The Effect of the Degree of Polymerization and Polymer Composition on the Temperature Responsiveness of Cholesteric Semi-Interpenetrating Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Substrates
2.3. Preparation of the Acrylate End-Capped Oligomer
2.4. Preparation of the Thiol End-Capped Oligomer
2.5. Preparation of the Polymer Films
2.6. Characterization
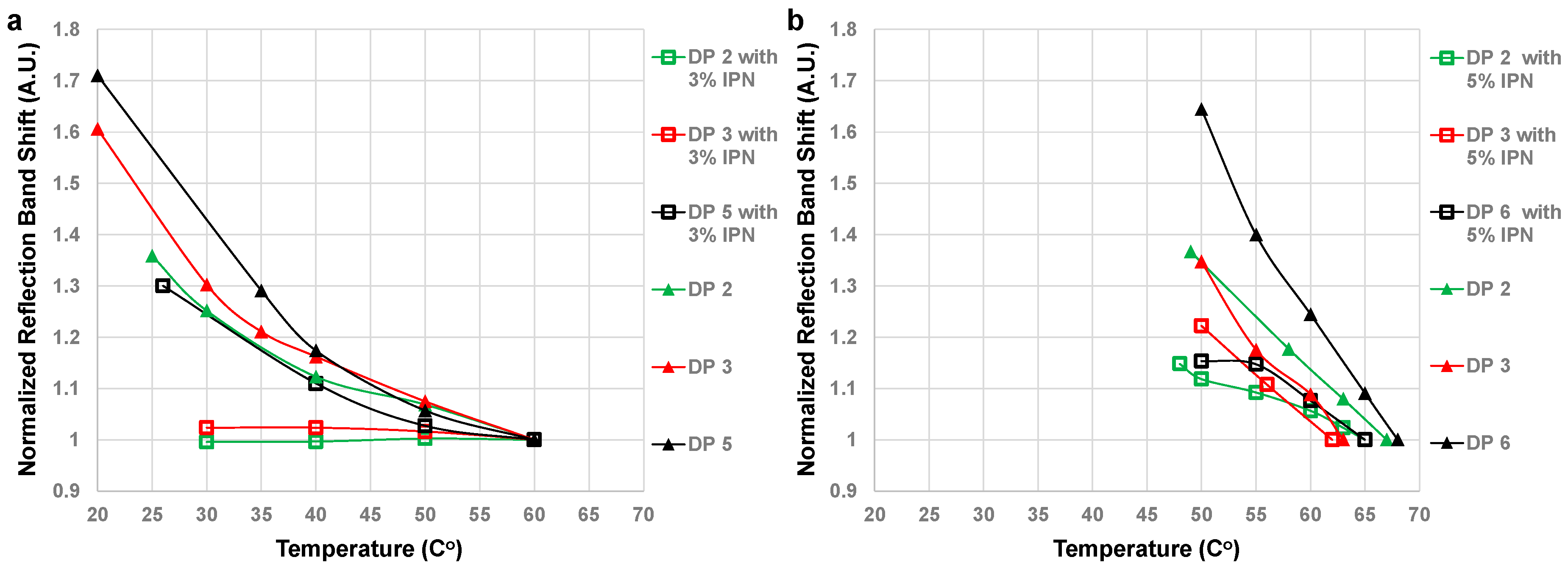
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potyrailo, R.A.; Bonam, R.K.; Hartley, J.G.; Starkey, T.A.; Vukusic, P.; Vasudev, M.; Bunning, T.; Naik, R.R.; Tang, Z.; Palacios, M.A.; et al. Towards outperforming conventional sensor arrays with fabricated individual photonic vapour sensors inspired by Morpho butterflies. Nat. Commun. 2015, 6, 7959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: Facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.F.; Arwin, H.; Järrendahl, K. Polarizing properties and structural characteristics of the cuticle of the scarab Beetle Chrysina gloriosa. Thin Solid Films 2014, 571, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Crne, M.; Park, J.O.; Srinivasarao, M. Bouligand Structures Underlie Circularly Polarized Iridescence of Scarab Beetles: A Closer View. Mater. Today Proc. 2014, 1, 161–171. [Google Scholar] [CrossRef]
- Zhang, P.; de Haan, L.T.; Debije, M.G.; Schenning, A.P.H.J. Liquid crystal-based structural color actuators. Light Sci. Appl. 2022, 11, 248. [Google Scholar] [CrossRef]
- Belmonte, A.; Bus, T.; Broer, D.J.; Schenning, A.P.H.J. Patterned Full-Color Reflective Coatings Based on Photonic Cholesteric Liquid-Crystalline Particles. ACS Appl. Mater. Interfaces 2019, 11, 14376–14382. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, C.I.; Reguera, E.; Stein, A. Tunable Colors in Opals and Inverse Opal Photonic Crystals. Adv. Funct. Mater. 2010, 20, 2565–2578. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, T.Y.; Lee, S.Y.; Lee, S.Y.; Kim, S.-H. Designing Multicolor Micropatterns of Inverse Opals with Photonic Bandgap and Surface Plasmon Resonance. Adv. Funct. Mater. 2018, 28, 1706664. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Arsenault, A.C.; Manners, I.; Ozin, G.A. Electroactive Inverse Opal: A Single Material for All Colors. Angew. Chem. Int. Ed. 2009, 48, 943–947. [Google Scholar] [CrossRef]
- White, T.J.; McConney, M.E.; Bunning, T.J. Dynamic color in stimuli-responsive cholesteric liquid crystals. J. Mater. Chem. 2010, 20, 9832–9847. [Google Scholar] [CrossRef]
- Ranjkesh, A.; Choi, Y.; Huh, J.W.; Oh, S.W.; Yoon, T.H. Flexible, broadband, super-reflective infrared reflector based on cholesteric liquid crystal polymer. Sol. Energy Mater. Sol. Cells 2021, 230, 111137. [Google Scholar] [CrossRef]
- Yelamaggad, C.V.; Shanker, G.; Hiremath, U.S.; Prasad, S.K. Cholesterol-based nonsymmetric liquid crystal dimers: An overview. J. Mater. Chem. 2008, 18, 2927–2949. [Google Scholar] [CrossRef]
- Chilaya, G.S. Effect of various external factors and pretransitional phenomena on structural transformations in cholesteric liquid crystals. Crystallogr. Rep. 2000, 45, 871–886. [Google Scholar] [CrossRef]
- Dhar, R. Twisted-grain-boundary (TGB) phases: Nanostructured liquid-crystal analogue of Abrikosov vortex lattices. Phase Transit. 2006, 79, 175–199. [Google Scholar] [CrossRef]
- Nam, S.; Wang, D.; Lee, G.; Choi, S.S. Broadband wavelength tuning of electrically stretchable chiral photonic gel. Nanophotonics 2022, 11, 2139–2148. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, X.; Schenning, A.P.H.J.; Zhou, G.; Haan, L.T. A Patterned Mechanochromic Photonic Polymer for Reversible Image Reveal. Adv. Mater. Interfaces 2020, 7, 1901878. [Google Scholar] [CrossRef]
- Shi, X.; Deng, Z.; Zhang, P.; Wang, Y.; Zhou, G.; de Haan, L.T. Wearable Optical Sensing of Strain and Humidity: A Patterned Dual-Responsive Semi-Interpenetrating Network of a Cholesteric Main-Chain Polymer and a Poly(ampholyte). Adv. Funct. Mater. 2021, 31, 2104641. [Google Scholar] [CrossRef]
- Van Heeswijk, E.P.A.; Yang, L.; Grossiord, N.; Schenning, A.P.H.J. Tunable Photonic Materials via Monitoring Step-Growth Polymerization Kinetics by Structural Colors. Adv. Funct. Mater. 2020, 30, 1906833. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-H.; Liu, M.-F.; Hwang, S.-J. Optical sensing of organic vapour based on polymer cholesteric liquid crystal film. Liq. Cryst. 2020, 47, 1390–1397. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Broer, D.J.; Schenning, A.P.H.J. Stimuli-responsive photonic polymer coatings. Chem. Commun. 2014, 50, 15839–15848. [Google Scholar] [CrossRef]
- Zhang, W.; Froyen, A.A.F.; Schenning, A.P.H.J.; Zhou, G.; Debije, M.G.; de Haan, L.T. Temperature-Responsive Photonic Devices Based on Cholesteric Liquid Crystals. Adv. Photonics Res. 2021, 2, 2100016. [Google Scholar] [CrossRef]
- Moirangthem, M.; Arts, R.; Merkx, M.; Schenning, A.P.H.J. An Optical Sensor Based on a Photonic Polymer Film to Detect Calcium in Serum. Adv. Funct. Mater. 2016, 26, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yamane, H.; Kikuchi, H.; Yamane, H.; Zhang, G.; Chen, X.; Tisato, K. Investigation of the Electrothermo-Optical Effect of a Smectic LCP-Nematic LC-Chiral Dopant Ternary Composite System Based on S A} N* Phase Transition. J. Appl. Polym. Sci. 1999, 73, 623–631. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, D.-K. Temperature dependence of pitch and twist elastic constant in a cholesteric to smectic A phase transition. Liq. Cryst. 2002, 29, 1497–1501. [Google Scholar] [CrossRef]
- Yoon, H.G.; Dierking, I.; Gleeson, H.F. Cholesteric pitch divergence near smectic phase transitions. Phys. Rev. E 2010, 82, 011705. [Google Scholar] [CrossRef]
- Zhang, W.; Schenning, A.P.H.J.; Kragt, A.J.J.; Zhou, G.; de Haan, L.T. Reversible thermochromic photonic coatings with a protective topcoat. ACS Appl. Mater. Interfaces 2021, 13, 3153–3160. [Google Scholar] [CrossRef]
- Keating, P.N. A Theory of the Cholesteric Mesophase. Mol. Cryst. 1969, 8, 315–326. [Google Scholar] [CrossRef]
- Tzeng, S.-Y.T.; Chen, C.-N.; Tzeng, Y. Thermal tuning band gap in cholesteric liquid crystals. Liq. Cryst. 2010, 37, 1221–1224. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Wang, L.; Yang, D.; Yu, H.; Yang, H. Polymeric infrared reflective thin films with ultra-broad bandwidth. Liq. Cryst. 2016, 43, 750–757. [Google Scholar] [CrossRef]
- Yue, L.; Shi, X.; Zhou, G.; de Haan, L.T. Controlling the Phase Behavior and Reflection of Main-Chain Cholesteric Oligomers Using a Smectic Monomer. Int. J. Mol. Sci. 2022, 23, 3275. [Google Scholar] [CrossRef]
- Wang, J.-W.; Zhang, B.-Y. Synthesis and optical properties of cholesteric liquid-crystalline oligomers displaying reversible thermochromism. J. Appl. Polym. Sci. 2013, 130, 1321–1327. [Google Scholar] [CrossRef]
- Nagai, H.; Urayama, K. Thermal response of cholesteric liquid crystal elastomers. Phys. Rev. E 2015, 92, 22501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lin, X.; You, Y.; Hu, X.; de Haan, L.; Zhao, W.; Zhou, G.; Yuan, D. Flexible thermal responsive infrared reflector based on cholesteric liquid crystals and polymer stabilized cholesteric liquid crystals. Opt. Express 2019, 27, 13516–13525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Schenning, A.P.H.J.; Kragt, A.J.J.; Zhou, G.; de Haan, L.T. Thermochromic Multicolored Photonic Coatings with Light Polarization- and Structural Color-Dependent Changes. ACS Appl. Polym. Mater. 2022, 4, 537–545. [Google Scholar] [CrossRef]
- Zhang, W.; Lub, J.; Schenning, A.P.H.J.; Zhou, G.; de Haan, L.T. Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings. Int. J. Mol. Sci. 2020, 21, 1803. [Google Scholar] [CrossRef] [Green Version]
- Dmochowska, E.; Herman, J.; Czerwiński, M.; Stulov, S.; Bubnov, A.; Kula, P. Self-assembling behaviour of chiral calamitic monoacrylates targeted for polymer stabilisation of polar smectic phases in chiral liquid crystals. J. Mol. Liq. 2021, 331, 115723. [Google Scholar] [CrossRef]
- Herman, J.; Dmochowska, E.; Czerwiński, M. Synthesis of new chiral mono- and diacrylates for ferro- and antiferroelectric liquid crystals. J. Mol. Liq. 2018, 271, 353–360. [Google Scholar] [CrossRef]



| No. | Compositions (mol%) | Average DP | |||
|---|---|---|---|---|---|
| Monomer 1 | Monomer 3 | EDDET 4 | DPA 6 | ||
| 1 | 62.4 | 2.9 | 32.7 | 2.0 | 2.0 |
| 2 | 55.7 | 2.9 | 39.0 | 2.4 | 3.0 |
| 3 | 51.2 | 2.9 | 43.3 | 2.6 | 5.0 |
| No. | Compositions (mol%) | Average DP | ||||
|---|---|---|---|---|---|---|
| Monomer 1 | Monomer 2 | Monomer 3 | EDDET 4 | DPA 6 | ||
| 1 | 46.0 | 15.3 | 3.4 | 32.0 | 3.3 | 2.0 |
| 2 | 41.3 | 13.7 | 3.4 | 38.9 | 2.7 | 3.0 |
| 3 | 38.1 | 12.7 | 2.9 | 42.8 | 3.5 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Zhou, G.; de Haan, L.T. The Effect of the Degree of Polymerization and Polymer Composition on the Temperature Responsiveness of Cholesteric Semi-Interpenetrating Networks. Crystals 2022, 12, 1614. https://doi.org/10.3390/cryst12111614
Yue L, Zhou G, de Haan LT. The Effect of the Degree of Polymerization and Polymer Composition on the Temperature Responsiveness of Cholesteric Semi-Interpenetrating Networks. Crystals. 2022; 12(11):1614. https://doi.org/10.3390/cryst12111614
Chicago/Turabian StyleYue, Lansong, Guofu Zhou, and Laurens T. de Haan. 2022. "The Effect of the Degree of Polymerization and Polymer Composition on the Temperature Responsiveness of Cholesteric Semi-Interpenetrating Networks" Crystals 12, no. 11: 1614. https://doi.org/10.3390/cryst12111614
APA StyleYue, L., Zhou, G., & de Haan, L. T. (2022). The Effect of the Degree of Polymerization and Polymer Composition on the Temperature Responsiveness of Cholesteric Semi-Interpenetrating Networks. Crystals, 12(11), 1614. https://doi.org/10.3390/cryst12111614






