Two Novel Pyrene Tetra-Sulfonate Europium Coordination Polymers: Structure Formation Mechanism Analysis and Sequential Modulation Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Eu6(μ6-O)(μ3-OH)8(NO3)6(1,3,6,8-H2PTS)(H2O)10 (1)
2.2. Synthesis of Eu(NO3)(1,3,6,8-PTS)0.5(H2O)3·0.5bipy (2)
2.3. X-ray Crystallographic Study
3. Results and Discussion
3.1. Crystal Structure
3.1.1. Eu6(μ6-O)(μ3-OH)8(NO3)6(1,3,6,8-H2PTS)(H2O)10 (1)
3.1.2. Eu(NO3)(1,3,6,8-PTS)0.5(H2O)3·0.5bipy (2)
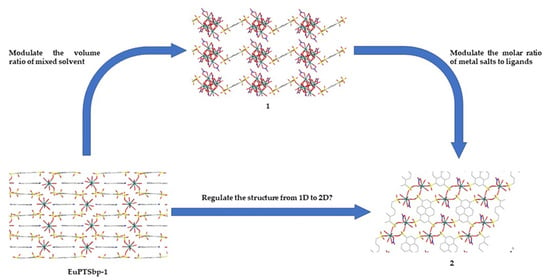
3.2. Structure Comparison and Formation Mechanism Speculation for EuPTS-CPs
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tu, M.; Xia, B.Z.; Kravchenko, D.E.; Tietze, M.L.; Cruz, A.J.; Stassen, I.; Hauffman, T.; Teyssandier, J.; De Feyter, S.; Wang, Z.; et al. Direct X-ray and electron-beam lithography of halogenated zeolitic imidazolate frameworks. Nat. Mater. 2021, 20, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.R.; Zhao, X.; Su, H.; Tang, F.M.; Che, W.; Zhang, H.; Liu, Q.H. Lattice-strained metal-organic-framework arrays for bifunctional oxygen electrocatalysis. Nat. Energy 2019, 4, 115–122. [Google Scholar] [CrossRef]
- Arora, H.; Dong, R.H.; Venanzi, T.; Zscharschuch, J.; Schneider, H.; Helm, M.; Feng, X.L.; Canovas, E.; Erbe, A. Demonstration of a Broadband Photodetector Based on a Two-Dimensional Metal-Organic Framework. Adv. Mater. 2020, 32, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, Y.; Yang, L.; Qin, L.; Dong, Y.H.; Zhou, Z.; Zhang, D.P.; Wang, S.N. Self-assembly of a cobalt(II)-based metal-organic framework as an effective water-splitting heterogeneous catalyst for light-driven hydrogen production. Acta Crystallogr. Sect. C-Struct. Chem. 2020, 76, 616–624. [Google Scholar] [CrossRef]
- Chen, S.S.; Han, S.S.; Ma, C.B.; Li, W.D.; Zhao, Y. A Series of Metal-Organic Frameworks: Syntheses, Structures and Luminescent Detection, Gas Adsorption, Magnetic Properties. Cryst. Growth Des. 2021, 21, 869–885. [Google Scholar] [CrossRef]
- Du, H.; Fu, J.; Liu, L.-X.; Ding, S.; Lyu, Z.; Chang, Y.-C.; Jin, X.; Kengara, F.O.; Song, B.; Min, Q.; et al. Recent progress in electrochemical reduction of carbon monoxide toward multi-carbon products. Mater. Today 2022, 59, 182–199. [Google Scholar] [CrossRef]
- Lu, T.Q.; Cheng, L.T.; Wang, X.T.; Chen, C.; Zheng, J.; Lu, D.F.; Zheng, X.Y. Lanthanide Metal-Organic Frameworks with High Chemical Stability as Multifunctional Materials: Cryogenic Magnetic Cooler and Luminescent Probe. Cryst. Growth Des. 2022, 22, 4917–4925. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, H.; Zhu, C.; Yue, C.; Zhu, W.; Liu, F.; Chen, Z.-X. Ultralow-Energy-Barrier H2O2 Dissociation on Coordinatively Unsaturated Metal Centers in Binary Ce-Fe Prussian Blue Analogue for Efficient and Stable Photo-Fenton Catalysis. Energy Environ. Mater. 2022, e12476. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, J.; Xi, L.; Xie, J.; Wang, X.F.; Ma, Y.; Li, L.C. Two Novel Lanthanide Metal-Organic Frameworks: Selective Luminescent Sensing for Nitrobenzene, Cu2+, and MnO4. Cryst. Growth Des. 2020, 20, 5225–5234. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal–organic framework based nanozymes: Promising materials for biochemical analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Lu, B.-B.; Han, X.; Feng, C.-J.; Wang, D.; Ye, F. Two Co(II)-Based MOFs Constructed from Resorcin[4]Arene Ligand: Syntheses, Structures, and Heterogeneous Catalyst for Conversion of CO2. Crystals 2021, 11, 574. [Google Scholar] [CrossRef]
- Mahmoud, E. Quantitative Structure–Property Relationships from Experiments for CH4 Storage and Delivery by Metal–Organic Frameworks. Crystals 2020, 10, 700. [Google Scholar] [CrossRef]
- Du, H.; Liu, L.-X.; Cai, Y.; Wang, Y.; Zhang, J.-R.; Min, Q.; Zhu, W. In situ formed N-containing copper nanoparticles: A high-performance catalyst toward carbon monoxide electroreduction to multicarbon products with high faradaic efficiency and current density. Nanoscale 2022, 14, 7262–7268. [Google Scholar] [CrossRef] [PubMed]
- Abdolalian, P.; Morsali, A. Flexible and breathing metal-organic framework with high and selective carbon dioxide storage versus nitrogen. Polyhedron 2019, 161, 56–62. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.a.H. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.; Park, S.; Oh, M. Coordination polymer nanorods of Fe-MIL-88B and their utilization for selective preparation of hematite and magnetite nanorods. Chem. Commun. 2011, 47, 4138–4140. [Google Scholar] [CrossRef]
- Kong, A.; Mao, C.; Lin, Q.; Wei, X.; Bu, X.; Feng, P. From cage-in-cage MOF to N-doped and Co-nanoparticle-embedded carbon for oxygen reduction reaction. Dalton Trans. 2015, 44, 6748–6754. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-Organic Framework as a Template for Porous Carbon Synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, M.; Fan, G.; Yang, A.; Meyer, J.R.; Ou, Y.; Yin, B.; Fortner, J.; Foston, M.; Li, Z.; et al. Facet-Dependent Enhancement in the Activity of Bismuth Vanadate Microcrystals for the Photocatalytic Conversion of Methane to Methanol. ACS Appl. Nano Mater. 2018, 1, 6683–6691. [Google Scholar] [CrossRef]
- He, Y.H.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.Y.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260. [Google Scholar] [CrossRef]
- Niu, X.; Shi, Q.; Zhu, W.; Liu, D.; Tian, H.; Fu, S.; Cheng, N.; Li, S.; Smith, J.N.; Du, D.; et al. Unprecedented peroxidase-mimicking activity of single-atom nanozyme with atomically dispersed Fe–Nx moieties hosted by MOF derived porous carbon. Biosens. Bioelectron. 2019, 142, 111495. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Nam, S.; Kim, T.; Im, J.H.; Jung, H.; Kang, J.H.; Wi, S.; Park, B.; Park, C.R. Preparation and Exceptional Lithium Anodic Performance of Porous Carbon-Coated ZnO Quantum Dots Derived from a Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 7394–7397. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of Core–Shell Metal–Organic Frameworks: A New Method for Selectively Functionalized Nanoporous Hybrid Carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Yang, X.; Liu, L.-X.; Cai, Y.; Wu, Y.; Wang, S.; Li, H.; Zhou, Y.; Wang, Y.; et al. Metal-Based Aerogels Catalysts for Electrocatalytic CO2 Reduction. Chem. A Eur. J. 2022, 28, e202201834. [Google Scholar] [CrossRef]
- Liu, J.; Cai, Y.; Song, R.; Ding, S.; Lyu, Z.; Chang, Y.-C.; Tian, H.; Zhang, X.; Du, D.; Zhu, W.; et al. Recent progress on single-atom catalysts for CO2 electroreduction. Mater. Today 2021, 48, 95–114. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like Mesoporous α-Fe2O3 Anode Material Prepared from MOF Template for High-Rate Lithium Batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-F.; He, T.; Yue, K.-F.; Liu, Y.-L.; Zhou, C.-S.; Yan, N.; Wang, Y.-Y. Temperature-Induced Syntheses, Iodine Elimination, Enantiomers Resolution, and Single-Crystal-to-Single-Crystal Transformation of Imidazole-Co(II) Coordination Polymers with Amino-isophthalic Acid as Co-Ligand. Cryst. Growth Des. 2016, 16, 3961–3968. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, K.; Gao, S.; Kobayashi, H. Formate-Based Magnetic Metal–Organic Frameworks Templated by Protonated Amines. Adv. Mater. 2010, 22, 1526–1533. [Google Scholar] [CrossRef]
- Mazaj, M.; Birsa Čelič, T.; Mali, G.; Rangus, M.; Kaučič, V.; Zabukovec Logar, N. Control of the Crystallization Process and Structure Dimensionality of Mg–Benzene–1,3,5-Tricarboxylates by Tuning Solvent Composition. Cryst. Growth Des. 2013, 13, 3825–3834. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, D.-B.; Wan, B.; Li, X.-H.; Shi, Q. Construction of 1D to 3D cadmium(II) coordination polymers from 4-(imidazol-1-yl)-benzoic acid: Effect of bridging anions. Inorg. Chim. Acta 2014, 413, 187–193. [Google Scholar] [CrossRef]
- Liu, K.; Hu, H.; Sun, J.; Zhang, Y.; Han, J.; Wang, L. pH–value-controlled assembly of photoluminescent zinc coordination polymers in the mixed-ligand system. J. Mol. Struct. 2017, 1134, 174–179. [Google Scholar] [CrossRef]
- Shimizu, G.K.; Enright, G.D.; Ratcliffe, C.I.; Preston, K.F.; Reid, J.L.; Ripmeester, J.A. A layered silver sulfonate incorporating nine-coordinate AgI in a hexagonal grid. Chem. Commun. 1999, 16, 1485–1486. [Google Scholar] [CrossRef]
- Cai, J. Structural chemistry and properties of metal arenesulfonates. Coord. Chem. Rev. 2004, 248, 1061–1083. [Google Scholar] [CrossRef]
- Côté, A.P.; Shimizu, G.K.H. The supramolecular chemistry of the sulfonate group in extended solids. Coord. Chem. Rev. 2003, 245, 49–64. [Google Scholar] [CrossRef]
- Meng, X.; Song, S.-Y.; Song, X.-Z.; Zhu, M.; Zhao, S.-N.; Wu, L.-L.; Zhang, H.-J. A tetranuclear copper cluster-based MOF with sulfonate–carboxylate ligands exhibiting high proton conduction properties. Chem. Commun. 2015, 51, 8150–8152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wei, G.; Liu, Z.; Oliver, S.R.J.; Fei, H. A Robust Sulfonate-Based Metal–Organic Framework with Permanent Porosity for Efficient CO2 Capture and Conversion. Chem. Mater. 2016, 28, 6276–6281. [Google Scholar] [CrossRef]
- D’Vries, R.F.; de la Peña-O’Shea, V.A.; Snejko, N.; Iglesias, M.; Gutiérrez-Puebla, E.; Monge, M.Á. Insight into the Correlation between Net Topology and Ligand Coordination Mode in New Lanthanide MOFs Heterogeneous Catalysts: A Theoretical and Experimental Approach. Cryst. Growth Des. 2012, 12, 5535–5545. [Google Scholar] [CrossRef]
- Li, H.; Sheng, T.; Xue, Z.; Zhu, X.; Hu, S.; Wen, Y.; Fu, R.; Zhuo, C.; Wu, X. Synthesis, structure, characterization, and multifunctional properties of a family of rare earth organic frameworks. Crystengcomm 2017, 19, 2106–2112. [Google Scholar] [CrossRef]
- Demel, J.; Kubát, P.; Millange, F.; Marrot, J.; Císařová, I.; Lang, K. Lanthanide-Porphyrin Hybrids: From Layered Structures to Metal–Organic Frameworks with Photophysical Properties. Inorg. Chem. 2013, 52, 2779–2786. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, H.; Zhu, X.; Li, H. A luminescence europium Metal-organic coordination polymer for Room-Temperature X-ray detection. Inorg. Chem. Commun. 2022, 136, 109182. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Xue, D. Selected Controlled Synthesis of Three-Dimensional 4d−4f Heterometallic Coordination Frameworks by Lanthanide Carboxylate Subunits and Silver Centers. Cryst. Growth Des. 2006, 6, 2551–2557. [Google Scholar] [CrossRef]
- Yan, C.; Li, K.; Wei, S.-C.; Wang, H.-P.; Fu, L.; Pan, M.; Su, C.-Y. Lanthanide homometallic and d–f heterometallic MOFs from the same tripodal ligand: Structural comparison, one photon (OP) vs. two photon (TP) luminescence and selective guest adsorption behavior. J. Mater. Chem. 2012, 22, 9846–9852. [Google Scholar] [CrossRef]
- Calvez, G.; Daiguebonne, C.; Guillou, O.; Le Dret, F. A New Series of Anhydrous Lanthanide-Based Octahedral Hexanuclear Complexes. Eur. J. Inorg. Chem. 2009, 2009, 3172–3178. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Bögge, H.; Schmidtmann, M.; Peters, F. Organizational Forms of Matter: An Inorganic Super Fullerene and Keplerate Based on Molybdenum Oxide. Angew. Chem. Int. Ed. 1998, 37, 3359–3363. [Google Scholar] [CrossRef]
- Beall, G.W.; Milligan, W.O.; Dillin, D.R.; Williams, R.J.; McCoy, J.J. Refinement of neodymium trihydroxide. Acta Crystallogr. Sect. B 1976, 32, 2227–2229. [Google Scholar] [CrossRef]
- Pelloquin, D.; Louër, M.; Louër, D. Powder Diffraction Studies in the YONO3-Y2O3 System. J. Solid State Chem. 1994, 112, 182–188. [Google Scholar] [CrossRef]
- Le Natur, F.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K.; Ledoux, J.; Le Polles, L.; Roiland, C. Coordination polymers based on heterohexanuclear rare earth complexes: Toward independent luminescence brightness and color tuning. Inorg. Chem. 2013, 52, 6720–6730. [Google Scholar] [CrossRef]
- Yao, H.; Calvez, G.; Daiguebonne, C.; Bernot, K.; Suffren, Y.; Guillou, O. Hetero-hexalanthanide Complexes: A New Synthetic Strategy for Molecular Thermometric Probes. Inorg. Chem. 2019, 58, 16180–16193. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Calvez, G.; Daiguebonne, C.; Bernot, K.; Suffren, Y.; Puget, M.; Lescop, C.; Guillou, O. Hexalanthanide Complexes as Molecular Precursors: Synthesis, Crystal Structure, and Luminescent and Magnetic Properties. Inorg. Chem. 2017, 56, 14632–14642. [Google Scholar] [CrossRef]
- Yao, H.; Calvez, G.; Daiguebonne, C.; Suffren, Y.; Bernot, K.; Guillou, O. Hexanuclear Molecular Precursors as Tools to Design Luminescent Coordination Polymers with Lanthanide Segregation. Inorg. Chem. 2021, 60, 16782–16793. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Calvez, G.; Daiguebonne, C.; Suffren, Y.; Bernot, K.; Guillou, O. Microwave-assisted synthesis of lanthanide coordination polymers with 2-bromobenzoic acid as ligand from hexa-lanthanide molecular precursors. J. Mol. Struct. 2022, 1250, 131918. [Google Scholar] [CrossRef]








| Compounds | 1 | 2 |
|---|---|---|
| Empirical formula | C16H36O49N6S4Eu6 * | C13H13O12N2S2Eu |
| Formula weight | 2136.51 | 605.33 |
| Color and habit | Colorless Prism | Colorless Flake |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a (Å) | 9.658(11) | 7.376(5) |
| b (Å) | 10.668(14) | 11.682(9) |
| c (Å) | 17.12(2) | 11.757(9) |
| α (°) | 89.46(4) | 110.813(10) |
| β (°) | 82.75(5) | 104.856(9) |
| γ (°) | 81.24(3) | 95.367(3) |
| V (Å3) | 1729(4) | 896.0(12) |
| Z | 1 | 2 |
| Dc (g cm−3) | 2.052 | 2.244 |
| μ (mm−1) | 5.578 | 3.806 |
| F (000) | 1008 | 592 |
| θ range (°) | 3.02 to 27.42 | 2.96 to 27.53 |
| h, k, l, ranges | −12 to 12, | −9 to 9, |
| −13 to 13, | −15 to 15, | |
| −21 to 21 | −15 to 15 | |
| T/K | 153(2) | 153(2) |
| R1, a wR2b [I > 2σ(I)] | 0.0868, 0.2394 | 0.0479, 0.1118 |
| GOF on F2 | 1.008 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sun, X.; Fu, J.; Zhu, W. Two Novel Pyrene Tetra-Sulfonate Europium Coordination Polymers: Structure Formation Mechanism Analysis and Sequential Modulation Strategy. Crystals 2022, 12, 1818. https://doi.org/10.3390/cryst12121818
Li H, Sun X, Fu J, Zhu W. Two Novel Pyrene Tetra-Sulfonate Europium Coordination Polymers: Structure Formation Mechanism Analysis and Sequential Modulation Strategy. Crystals. 2022; 12(12):1818. https://doi.org/10.3390/cryst12121818
Chicago/Turabian StyleLi, Haoran, Xiaolian Sun, Jiaju Fu, and Wenlei Zhu. 2022. "Two Novel Pyrene Tetra-Sulfonate Europium Coordination Polymers: Structure Formation Mechanism Analysis and Sequential Modulation Strategy" Crystals 12, no. 12: 1818. https://doi.org/10.3390/cryst12121818
APA StyleLi, H., Sun, X., Fu, J., & Zhu, W. (2022). Two Novel Pyrene Tetra-Sulfonate Europium Coordination Polymers: Structure Formation Mechanism Analysis and Sequential Modulation Strategy. Crystals, 12(12), 1818. https://doi.org/10.3390/cryst12121818