Oxidation Behavior of FeCoNiCrMo High-Entropy Coatings by Atmospheric Plasma Spraying on Zircaloy-4 in Steam at 1100 °C
Abstract
:1. Introduction
2. Experimental Steps
2.1. Materials
2.2. Pretreatment and Preparation of Coatings
2.3. Oxidation
2.4. Characterization
3. Results and Discussion
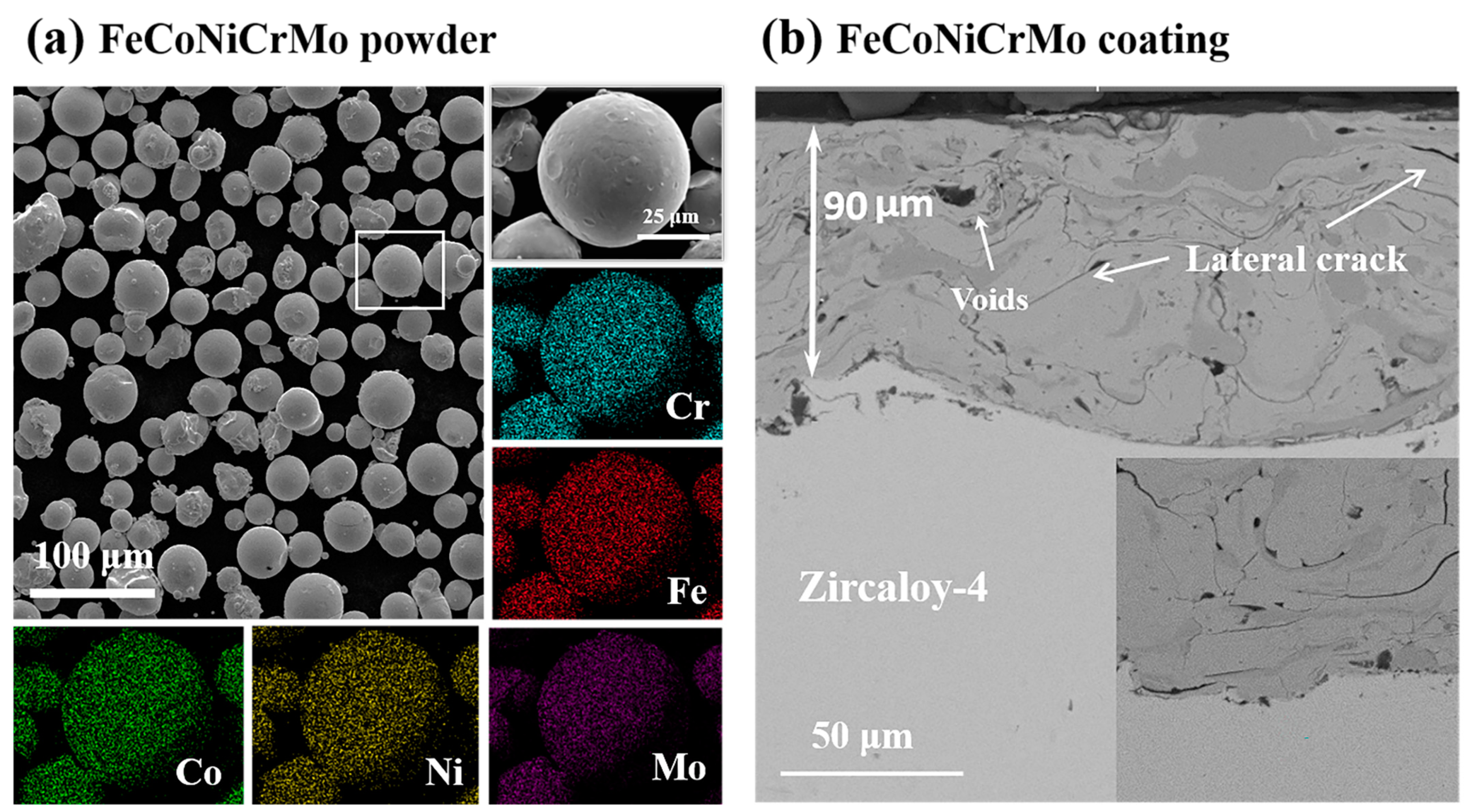
3.1. Microstructure of the Coatings
3.2. Oxidation Tests
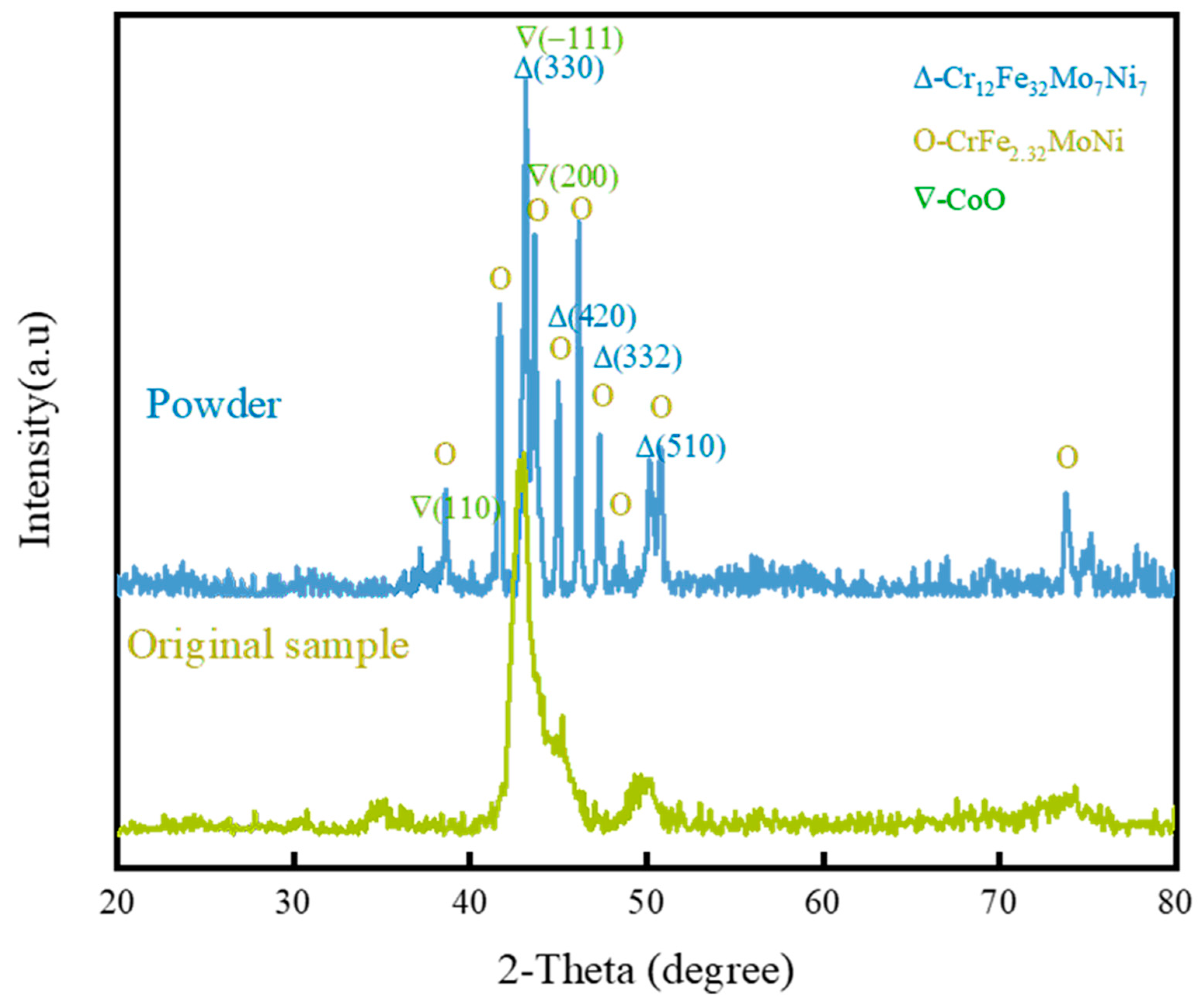
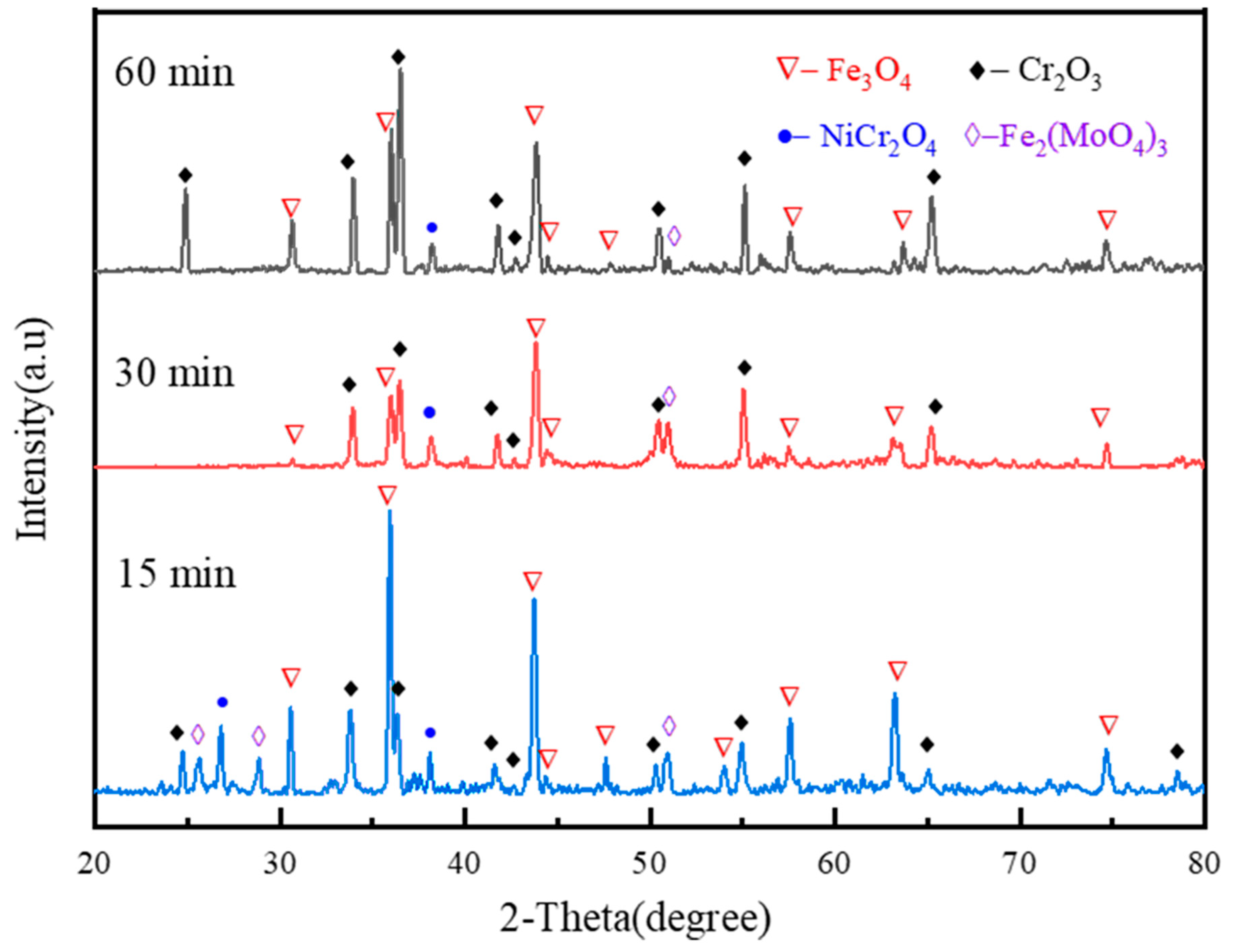
3.2.1. Surface XRD Analysis
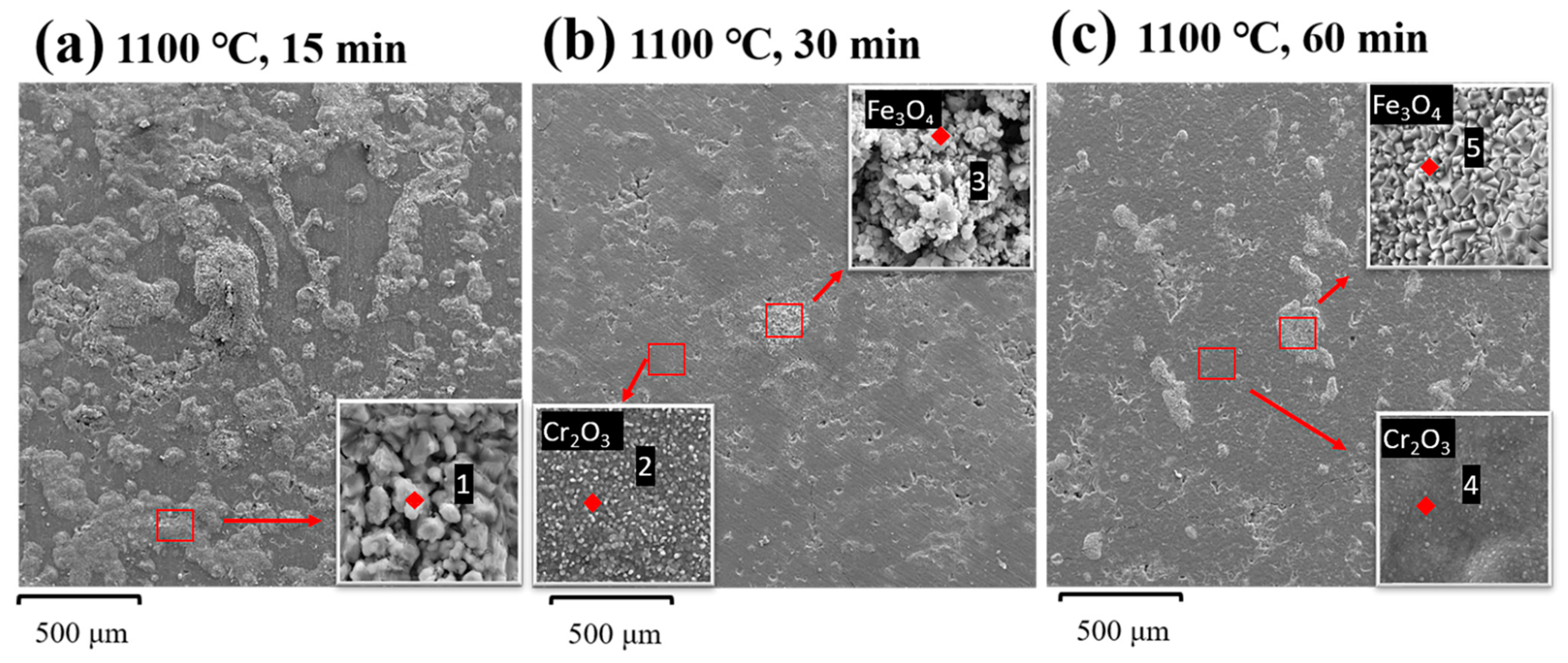
3.2.2. Surface Micromorphology and Oxidation Analysis
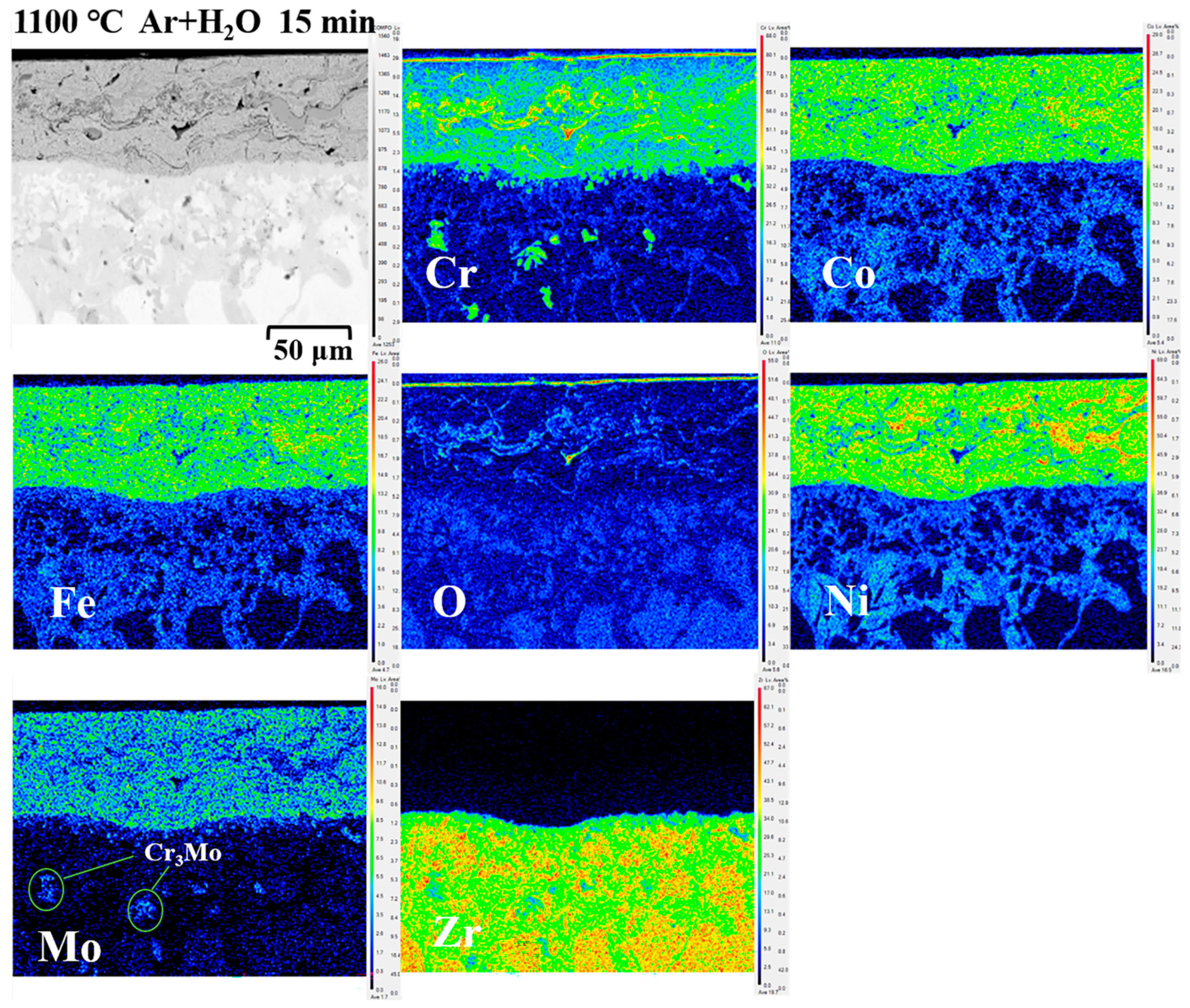
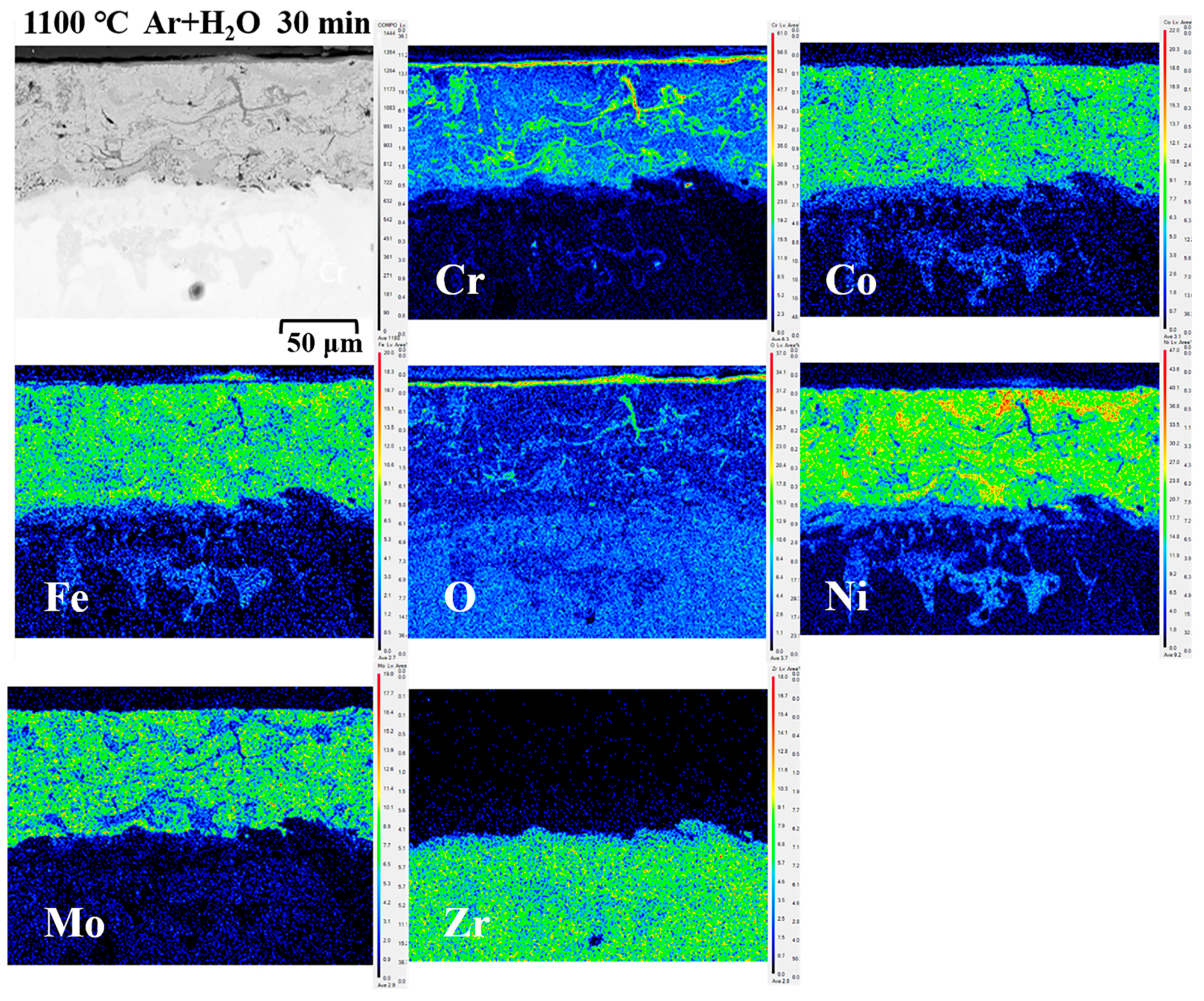
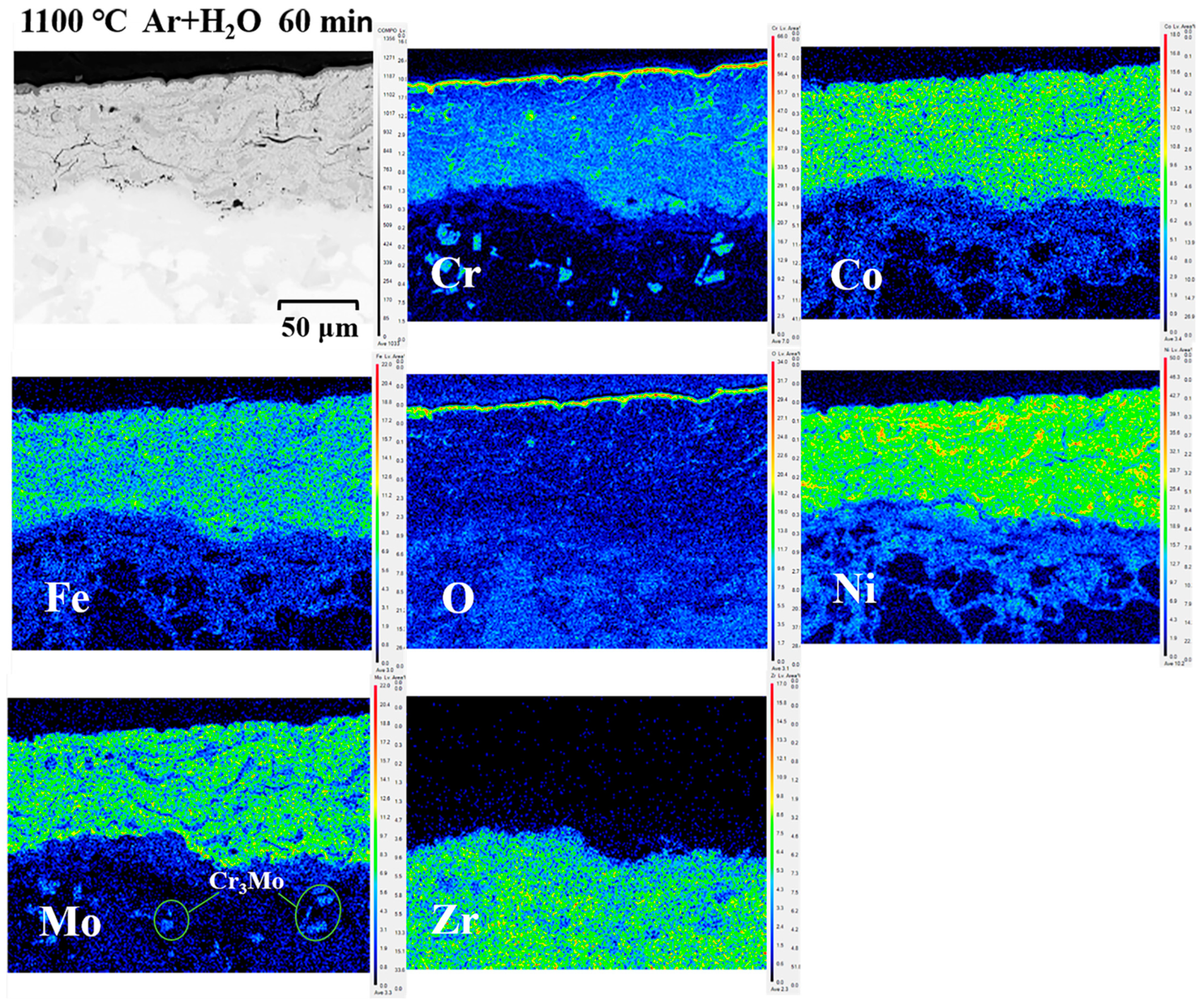
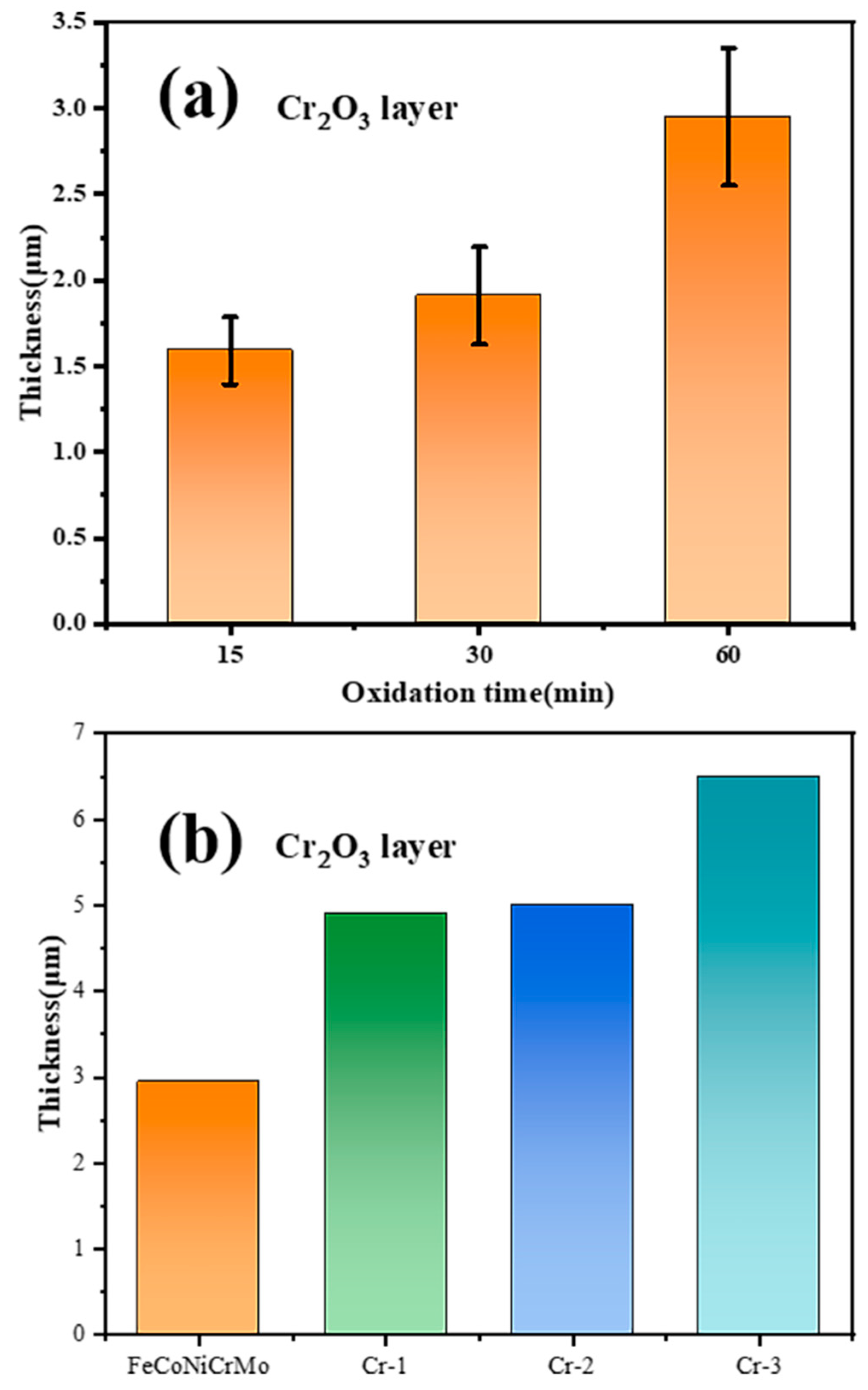
3.2.3. Cross-Sectional Micromorphology and Oxidation Analysis
3.3. Diffusion of Coatings/Zircaloy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.-F.; Wu, Y.-W.; Tan, J.; Meng, C.-Y.; Yang, L.; Dang, W.-A.; He, X.-J. Evaluation of corrosion and oxidation behaviors of TiAlCrN coatings for nuclear fuel cladding. Surf. Coat. Technol. 2019, 358, 521–530. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Ceramic coating for corrosion (c3) resistance of nuclear fuel cladding. Surf. Coat. Technol. 2015, 281, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Koo, Y.H. Development of Surface Modified Zr Cladding by Coating Technology for ATF. In Proceedings of the Top Fuel, Boise, ID, USA, 11–15 September 2016. [Google Scholar]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Koo, Y.H. Progress of surface modified Zr cladding development for ATF in Korea. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017. [Google Scholar]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Chen, Q.S.; Liu, C.H.; Zhang, R.Q.; Yang, H.Y.; Wei, T.G.; Wang, Y.; Li, Z.; He, L.X.; Wang, J.; Wang, L.; et al. Microstructure and high-temperature steam oxidation properties of thick Cr coatings prepared by magnetron sputtering for accident tolerant fuel claddings: The role of bias in the deposition process. Corros. Sci. 2020, 165, 108378. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, H.-G.; Park, J.-y.; Jung, Y.-I.; Park, D.-J.; Koo, Y.-H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Du, P.; Song, P.; Zhang, R.; Li, Z.; Lu, J. Oxidation properties and microstructure of a chromium coating on zircaloy-4 fuel cladding material applied by atmospheric plasma spraying. J. Nucl. Mater. 2022, 560, 153496. [Google Scholar] [CrossRef]
- Li, Q.; Song, P.; Zhang, R.; Li, Z.; Wang, Y.; Du, P.; Lu, J. Oxidation behavior and Cr-Zr diffusion of Cr coatings prepared by atmospheric plasma spraying on zircaloy-4 cladding in steam at 1300 °C. Corros. Sci. 2022, 203, 110378. [Google Scholar] [CrossRef]
- Cheng, B.; Kim, Y.-J.; Chou, P. Improving Accident Tolerance of Nuclear Fuel with Coated Mo-alloy Cladding. Nucl. Eng. Technol. 2016, 48, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Dabney, T.; Johnson, G.; Yeom, H.; Maier, B.; Walters, J.; Sridharan, K. Experimental evaluation of cold spray FeCrAl alloys coated zirconium-alloy for potential accident tolerant fuel cladding. Nucl. Mater. Energy 2019, 21, 100715. [Google Scholar] [CrossRef]
- Gigax, J.G.; Kennas, M.; Kim, H.; Wang, T.; Maier, B.R.; Yeom, H.; Johnson, G.O.; Sridharan, K.; Shao, L. Radiation response of Ti2AlC MAX phase coated Zircaloy-4 for accident tolerant fuel cladding. J. Nucl. Mater. 2019, 523, 26–32. [Google Scholar] [CrossRef]
- Maier, B.R.; Garcia-Diaz, B.L.; Hauch, B.; Olson, L.C.; Sindelar, R.L.; Sridharan, K. Cold spray deposition of Ti2AlC coatings for improved nuclear fuel cladding. J. Nucl. Mater. 2015, 466, 712–717. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Bryk, V.V.; Vasilenko, R.L.; Ovcharenko, V.D.; Reshetnyak, E.N.; Tolmachova, G.N.; V’Yugov, P.N. Vacuum-arc chromium-based coatings for protection of zirconium alloys from the high-temperature oxidation in air. J. Nucl. Mater. 2015, 465, 400–406. [Google Scholar] [CrossRef]
- Zou, Y.; Wheeler, J.M.; Ma, H.; Okle, P.; Spolenak, R. Nanocrystalline High-Entropy Alloys: A New Paradigm in High-Temperature Strength and Stability. Nano Lett. 2017, 17, 1569–1574. [Google Scholar] [CrossRef]
- Munitz, A.; Meshi, L.; Kaufman, M.J. Heat treatments’ effects on the microstructure and mechanical properties of an equiatomic Al-Cr-Fe-Mn-Ni high entropy alloy. Mater. Sci. Eng. A 2017, 689, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Hsu, W.L.; Murakami, H.; Yeh, J.W.; Yeh, A.C.; Shimoda, K. A Heat-Resistant NiCo0.6Fe0.2Cr1.5SiAlTi0.2 Overlay Coating for High-Temperature Applications. J. Electrochem. Soc. 2016, 163, C752–C758. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, Y.; Li, J.; Liu, Y.; Liu, Y. Microstructure and mechanical properties of FeCoCrNiNbX high-entropy alloy coatings. Phys. B Condens. Matter 2018, 550, 112–116. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Long, J.; Wang, J.; Zhang, R.; Yang, H.; Zhang, W.; Yao, F.; Zhao, S.; Zhang, Q. Microstructure and corrosion characteristics of CrCuFeMoNi HEA coatings with different compositions in high-temperature and high-pressure water. Mater. Res. Express 2019, 6, 086511. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, L.; Lui, H.-W.; Cai, M.-H.; Yeh, J.-W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, C.; Shen, Y.; Feng, X. Microstructure and corrosion property of CrMnFeCoNi high entropy alloy coating on Q235 substrate via mechanical alloying method. Surf. Interfaces 2019, 15, 135–140. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Zhang, Y.-P.; He, L.; Liu, C.-G. Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloys Compd. 2013, 549, 195–199. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Shen, J.; Vilar, R. Thermal stability and oxidation resistance of laser clad TiVCrAlSi high entropy alloy coatings on Ti–6Al–4V alloy. Surf. Coat. Technol. 2011, 206, 1389–1395. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Araki, H.; Watanabe, M.; Kuroda, S.; Yeh, A.-C.; Yeh, J.-W. A Study of NiCo0.6Fe0.2CrxSiAlTiyHigh-Entropy Alloys for Applications as a High-Temperature Protective Coating and a Bond Coat in Thermal Barrier Coating Systems. J. Electrochem. Soc. 2018, 165, C524–C531. [Google Scholar] [CrossRef]
- Mohanty, A.; Sampreeth, J.K.; Bembalge, O.; Hascoet, J.Y.; Marya, S.; Immanuel, R.J.; Panigrahi, S.K. High temperature oxidation study of direct laser deposited AlXCoCrFeNi (X = 0.3,0.7) high entropy alloys. Surf. Coat. Technol. 2019, 380, 125028. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Zhang, Y.; Liu, S.-H.; Li, C.-J.; Li, C.-X. High-temperature oxidation behavior of CuAlNiCrFe high-entropy alloy bond coats deposited using high-speed laser cladding process. Surf. Coat. Technol. 2020, 398, 126093. [Google Scholar] [CrossRef]
- Daoud, H.M.; Manzoni, A.M.; Völkl, R.; Wanderka, N.; Glatzel, U. Oxidation Behavior of Al8Co17Cr17Cu8Fe17Ni33, Al23Co15Cr23Cu8Fe15Ni15, and Al17Co17Cr17Cu17Fe17Ni17 Compositionally Complex Alloys (High-Entropy Alloys) at Elevated Temperatures in Air. Adv. Eng. Mater. 2015, 17, 1134–1141. [Google Scholar] [CrossRef]
- Laplanche, G.; Volkert, U.; Eggeler, G.; George, E. Oxidation behavior of the CrMnFeCoNi high-entropy alloy. Oxid. Met. 2016, 85, 629–645. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J.; Dietrich, J.R.; Senkov, O.N. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb20Mo20Cr20Ti20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, Y.; Chen, Z.; Zhang, J.; Shen, B. Oxidation response of a vacuum arc melted NbZrTiCrAl refractory high entropy alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Barin, I.; Platzki, G. Thermochemical Data of Pure Substances; Wiley Online Library: Hoboken, NJ, USA, 1989; Volume 304. [Google Scholar]
- Liu, Y.-x.; Cheng, C.-q.; Shang, J.-l.; Wang, R.; Li, P.; Zhao, J. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferrous Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Syrtanov, M.S.; Kashkarov, E.B.; Abdulmenova, A.V.; Sidelev, D.V. High-temperature oxidation of Zr1Nb zirconium alloy with protective Cr/Mo coating. Surf. Coat. Technol. 2022, 439, 128459. [Google Scholar] [CrossRef]
- Han, X.; Xue, J.; Peng, S.; Zhang, H. An interesting oxidation phenomenon of Cr coatings on Zry-4 substrates in high temperature steam environment. Corros. Sci. 2019, 156, 117–124. [Google Scholar] [CrossRef]
- Ma, H.-B.; Zhao, Y.-H.; Liu, Y.; Zhu, J.-T.; Yan, J.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Liu, G.; Lin, X.-D.; et al. Self-healing behavior of Cr-coated Zr alloy cladding in high temperature steam oxidation process. J. Nucl. Mater. 2022, 558, 153327. [Google Scholar] [CrossRef]
- Hu, X.; Dong, C.; Wang, Q.; Chen, B.; Yang, H.; Wei, T.; Zhang, R.; Gu, W.; Chen, D. High-temperature oxidation of thick Cr coating prepared by arc deposition for accident tolerant fuel claddings. J. Nucl. Mater. 2019, 519, 145–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Yu, X.; Tang, D. Role of Cr in the high-temperature oxidation behavior of CrxMnFeNi high-entropy alloys at 800 °C in air. Corros. Sci. 2022, 200, 110211. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Kai, W.; Li, C.C.; Cheng, F.P.; Chu, K.P.; Huang, R.T.; Tsay, L.W.; Kai, J.J. Air-oxidation of FeCoNiCr-based quinary high-entropy alloys at 700–900 °C. Corros. Sci. 2017, 121, 116–125. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Sweeney, W.E.; Batt, A.P. Electron probe and X-ray diffraction measurements of intermediate phases in Zr diffused with Cr, Fe, Ni, Cu and Mo. J. Nucl. Mater. 1964, 13, 87–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, E.H.; Xiang, C. Irradiation behaviors of two novel single-phase bcc-structure high-entropy alloys for accident-tolerant fuel cladding. J. Mater. Sci. Technol. 2021, 84, 230–238. [Google Scholar] [CrossRef]
- Xia, S.Q.; Zhen, W.A.N.G.; Yang, T.F.; Zhang, Y. Irradiation Behavior in High Entropy Alloys. J. Iron Steel Res. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Ayyagari, A.; Muskeri, S.; Hasannaeimi, V.; Salloom, R.; Chen, W.-Y.; Mukherjee, S. Ion irradiation response and mechanical behavior of reduced activity high entropy alloy. J. Nucl. Mater. 2020, 529, 151955. [Google Scholar] [CrossRef]


| Zr | Sn | Fe | Cr | Ni | Si | O | |
|---|---|---|---|---|---|---|---|
| Zr-4 alloy composition | Base | 1.2–1.7 | 0.18–0.24 | 0.07–0.13 | - | - | 1400 ppm |
| Measuring composition | Base | 1.45 | 0.23 | 0.12 | 0.03 | 0.9 | 0.13 |
| Parameter | Magnitude |
|---|---|
| Spraying voltage | 50 V |
| Spraying current | 610 A |
| Powder feed voltage | 3.5 V |
| Standoff distance | 100 mm |
| Moving rate of spray gun | 12 mm/s |
| H2 pressure | 0.4 MPa |
| Ar pressure | 0.6 MPa |
| Phase | Fe3O4 | Cr2O3 | NiCr2O4 | Fe2(MoO4)3 |
|---|---|---|---|---|
| 15 min | 28.1% | 25.6% | 34.6% | 11.7% |
| 30 min | 25.2% | 35.8% | 25.6% | 13.4% |
| 60 min | 20.9% | 55.5% | 19.1% | 4.4% |
| At% | O | Fe | Cr | Co | Mo | Ni |
|---|---|---|---|---|---|---|
| 1 | 65.3 | 15.0 | 0.5 | 14.2 | 4.9 | 0.1 |
| 2 | 64.4 | 7.1 | 24.3 | 3.2 | 0.0 | 1.0 |
| 3 | 55.6 | 22.4 | 6.7 | 9.2 | 0.0 | 0.7 |
| 4 | 60.3 | 4.7 | 32.3 | 2.0 | 0.9 | 5.2 |
| 5 | 68.1 | 21.4 | 1.7 | 0.1 | 6.5 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Li, Q.; Yang, B.; Yang, Z.; Wang, J.; Song, P. Oxidation Behavior of FeCoNiCrMo High-Entropy Coatings by Atmospheric Plasma Spraying on Zircaloy-4 in Steam at 1100 °C. Crystals 2022, 12, 1529. https://doi.org/10.3390/cryst12111529
Wen L, Li Q, Yang B, Yang Z, Wang J, Song P. Oxidation Behavior of FeCoNiCrMo High-Entropy Coatings by Atmospheric Plasma Spraying on Zircaloy-4 in Steam at 1100 °C. Crystals. 2022; 12(11):1529. https://doi.org/10.3390/cryst12111529
Chicago/Turabian StyleWen, Lei, Qing Li, Bixiao Yang, Zhennan Yang, Jianrui Wang, and Peng Song. 2022. "Oxidation Behavior of FeCoNiCrMo High-Entropy Coatings by Atmospheric Plasma Spraying on Zircaloy-4 in Steam at 1100 °C" Crystals 12, no. 11: 1529. https://doi.org/10.3390/cryst12111529






