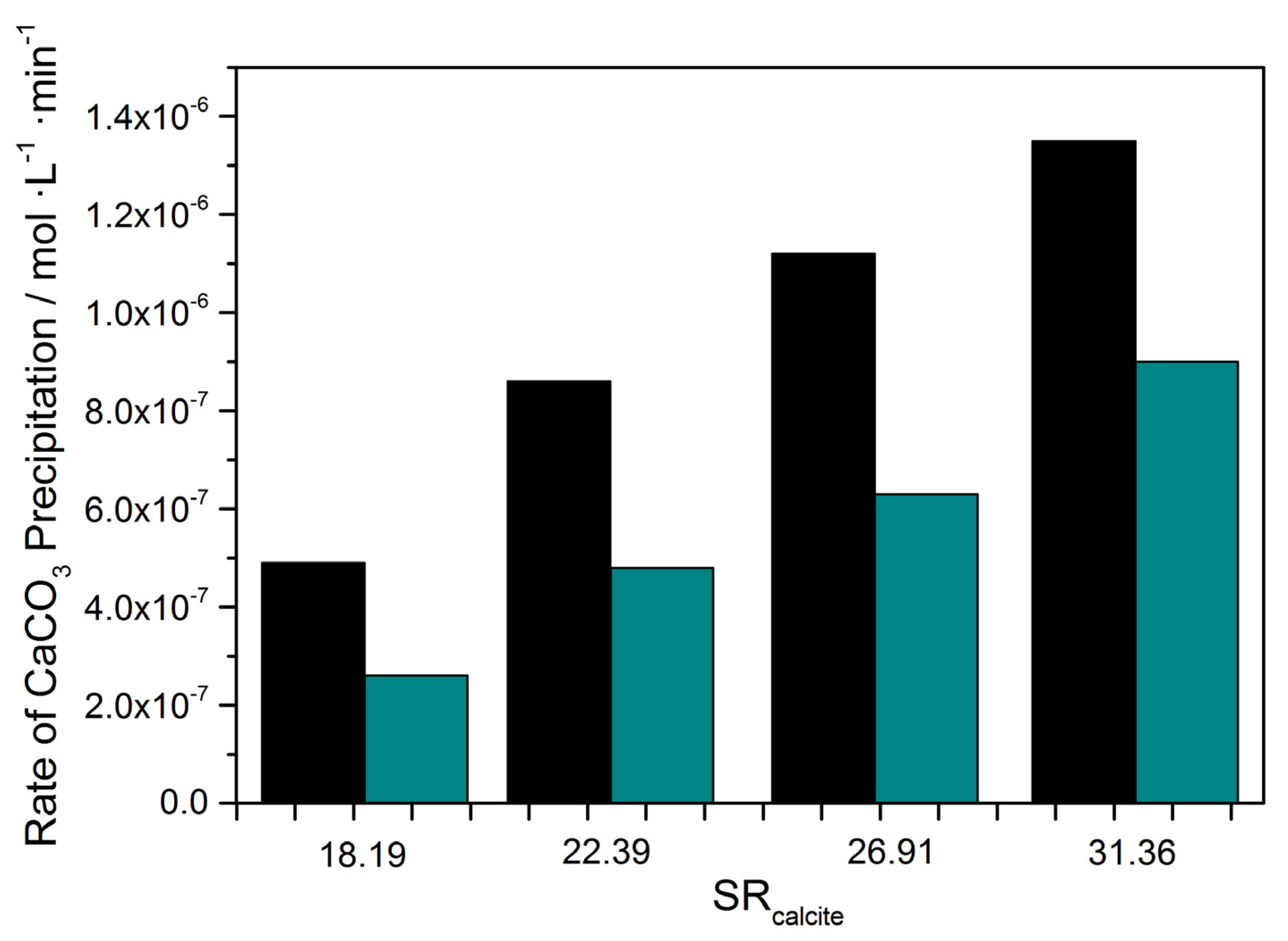
| Metal in Solution (10 μM) | CaCO3 Precipitation Rate, Rp/×10−7 mol·L−1 min−1 | Inhibition (%) | CaCO3 Precipitation Rate, Rp/×10−7 mol·L−1·min−1 | Inhibition (%) |
| None (Blank) | 4.90 | - | 4.88 | - |
| Zn | 1.32 | 73 | 1.29 | 74 |
| Cd | 1.00 | 79 | 1.67 | 66 |
| Ni | 0.61 | 89 | 0.90 | 81 |
| Cu | 0.31 | 36 | 3.06 | 37 |
| Zn | Ni | Cd | ||||||
|---|---|---|---|---|---|---|---|---|
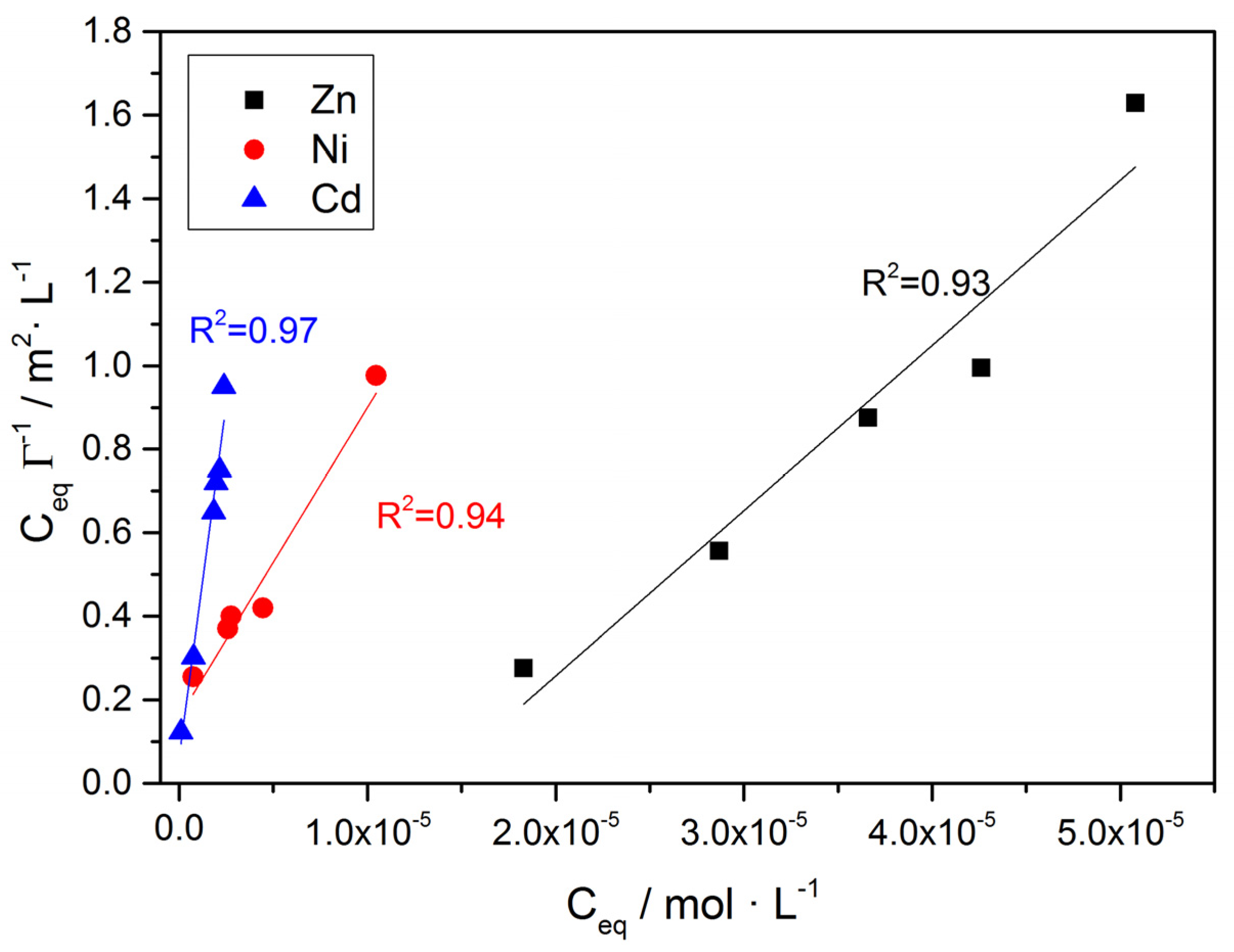
| Co /×10−5∙mol∙L−1 | Ceq /×10−5∙mol∙L−1 | ΓZn /×10−5∙mol∙m−2 | Co /×10−5∙mol∙L−1 | Ceq /×10−5∙mol∙L−1 | ΓNi /×10−5∙mol∙m−2 | Co /×10−5∙mol∙L−1 | Ceq /×10−5∙mol∙L−1 | ΓCd /×10−5∙mol∙m−2 |
| 2.19 | 1.46 | 2.28 | 1.65 | 0.73 | 2.86 | 0.56 | 0.089 | 0.72 |
| 3.95 | 1.83 | 6.62 | 3.98 | 2.91 | 3.33 | 2.40 | 0.76 | 2.51 |
| 4.52 | 2.87 | 5.16 | 5.59 | 2.76 | 8.84 | 4.15 | 1.84 | 3.55 |
| 5.00 | 3.66 | 4.18 | 8.60 | 4.46 | 12.9 | 5.42 | 2.14 | 5.02 |
| 5.63 | 4.26 | 4.28 | 13.90 | 10.46 | 1.07 | 6.34 | 1.98 | 6.69 |
| 6.07 | 5.08 | 3.12 | 7.91 | 2.38 | 2.08 | |||
| Co /×10−5∙mol∙L−1 | Ceq /×10−5∙mol∙L−1 | Γ /×10−5∙mol∙m−2 |
|---|---|---|
| 2.00 | 1.53 | 0.67 |
| 4.00 | 3.12 | 1.53 |
| 4.50 | 3.58 | 1.38 |
| 5.60 | 4.56 | 1.57 |
| 6.00 | 4.91 | 1.62 |
| 10.00 | 8.34 | 2.52 |
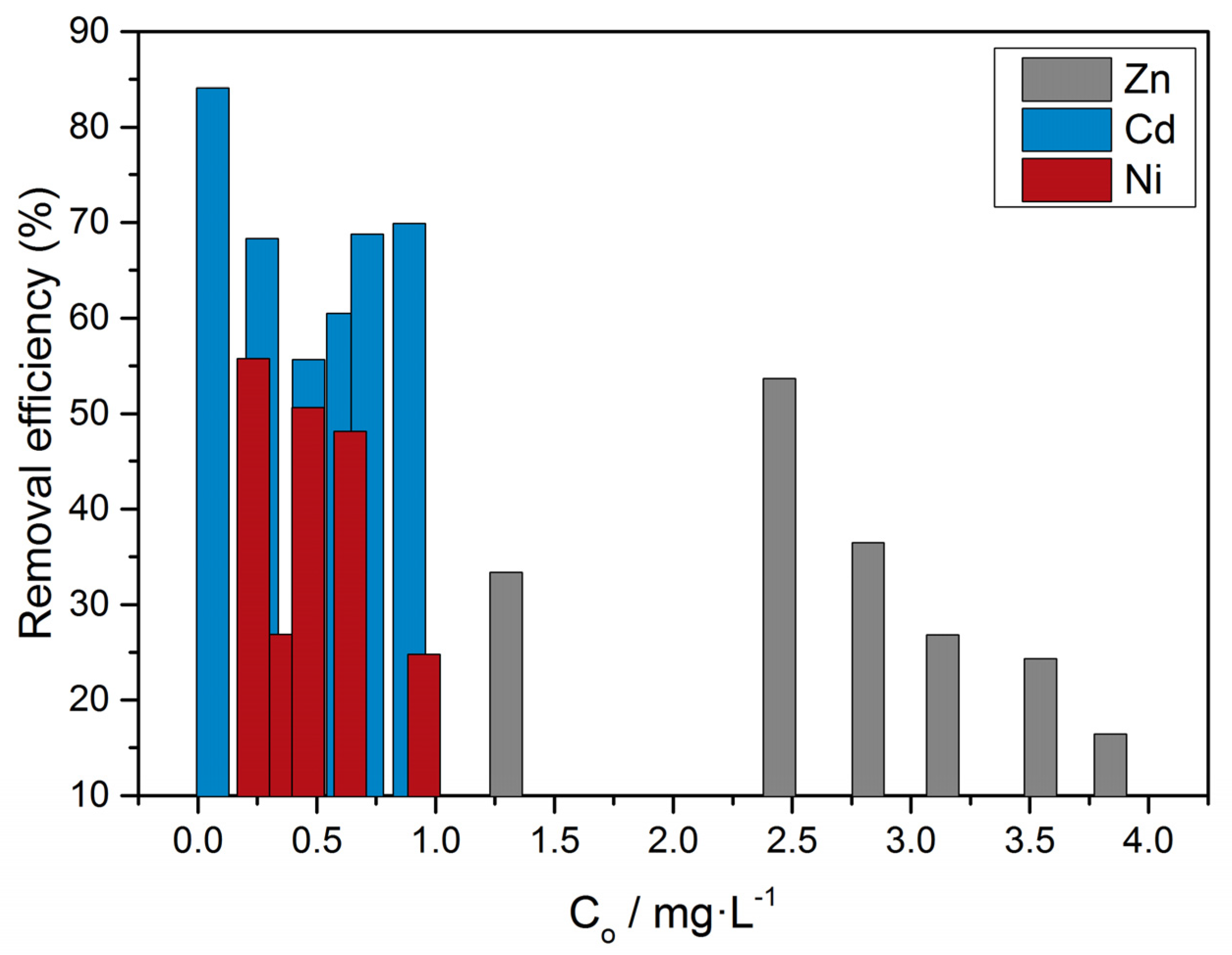
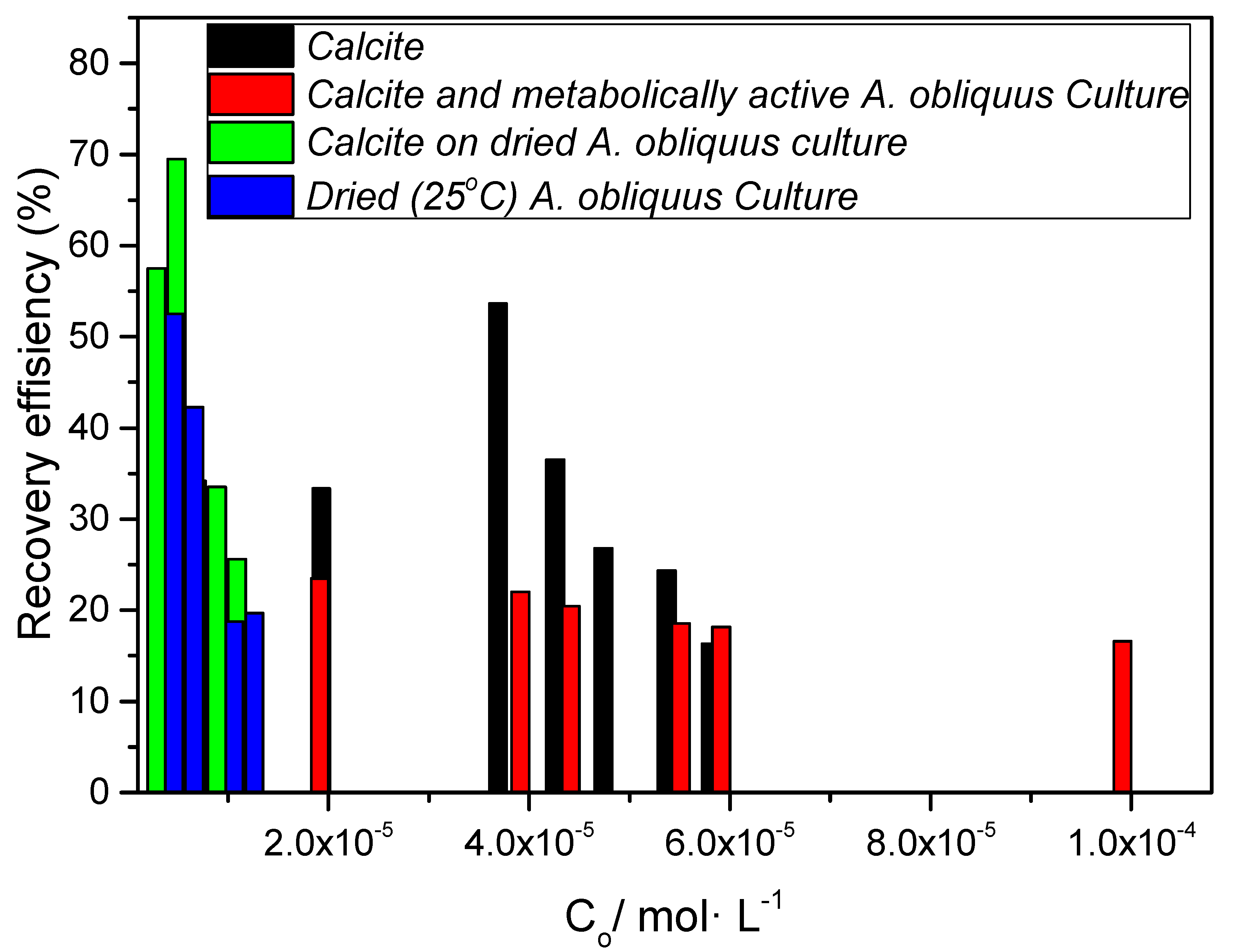
| Substrate | CZn/mol L−1 | ||||
|---|---|---|---|---|---|
| 2 × 10−6 | 4.0 × 10−6 | 6.0 × 10−6 | 8.0 × 10−6 | 10.0 × 10−6 | |
| 50 mg of calcite with metabolically active A. Obliquus culture | S1 | S2 | S3 | S4 | S5 |
| Composite material: 50 mg of precipitated calcite on air-dried A. Obliquus culture | S6 | S7 | S8 | S9 | S10 |
| 50 mg of air-dried A. Obliquus culture | S11 | S12 | S13 | S14 | S15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natsi, P.D.; Koutsoukos, P.G. Calcium Carbonate Crystallization on a Microalgal Matrix: The Effects of Heavy Metal Presence. Crystals 2022, 12, 1424. https://doi.org/10.3390/cryst12101424
Natsi PD, Koutsoukos PG. Calcium Carbonate Crystallization on a Microalgal Matrix: The Effects of Heavy Metal Presence. Crystals. 2022; 12(10):1424. https://doi.org/10.3390/cryst12101424
Chicago/Turabian StyleNatsi, Panagiota D., and Petros G. Koutsoukos. 2022. "Calcium Carbonate Crystallization on a Microalgal Matrix: The Effects of Heavy Metal Presence" Crystals 12, no. 10: 1424. https://doi.org/10.3390/cryst12101424
APA StyleNatsi, P. D., & Koutsoukos, P. G. (2022). Calcium Carbonate Crystallization on a Microalgal Matrix: The Effects of Heavy Metal Presence. Crystals, 12(10), 1424. https://doi.org/10.3390/cryst12101424







