Crystalline Phase Segregation of Quantum-Dots-Passivated CH3NH3PbI3 Film via Argon Plasma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Process of MAPbI3 and Cs-Oleate Precursor
2.3. Synthesis and Purification Process of CsPbI3 QDs
2.4. Growth of the Composite Perovskite Film
2.5. Characteristic Measurements
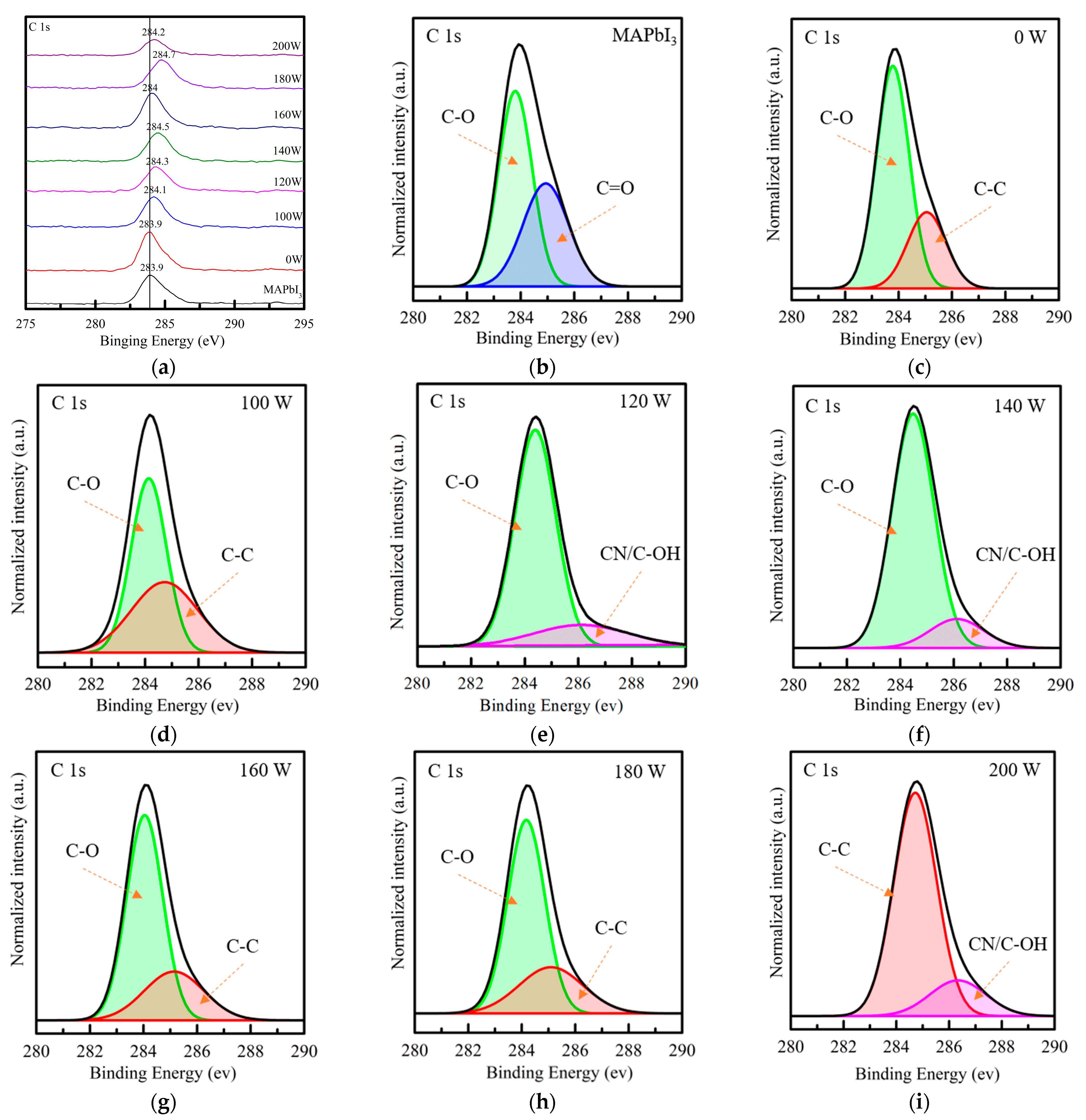
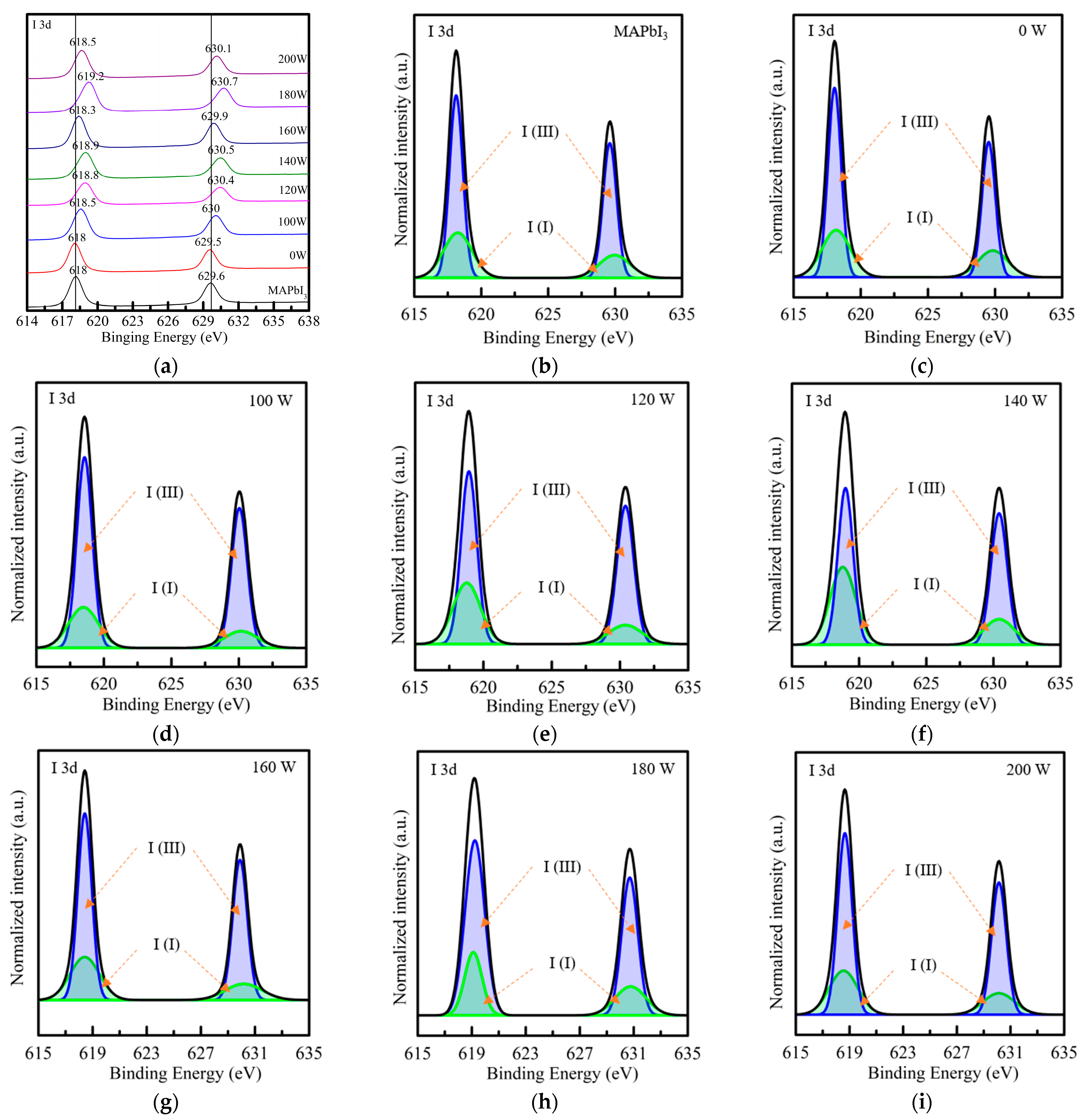
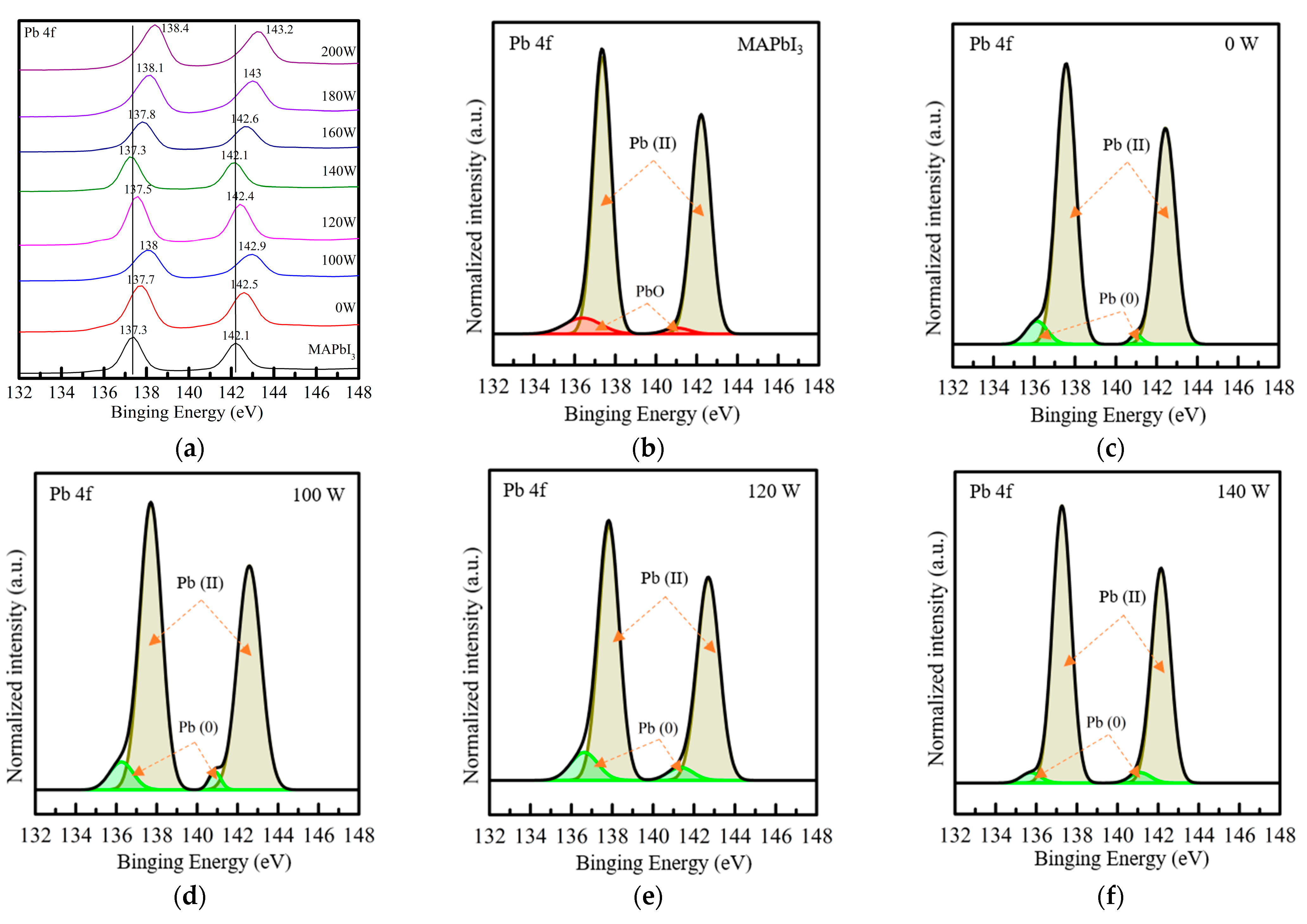
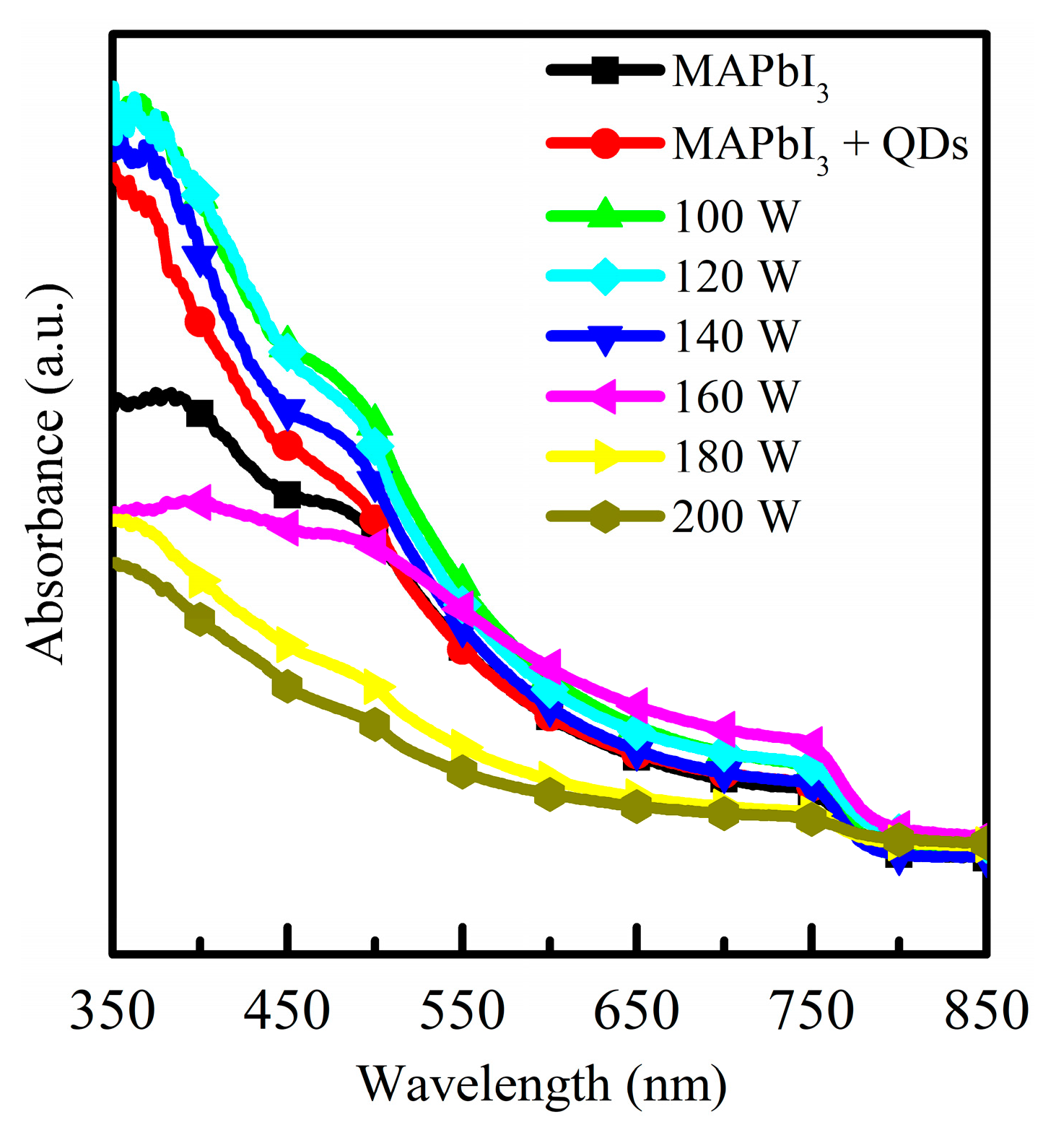
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, F.; Qiu, S.; Hu, J.; Wang, H.; Cai, B.; Li, J.; Yuan, X.; Liu, X.; Forberich, K.; Brabec, C.J.; et al. A Generalized Crystallization Protocol for Scalable Deposition of High—Quality Perovskite Thin Films for Photovoltaic Applications. Adv. Sci. 2019, 6, 1901067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shi, J.; Zheng, J.; Bing, J.; Yuan, J.; Cho, Y.; Tang, S.; Zhang, M.; Yao, Y.; Lau, C.F.J.; et al. Acetic Acid Assisted Crystallization Strategy for High Efficiency and Long—Term Stable Perovskite Solar Cell. Adv. Sci. 2020, 7, 1903368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-C.; Tien, C.-H.; Tseng, Z.-L.; Ruan, J.-H. Enhanced Efficiency of MAPbI3 Perovskite Solar Cells with FAPbX3 Perovskite Quantum Dots. Nanomaterials 2019, 9, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Yuan, F.; Johnston, A.; Gao, C.; Choubisa, H.; Gao, Y.; Wang, Y.; Sagar, L.K.; Sun, B.; Li, P.; et al. High Color Purity Lead—Free Perovskite Light—Emitting Diodes via Sn Stabilization. Adv. Sci. 2020, 7, 1903213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Lin, C.-H.; Hyun, B.-R.; Sher, C.-W.; Lv, Z.; Luo, B.; Jiang, F.; Wu, T.; Ho, C.-H.; Kuo, H.-C.; et al. Micro-Light-Emitting Diodes with Quantum Dots in Display Technology. Light Sci. Appl. 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Liu, X.; Shi, Z.; Fang, X.; He, J. Low-Dimensional Metal Halide Perovskite Photodetectors. Adv. Mater. 2021, 33, 2003309. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Q.; Zhang, Z.; Wu, D.; Yuan, H.; Li, Y.; Qarony, W.; Lau, S.P.; Luo, L.; Tsang, Y.H. Multilayered PdSe2/Perovskite Schottky Junction for Fast, Self—Powered, Polarization-Sensitive, Broadband Photodetectors, and Image Sensor Application. Adv. Sci. 2019, 6, 1901134. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Bucknall, M.P.; Young, T.L.; Zhang, M.; Hu, L.; Bing, J.; Lee, D.S.; Kim, J.; Wu, T.; Takamure, N.; et al. Gas Chromatography–Mass Spectrometry Analyses of Encapsulated Stable Perovskite Solar Cells. Science 2020, 368, eaba2412. [Google Scholar] [CrossRef]
- Xu, J.; Boyd, C.C.; Yu, Z.J.; Palmstrom, A.F.; Witter, D.J.; Larson, B.W.; France, R.M.; Werner, J.; Harvey, S.P.; Wolf, E.J.; et al. Triple-Halide Wide–Band Gap Perovskites with Suppressed Phase Segregation for Efficient Tandems. Science 2020, 367, 1097–1104. [Google Scholar] [CrossRef]
- Hu, L.; Peng, J.; Wang, W.; Xia, Z.; Yuan, J.; Lu, J.; Huang, X.; Ma, W.; Song, H.; Chen, W.; et al. Sequential Deposition of CH 3 NH 3 PbI 3 on Planar NiO Film for Efficient Planar Perovskite Solar Cells. ACS Photonics 2014, 1, 547–553. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; Wang, F.; Ye, J.; He, T.; Wu, F.; Peng, M.; Wu, P.; Chen, Y.; Zhong, F.; et al. A Noble Metal Dichalcogenide for High-Performance Field—Effect Transistors and Broadband Photodetectors. Adv. Funct. Mater. 2020, 30, 1907945. [Google Scholar] [CrossRef]
- Kim, M.; Motti, S.G.; Sorrentino, R.; Petrozza, A. Enhanced Solar Cell Stability by Hygroscopic Polymer Passivation of Metal Halide Perovskite Thin Film. Energy Environ. Sci. 2018, 11, 2609–2619. [Google Scholar] [CrossRef]
- Ni, Z.; Bao, C.; Liu, Y.; Jiang, Q.; Wu, W.-Q.; Chen, S.; Dai, X.; Chen, B.; Hartweg, B.; Yu, Z.; et al. Resolving Spatial and Energetic Distributions of Trap States in Metal Halide Perovskite Solar Cells. Science 2020, 367, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Walter, D.; Ren, Y.; Tebyetekerwa, M.; Wu, Y.; Duong, T.; Lin, Q.; Li, J.; Lu, T.; Mahmud, M.A.; et al. Nanoscale Localized Contacts for High Fill Factors in Polymer-Passivated Perovskite Solar Cells. Science 2021, 371, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Zhang, X.; Yuan, J.; Wang, Y.; Shi, G.; Patterson, R.; Shi, J.; Ling, X.; Hu, L.; Wu, T.; et al. Tuning the Surface-Passivating Ligand Anchoring Position Enables Phase Robustness in CsPbI 3 Perovskite Quantum Dot Solar Cells. ACS Energy Lett. 2020, 5, 3322–3329. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Q.; Huang, S.; Zheng, J.; Guan, X.; Patterson, R.; Kim, J.; Shi, L.; Lin, C.-H.; Lei, Q.; et al. Flexible and Efficient Perovskite Quantum Dot Solar Cells via Hybrid Interfacial Architecture. Nat. Commun. 2021, 12, 466. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, W.; Yue, Y.; Cai, M.; Xie, F.; Bi, E.; Islam, A.; Han, L. Perovskite Solar Cells with 18.21% Efficiency and Area over 1 Cm2 Fabricated by Heterojunction Engineering. Nat. Energy 2016, 1, 16148. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Li, L.; Wang, H.; Huang, Z.; Zhang, Y.; Chen, Y.; Zhang, X.; Zhu, C.; Zai, H.; et al. Liquid Medium Annealing for Fabricating Durable Perovskite Solar Cells with Improved Reproducibility. Science 2021, 373, 561–567. [Google Scholar] [CrossRef]
- Huang, P.-H.; Wang, Y.-H.; Ke, J.-C.; Huang, C.-J. The Effect of Solvents on the Performance of CH3NH3PbI3 Perovskite Solar Cells. Energies 2017, 10, 599. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-Efficiency Rubidium-Incorporated Perovskite Solar Cells by Gas Quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Lau, C.F.J.; Deng, X.; Zheng, J.; Kim, J.; Zhang, Z.; Zhang, M.; Bing, J.; Wilkinson, B.; Hu, L.; Patterson, R.; et al. Enhanced Performance via Partial Lead Replacement with Calcium for a CsPbI3 Perovskite Solar Cell Exceeding 13% Power Conversion Efficiency. J. Mater. Chem. A 2018, 6, 5580–5586. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Y.; Zhao, W.; Han, R.; Wen, J.; Liu, S. (Frank) Molten-Salt-Assisted CsPbI 3 Perovskite Crystallization for Nearly 20%-Efficiency Solar Cells. Adv. Mater. 2021, 33, 2103770. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, Z.; Li, C.; Wang, S.; Hao, F. Benzotriazole Derivative Inhibits Nonradiative Recombination and Improves the UV-Stability of Inverted MAPbI3 Perovskite Solar Cells. J. Energy Chem. 2022, 65, 592–599. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Li, J.; Liang, X.; Wang, L. Enhancement of Thermal Stability for Perovskite Solar Cells through Cesium Doping. RSC Adv. 2017, 7, 17473–17479. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Wang, H.; Ye, F.; Wang, C.; Liu, C.; Zhou, S.; Tao, C.; Fang, G. A Multi-Functional Halogen-Free Cesium Salt Bulk-Doping Treatment toward Performance-Enhancement of Perovskite Solar Cells. J. Power Sources 2022, 520, 230900. [Google Scholar] [CrossRef]
- Huang, P.-H.; Wang, C.-W.; Lien, S.-Y.; Lee, K.-W.; Wang, N.-F.; Huang, C.-J. Investigation of the Stability of Methylammonium Lead Iodide (MAPbI3) Film Doped with Lead Cesium Triiodide (CsPbI3) Quantum Dots under an Oxygen Plasma Atmosphere. Molecules 2021, 26, 2678. [Google Scholar] [CrossRef]
- Zheng, X.; Troughton, J.; Gasparini, N.; Lin, Y.; Wei, M.; Hou, Y.; Liu, J.; Song, K.; Chen, Z.; Yang, C.; et al. Quantum Dots Supply Bulk- and Surface-Passivation Agents for Efficient and Stable Perovskite Solar Cells. Joule 2019, 3, 1963–1976. [Google Scholar] [CrossRef]
- Cheng, F.; He, R.; Nie, S.; Zhang, C.; Yin, J.; Li, J.; Zheng, N.; Wu, B. Perovskite Quantum Dots as Multifunctional Interlayers in Perovskite Solar Cells with Dopant-Free Organic Hole Transporting Layers. J. Am. Chem. Soc. 2021, 143, 5855–5866. [Google Scholar] [CrossRef]
- Elmelund, T.; Scheidt, R.A.; Seger, B.; Kamat, P.V. Bidirectional Halide Ion Exchange in Paired Lead Halide Perovskite Films with Thermal Activation. ACS Energy Lett. 2019, 4, 1961–1969. [Google Scholar] [CrossRef]
- Pan, D.; Fu, Y.; Chen, J.; Czech, K.J.; Wright, J.C.; Jin, S. Visualization and Studies of Ion-Diffusion Kinetics in Cesium Lead Bromide Perovskite Nanowires. Nano Lett. 2018, 18, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Akriti; Shi, E.; Shiring, S.B.; Yang, J.; Atencio-Martinez, C.L.; Yuan, B.; Hu, X.; Gao, Y.; Finkenauer, B.P.; Pistone, A.J.; et al. Layer-by-Layer Anionic Diffusion in Two-Dimensional Halide Perovskite Vertical Heterostructures. Nat. Nanotechnol. 2021, 16, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V.; Kuno, M. Halide Ion Migration in Perovskite Nanocrystals and Nanostructures. Acc. Chem. Res. 2021, 54, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.B.; Zaiats, G.; Wappes, I.; Kamat, P.V. CsPbBr 3 Solar Cells: Controlled Film Growth through Layer-by-Layer Quantum Dot Deposition. Chem. Mater. 2017, 29, 9767–9774. [Google Scholar] [CrossRef] [Green Version]
- Scheidt, R.A.; Atwell, C.; Kamat, P.V. Tracking Transformative Transitions: From CsPbBr 3 Nanocrystals to Bulk Perovskite Films. ACS Mater. Lett. 2019, 1, 8–13. [Google Scholar] [CrossRef]
- Song, H.; Lin, Y.; Zhang, Z.; Rao, H.; Wang, W.; Fang, Y.; Pan, Z.; Zhong, X. Improving the Efficiency of Quantum Dot Sensitized Solar Cells beyond 15% via Secondary Deposition. J. Am. Chem. Soc. 2021, 143, 4790–4800. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Zhang, Z.-X.; Huang, P.-H.; Wu, W.-Y.; Ou, S.-L.; Lien, S.-Y.; Huang, C.-J.; Lee, M.-K.; Zhu, W.-Z. Effect of Plasma Power on the Structural Properties of Tin Oxide Prepared by Plasma-Enhanced Atomic Layer Deposition. Ceram. Int. 2021, 47, 8634–8641. [Google Scholar] [CrossRef]
- Hippler, R.; Cada, M.; Stranak, V.; Hubicka, Z. Time-Resolved Optical Emission Spectroscopy of a Unipolar and a Bipolar Pulsed Magnetron Sputtering Discharge in an Argon/Oxygen Gas Mixture with a Cobalt Target. Plasma Sources Sci. Technol. 2019, 28, 115020. [Google Scholar] [CrossRef]
- Berenguer, C.; Katsonis, K. Plasma Reactors and Plasma Thrusters Modeling by Ar Complete Global Models. Int. J. Aerosp. Eng. 2012, 2012, 740869. [Google Scholar] [CrossRef]
- Lien, S.-Y.; Liu, S.-Y.; Chen, W.-R.; Liu, C.-H.; Sze, P.-W.; Wang, N.-F.; Huang, C.-J. The Influence of Argon Plasma on Organic Perovskite MAPbI3 Film Doped with Inorganic Perovskite CsPbI3 Quantum Dots (QDs). Crystals 2022, 12, 799. [Google Scholar] [CrossRef]
- Luo, S.; Kazes, M.; Lin, H.; Oron, D. Strain-Induced Type II Band Alignment Control in CdSe Nanoplatelet/ZnS-Sensitized Solar Cells. J. Phys. Chem. C 2017, 121, 11136–11143. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Shahzad, N.; Tariq, M.A.; Sattar, A.; Pugliese, D. Investigating the Sequential Deposition Route for Mixed Cation Mixed Halide Wide Bandgap Perovskite Absorber Layer. Energies 2021, 14, 8401. [Google Scholar] [CrossRef]
- Ma, X.-X.; Li, Z.-S. Substituting Cs for MA on the Surface of MAPbI3 Perovskite: A First-Principles Study. Comput. Mater. Sci. 2018, 150, 411–417. [Google Scholar] [CrossRef]
- Zi, M.; Li, J.; Zhang, Z.; Wang, X.; Han, J.; Yang, X.; Qiu, Z.; Gong, H.; Ji, Z.; Cao, B. Effect of Deposition Temperature on Transparent Conductive Properties of γ-CuI Film Prepared by Vacuum Thermal Evaporation: Effect of Deposition Temperature on Transparent Conductive Properties of γ-CuI Film. Phys. Status Solidi A 2015, 212, 1466–1470. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Park, W.-T.; Kang, S.-J.; Xu, Y.; Kim, M.-G.; Noh, Y.-Y. Room-Temperature Solution-Synthesized p-Type Copper(I) Iodide Semiconductors for Transparent Thin-Film Transistors and Complementary Electronics. Adv. Mater. 2018, 30, 1802379. [Google Scholar] [CrossRef]
- Rocks, C.; Svrcek, V.; Maguire, P.; Mariotti, D. Understanding Surface Chemistry during MAPbI3 Spray Deposition and Its Effect on Photovoltaic Performance. J. Mater. Chem. C 2017, 5, 902–916. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Mohs, A.M.; Nie, S. Tuning the Optical and Electronic Properties of Colloidal Nanocrystals by Lattice Strain. Nat. Nanotech. 2009, 4, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Luo, S.; Yin, X.; Zhou, Y.; Nan, H.; Li, J.; Li, X.; Oron, D.; Shen, H.; Lin, H. Hybrid PbS Quantum-Dot-in-Perovskite for High-Efficiency Perovskite Solar Cell. Small 2018, 14, 1801016. [Google Scholar] [CrossRef]
- Senocrate, A.; Acarturk, T.; Kim, G.Y.; Merkle, R.; Starke, U.; Gratzel, M.; Maier, J. Interaction of Oxygen with Halide Perovskites. J. Mater. Chem. A 2018, 6, 10847–10855. [Google Scholar] [CrossRef]
- Huang, P.-H.; Chen, Y.-H.; Lien, S.-Y.; Lee, K.-W.; Wang, N.-F.; Huang, C.-J. Effect of Annealing on Innovative CsPbI3-QDs Doped Perovskite Thin Films. Crystals 2021, 11, 101. [Google Scholar] [CrossRef]


| Materials | Value | Units | Note |
|---|---|---|---|
| methylammonium iodide | 198.75 | mg | |
| cesium carbonate (Cs2CO3) | 0.1 | g | 99.9% |
| lead (II) iodide (PbI2) | 576.25 | mg | 99.9985% |
| oleic acid (C18H34O2) | 0.5 | ml | 90% (analytical reagent) |
| oleyl amine (C18H35NH2) | 1 | ml | 90% |
| 1-octadecene (ODE) | 10 | ml | 90% (technical grade) |
| toluene | 99.8% (anhydrous) | ||
| hexane | 97% (analytical reagent) | ||
| methyl acetate (MeOAc) | 99.5% (anhydrous) | ||
| methylammonium iodide (CH3NH3I) | 99% | ||
| Dimethyl sulfoxide ((CH3)2SO) | 99% | ||
| gamma-Butyrolactone (C4H6O2) | 99.9% | ||
| C2H6OS | 0.5 | ml | |
| C4H6O2 | 0.5 | ml | |
| PbI2 | 0.173 | g | |
| annealing time/temperature | 15/80 | min/°C | |
| Plasma parameters | Value | Units | Note |
| gas | Ar | 99.95% | |
| working power | 100 to 200 | W | 100/120/140/160/180/200 |
| working time | 2 | sec | |
| working pressure | 1.28 | torr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-H.; Liu, S.-Y.; Liu, C.-H.; Wang, N.-F.; Huang, C.-J. Crystalline Phase Segregation of Quantum-Dots-Passivated CH3NH3PbI3 Film via Argon Plasma Treatment. Crystals 2022, 12, 1556. https://doi.org/10.3390/cryst12111556
Huang P-H, Liu S-Y, Liu C-H, Wang N-F, Huang C-J. Crystalline Phase Segregation of Quantum-Dots-Passivated CH3NH3PbI3 Film via Argon Plasma Treatment. Crystals. 2022; 12(11):1556. https://doi.org/10.3390/cryst12111556
Chicago/Turabian StyleHuang, Pao-Hsun, Shao-Yu Liu, Chuan-Hsi Liu, Na-Fu Wang, and Chien-Jung Huang. 2022. "Crystalline Phase Segregation of Quantum-Dots-Passivated CH3NH3PbI3 Film via Argon Plasma Treatment" Crystals 12, no. 11: 1556. https://doi.org/10.3390/cryst12111556







