Continuous Isolation of Particles with Varying Aspect Ratios up to Thin Needles Achieving Free-Flowing Products
Abstract
:1. Introduction
- Maintaining ALD through particle isolation process
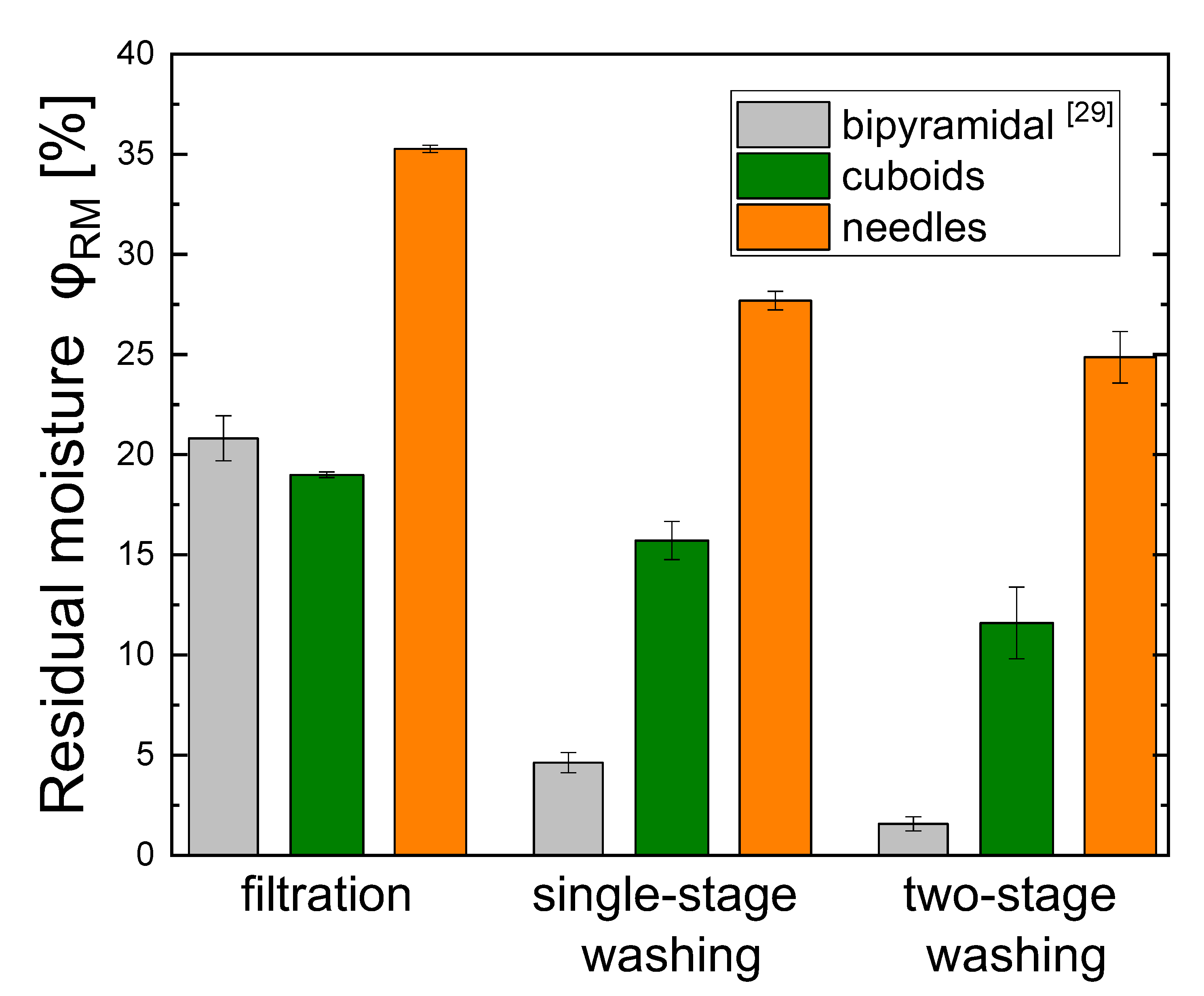
- Reducing residual moisture also for higher aspect ratios
2. Materials and Methods
2.1. Substances Used
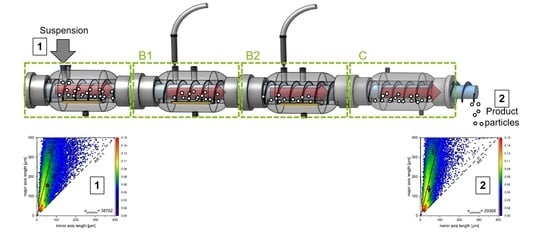
2.2. Experimental CVSF Setup
2.3. Experimental Procedures
2.3.1. Procedure for Preparation of Inlet Suspension with Particles of Varying Shape
2.3.2. Procedures of CVSF Experiments
2.4. Analytics
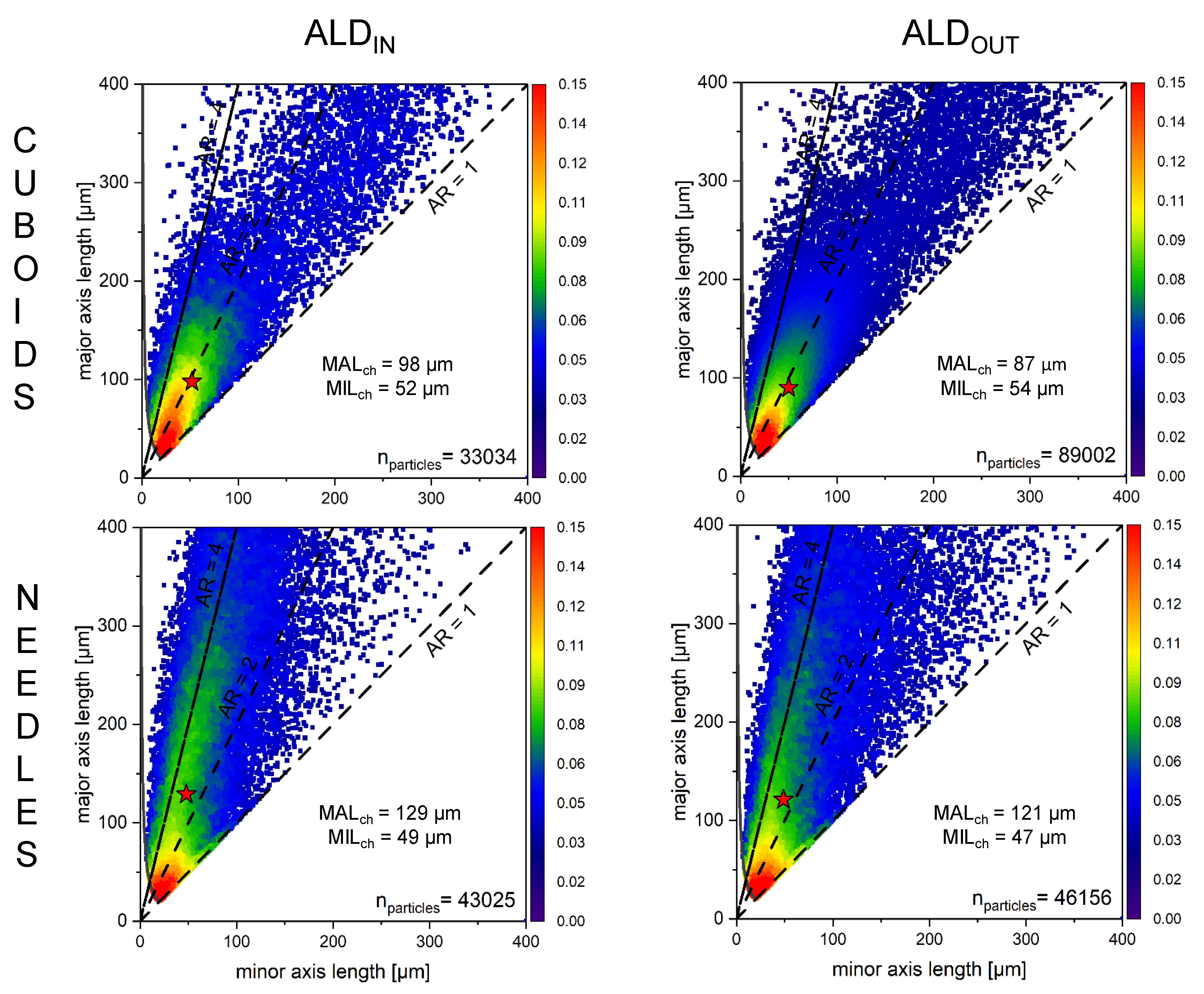
3. Extension of ALD Approach of Non-Spherical Particles
4. Results and Discussion
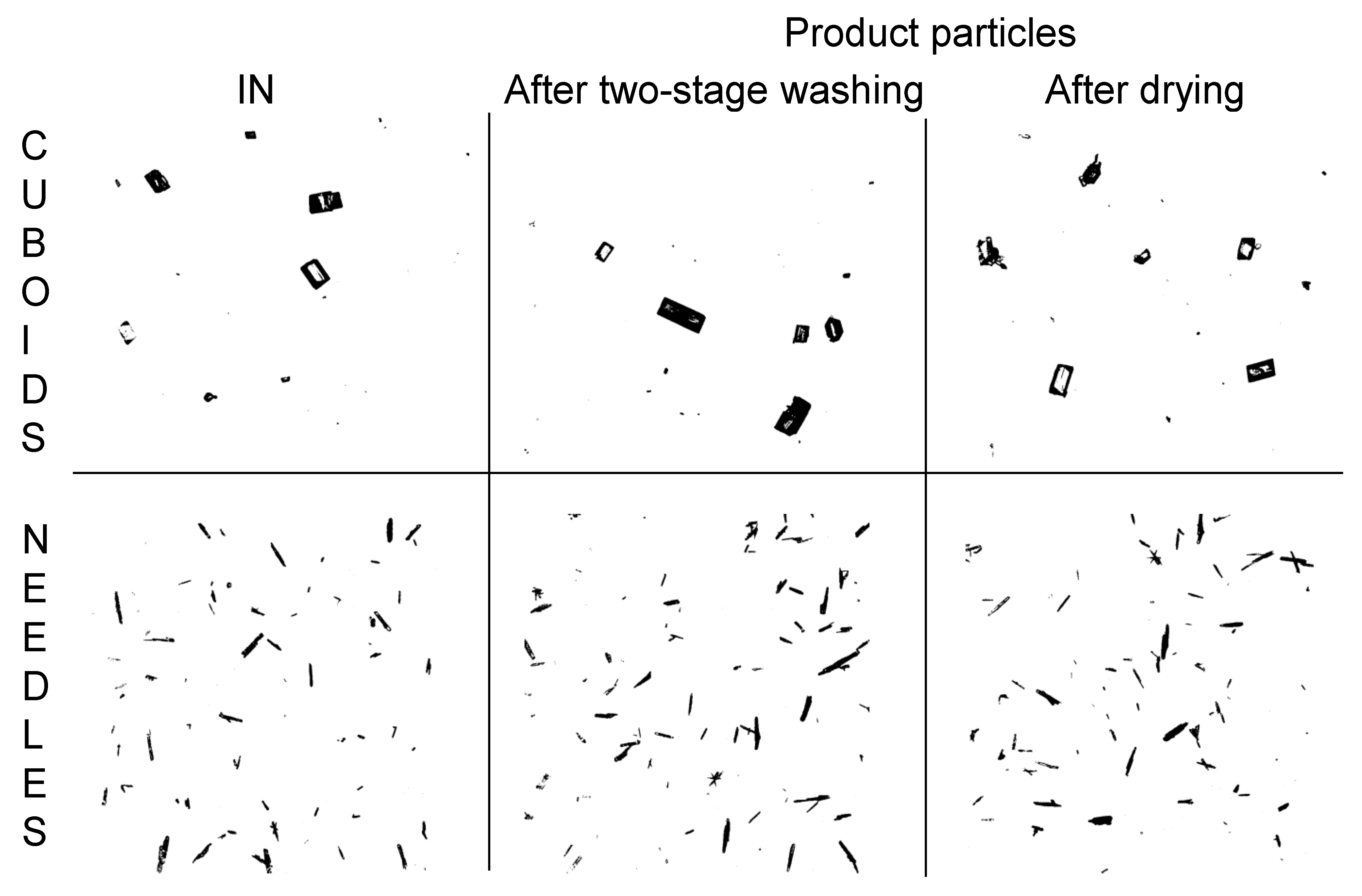
4.1. Maintaining ALD through Integrated Process
4.2. Residual Moisture
4.3. Operation with Added Drying Module
5. Conclusions and Outlook
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cote, A.; Erdemir, D.; Girard, K.P.; Green, D.A.; Lovette, M.A.; Sirota, E.; Nere, N.K. Perspectives on the current state, challenges, and opportunities in pharmaceutical crystallization process development. Cryst. Growth Des. 2020, 20, 7568–7581. [Google Scholar] [CrossRef]
- Hofmann, G. Kristallisation in der Industriellen Praxis; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Beckmann, W. Crystallization: Basic Concepts and Industrial Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Wood, B.; Girard, K.; Polster, C.S.; Croker, D. Progress to date in the design and operation of continuous crystallization processes for pharmaceutical applications. Org. Process Res. Dev. 2019, 23, 122–144. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous crystallization processes in pharmaceutical manufacturing: A review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, S.; Du, S.; Wang, J.; Gong, J. Progress of pharmaceutical continuous crystallization. Engineering 2017, 3, 354–364. [Google Scholar] [CrossRef]
- Wang, T.; Lu, H.; Wang, J.; Xiao, Y.; Zhou, Y.; Bao, Y.; Hao, H. Recent progress of continuous crystallization. J. Ind. Eng. Chem. 2017, 54, 14–29. [Google Scholar] [CrossRef]
- Kleinebudde, P.; Khinast, H.; Rantanen, J. Continuous Manufacturing of Pharmaceuticals; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Acevedo, D.; Peña, R.; Yang, Y.; Barton, A.; Firth, P.; Nagy, Z.K. Evaluation of mixed suspension mixed product removal crystallization processes coupled with a continuous filtration system. Chem. Eng. Process. Process. Intensif. 2016, 108, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Mascia, S.; Heider, P.L.; Zhang, H.; Lakerveld, R.; Benyahia, B.; Barton, P.I.; Braatz, R.D.; Cooney, C.L.; Evans, J.M.B.; Jamison, T.F.; et al. End-to-end continuous manufacturing of pharmaceuticals: Integrated synthesis, purification, and final dosage formation. Angew. Chem. Int. Ed. 2013, 52, 12359–12363. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, D.; Féraud, J.; Colson, D.; Mandrick, K.; Ode, D.; Brackx, E.; Puel, F. Influence of particle size and shape properties on cake resistance and compressibility during pressure filtration. Chem. Eng. Sci. 2016, 144, 176–187. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, S.; Macaringue, E.; Zhang, T.; Gong, J.; Wang, J. Recent Progress in Continuous Crystallization of Pharmaceutical Products: Precise Preparation and Control. Org. Process Res. Dev. 2021, 24, 1785–1801. [Google Scholar] [CrossRef]
- Domokos, A.; Nagy, B.; Szilágyi, B.; Marosi, G.; Nagy, Z.K. Integrated continuous pharmaceutical technologies—A review. Org. Process Res. Dev. 2021, 25, 721–739. [Google Scholar] [CrossRef]
- Johnson, M.D.; Burcham, C.L.; May, S.A.; Calvin, J.R.; Groh, J.M.; Myers, S.S.; Webster, L.P.; Roberts, J.C.; Reddy, V.R.; Luciani, C.V.; et al. API continuous cooling and antisolvent crystallization for kinetic impurity rejection in cGMP manufacturing. Org. Process Res. Dev. 2021, 25, 1284–1351. [Google Scholar] [CrossRef]
- Nagy, B.; Szilágyi, B.; Domokos, A.; Tacsi, K.; Pataki, H.; Marosi, G.; Nagy, Z.K.; Nagy, Z.K. Modeling of pharmaceutical filtration and continuous integrated crystallization-filtration processes. Chem. Eng. J. 2021, 413, 127566. [Google Scholar] [CrossRef]
- Domokos, A.; Nagy, B.; Gyürkés, M.; Farkas, A.; Tacsi, K.; Pataki, H.; Liu, Y.C.; Balogh, A.; Firth, P.; Szilágyi, B.; et al. End-to-end continuous manufacturing of conventional compressed tablets: From flow synthesis to tableting through integrated crystallization and filtration. Int. J. Pharm. 2020, 581, 119297. [Google Scholar] [CrossRef]
- Liu, Y.C.; Domokos, A.; Coleman, S.; Firth, P.; Nagy, Z.K. Development of continuous filtration in a novel continuous filtration carousel integrated with continuous crystallization. Org. Process Res. Dev. 2019, 23, 2655–2665. [Google Scholar] [CrossRef]
- Ottoboni, S.; Price, C.J.; Steven, C.; Meehan, E.; Barton, A.; Firth, P.; Mitchell, A.; Tahir, F. Development of a novel continuous filtration unit for pharmaceutical process development and manufacturing. J. Pharm. Sci. 2019, 108, 372–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottoboni, S.; Shahid, M.; Steven, C.; Coleman, S.; Meehan, E.; Barton, A.; Firth, P.; Sutherland, R.; Price, C.J. Developing a batch isolation procedure and running it in an automated semicontinuous unit: AWL CFD25 case study. Org. Process Res. Dev. 2020, 24, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, S.; Coleman, S.J.; Steven, C.; Siddique, M.; Fraissinet, M.; Joannes, M.; Laux, A.; Barton, A.; Firth, P.; Price, C.J.; et al. Understanding API static drying with hot gas flow: Design and test of a drying rig prototype and drying modeling development. Org. Process Res. Dev. 2020, 24, 2505–2520. [Google Scholar] [CrossRef] [PubMed]
- Destro, F.; Hur, I.; Wang, V.; Abdi, M.; Feng, X.; Wood, E.; Coleman, S.; Firth, P.; Barton, A.; Barolo, M.; et al. Mathematical modeling and digital design of an intensified filtration-washing-drying unit for pharmaceutical continuous manufacturing. Chem. Eng. Sci. 2021, 244, 116803. [Google Scholar] [CrossRef]
- Barton, A. Filtration Apparatus. WO 2015/033117 A1, 12 March 2015. [Google Scholar]
- Born, S.; Dittrich, J.; Takizawa, B.T.; Mascia, S. Continuous Rotary Plate Filter and Methods of Use Thereof. WO 2017/136779 A1, 10 August 2017. [Google Scholar]
- Testa, C.J.; Hu, C.; Shvedova, K.; Wu, W.; Sayin, R.; Casati, F.; Halkude, B.S.; Hermant, P.; Shen, D.E.; Ramnath, A.; et al. Design and commercialization of an end-to-end continuous pharmaceutical production process: A pilot plant case study. Org. Process Res. Dev. 2020, 24, 2874–2889. [Google Scholar] [CrossRef]
- Hu, C.; Testa, C.J.; Born, S.C.; Wu, W.; Shvedova, K.; Sayin, R.; Halkude, B.S.; Casati, F.; Ramnath, A.; Hermant, P.; et al. E-factor analysis of a pilot plant for end-to-end integrated continuous manufacturing (ICM) of pharmaceuticals. Green Chem. 2020, 22, 4350–4356. [Google Scholar] [CrossRef]
- Testa, C.J.; Shvedova, K.; Hu, C.; Wu, W.; Born, S.C.; Takizawa, B.; Mascia, S. Heterogeneous crystallization as a process intensification technology in an integrated continuous manufacturing process for pharmaceuticals. Org. Process Res. Dev. 2021, 25, 225–238. [Google Scholar] [CrossRef]
- Steenweg, C.; Wohlgemuth, K.; Schembecker, G. Rotating-Screw Drying Reactor—Schneckenfördertrocknungsreaktor. WO2021148108 A1, 29 July 2021. [Google Scholar]
- Steenweg, C.; Seifert, A.I.; Schembecker, G.; Wohlgemuth, K. Characterization of a modular continuous vacuum screw filter for small-scale solid–liquid separation of suspensions. Org. Process Res. Dev. 2021, 25, 926–940. [Google Scholar] [CrossRef]
- Steenweg, C.; Seifert, A.I.; Böttger, N.; Wohlgemuth, K. Process intensification enabling continuous manufacturing processes using modular continuous vacuum screw filter. Org. Process Res. Dev. 2021, 25, 2525–2536. [Google Scholar] [CrossRef]
- Steenweg, C.; Wohlgemuth, K. Von der Suspension zum getrockneten Produkt mittels neuartigem Vakuumschraubenfilter. Filtr. Sep. 2021, 4, 4–11. [Google Scholar]
- Steenweg, C.; Kufner, A.C.; Habicht, J.; Wohlgemuth, K. Towards continuous primary manufacturing processes—Particle design through combined crystallization and particle isolation. Processes 2021, 9, 2187. [Google Scholar] [CrossRef]
- FDA. Quality Considerations for Continuous Manufacturing: Guidance for Industry; Department of Health and Human Services: Washington, DC, USA, 2019.
- Wakeman, R. The influence of particle properties on filtration. Sep. Purif. Technol. 2007, 58, 234–241. [Google Scholar] [CrossRef]
- Pudasaini, N.; Upadhyay, P.P.; Parker, C.R.; Hagen, S.U.; Bond, A.D.; Rantanen, J. Downstream processability of crystal habit-modified active pharmaceutical ingredient. Org. Process Res. Dev. 2017, 21, 571–577. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G.; The Manufacturing Classification System (MCS) Working Group. A proposal for a drug product manufacturing classification system (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- MacLeod, C.S.; Muller, F. On the fracture of pharmaceutical needle-shaped crystals during pressure filtration: Case studies and mechanistic understanding. Org. Process Res. Dev. 2012, 16, 425–434. [Google Scholar] [CrossRef]
- Eren, A.; Szilagyi, B.; Quon, J.L.; Papageorgiou, C.D.; Nagy, Z.K. Experimental investigation of an integrated crystallization and wet-milling system with temperature cycling to control the size and aspect ratio of needle-shaped pharmaceutical crystals. Cryst. Growth Des. 2021, 21, 3981–3993. [Google Scholar] [CrossRef]
- Perini, G.; Salvatori, F.; Ochsenbein, D.; Mazzotti, M.; Vetter, T. Filterability prediction of needle-like crystals based on particle size and shape distribution data. Sep. Purif. Technol. 2019, 211, 768–781. [Google Scholar] [CrossRef]
- Lekhal, A.; Girard, K.; Brown, M.; Kiang, S.; Khinast, J.; Glasser, B. The effect of agitated drying on the morphology of l-threonine (needle-like) crystals. Int. J. Pharm. 2004, 270, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.; Kempkes, M.; Mazzotti, M. Measurement of size and shape distributions of particles through image analysis. Chem. Eng. Sci. 2008, 63, 5513–5521. [Google Scholar] [CrossRef]
- Kempkes, M.; Eggers, J.; Mazzotti, M. Measurement of particle size and shape by FBRM and in situ microscopy. Chem. Eng. Sci. 2008, 63, 4656–4675. [Google Scholar] [CrossRef]
- Kempkes, M.; Vetter, T.; Mazzotti, M. Monitoring the particle size and shape in the crystallization of paracetamol from water. Chem. Eng. Res. Des. 2010, 88, 447–454. [Google Scholar] [CrossRef]
- Briesen, H. Simulation of crystal size and shape by means of a reduced two-dimensional population balance model. Chem. Eng. Sci. 2006, 61, 104–112. [Google Scholar] [CrossRef]
- Wohlgemuth, K.; Schembecker, G. Modeling induced nucleation processes during batch cooling crystallization: A sequential parameter determination procedure. Comput. Chem. Eng. 2013, 52, 216–229. [Google Scholar] [CrossRef]
- Lührmann, M.-C.; Timmermann, J.; Schembecker, G.; Wohlgemuth, K. Enhanced Product Quality Control through Separation of Crystallization Phenomena in a Four-Stage MSMPR Cascade. Cryst. Growth Des. 2018, 18, 7323–7334. [Google Scholar] [CrossRef]
- Saal, S. Begasungskristallisation in Wässrigen Aminosäuresystemen. Master’s Thesis, Technische Universität Dortmund, Dortmund, Germany, 2017. [Google Scholar]
- Heisel, S.; Ernst, J.; Emshoff, A.; Schembecker, G.; Wohlgemuth, K. Shape-independent particle classification for discrimination of single crystals and agglomerates. Powder Technol. 2019, 345, 425–437. [Google Scholar] [CrossRef]
- Terdenge, L.-M.; Wohlgemuth, K. Impact of agglomeration on crystalline product quality within the crystallization process chain. Cryst. Res. Technol. 2016, 51, 513–523. [Google Scholar] [CrossRef]
- Heisel, S.; Rolfes, M.; Wohlgemuth, K. Discrimination between single crystals and agglomerates during the crystallization process. Chem. Eng. Technol. 2018, 41, 1218–1225. [Google Scholar] [CrossRef]
- De Albuquerque, I.; Mazzotti, M.; Ochsenbein, D.R.; Morari, M. Effect of needle-like crystal shape on measured particle size distributions. AIChE J. 2016, 62, 2974–2985. [Google Scholar] [CrossRef]
- Schorsch, S.; Vetter, T.; Mazzotti, M. Measuring multidimensional particle size distributions during crystallization. Chem. Eng. Sci. 2012, 77, 130–142. [Google Scholar] [CrossRef]
- Steger, C. On the Calculation of Arbitrary Moments of Polygons; Technical Report FGBV-96-05; Technische Universität München: Munich, Germany, 1996. [Google Scholar]
- Yu, W.; Hancock, B. Evaluation of dynamic image analysis for characterizing pharmaceutical excipient particles. Int. J. Pharm. 2008, 361, 150–157. [Google Scholar] [CrossRef] [PubMed]
- ISO 13322-2; Particle Size Analysis—Image Analysis Methods; Dynamic Image Analysis Methods. International Organization for Standardization: Geneva, Switzerland, 2006.
- Origin-Help: Density Dots. Available online: https://www.originlab.com/doc/Origin-Help/Density_Dots (accessed on 13 October 2021).
- Botev, Z.I.; Grotowski, J.F.; Kroese, D. Kernel density estimation via diffusion. Ann. Stat. 2010, 38, 2916–2957. [Google Scholar] [CrossRef] [Green Version]
- Kleppmann, W. Versuchsplanung: Produkte und Prozesse Optimieren, 8th ed.; Praxisreihe Qualitätswissen, Hanser: Münich, Germany, 2013. [Google Scholar]
- Papageorgiou, C.D.; Mitchell, C.; Quon, J.L.; Langston, M.; Borg, S.; Hicks, F.; Ende, D.J.A.; Breault, M. Development of a novel screening methodology for the assessment of the risk of particle size attrition during agitated drying. Org. Process Res. Dev. 2020, 24, 242–254. [Google Scholar] [CrossRef]
- Birch, M.; Marziano, I. Understanding and avoidance of agglomeration during drying processes: A case study. Org. Process Res. Dev. 2013, 17, 1359–1366. [Google Scholar] [CrossRef]
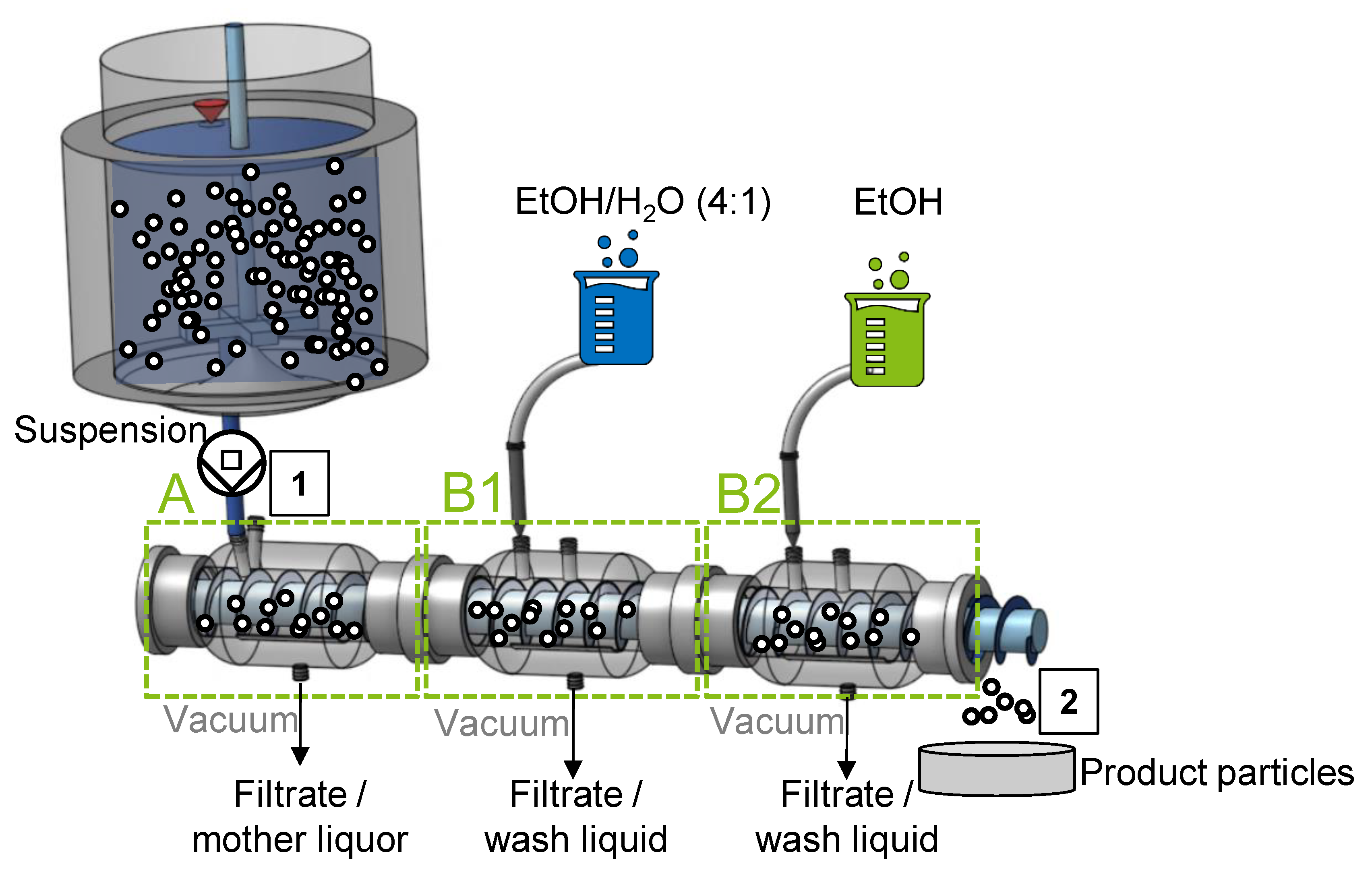
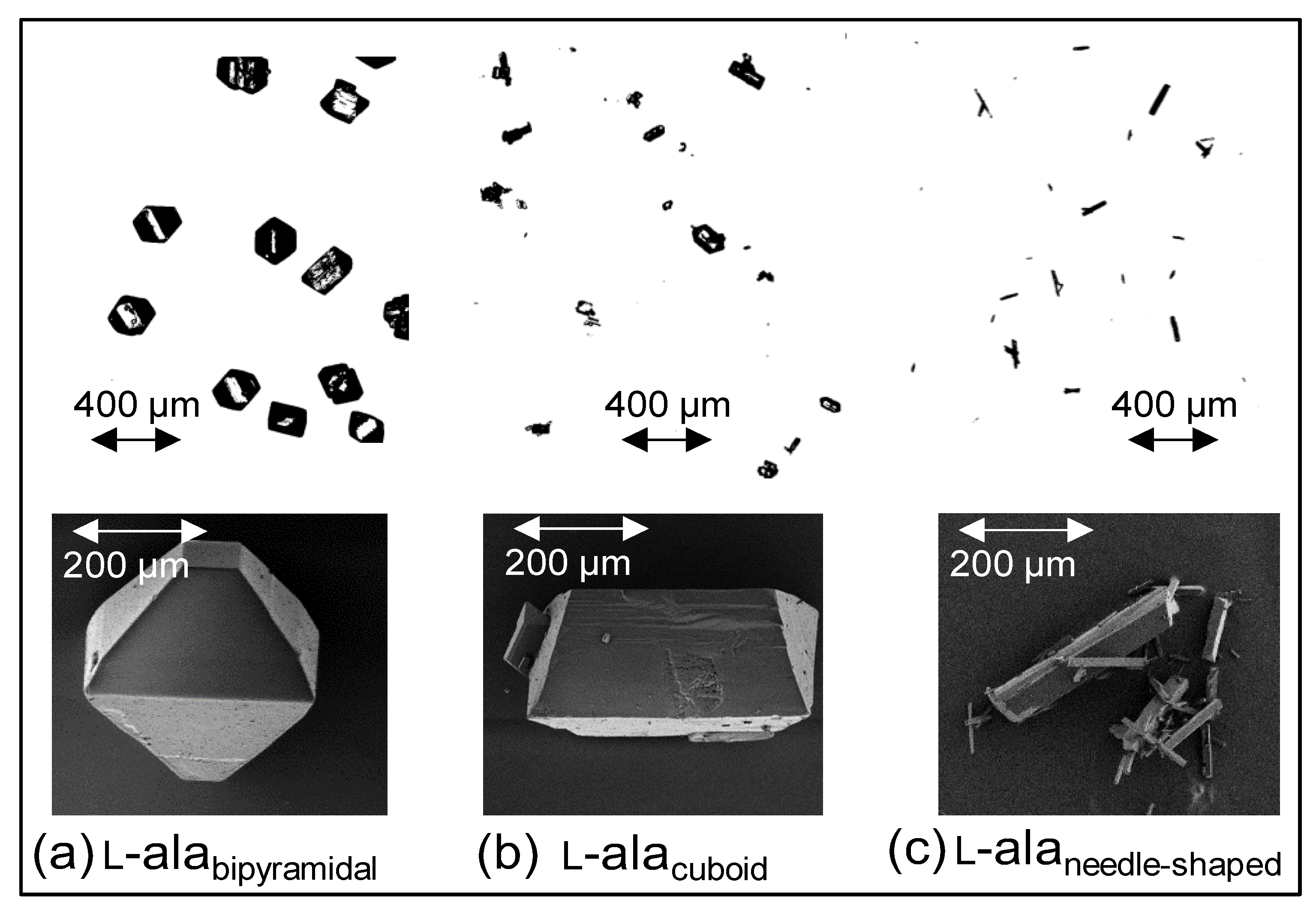

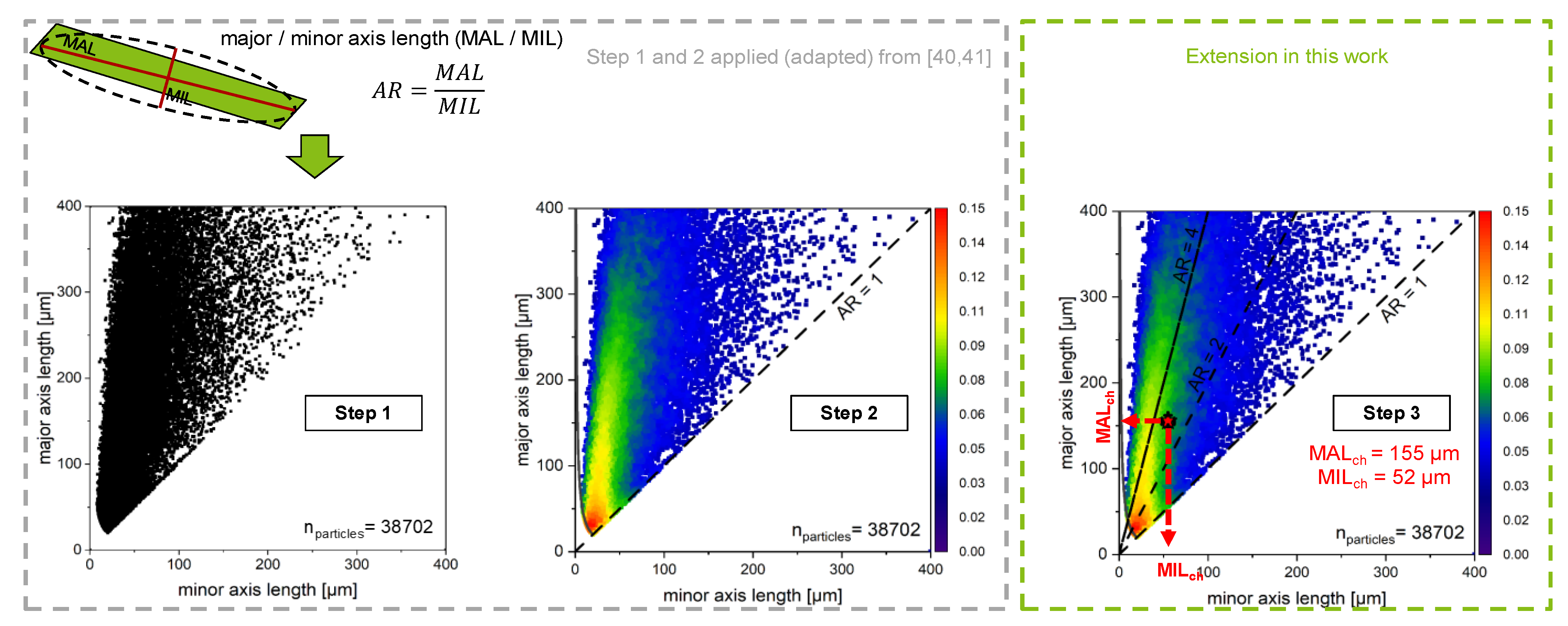


| Operating Parameters | Value |
|---|---|
| Qsusp [mL min−1] | 60 |
| ws [wt.%, g gsol−1] | 6 |
| nscrew [rpm] | 3 |
| pset [mbar] | 400 |
| Qwash [mL min−1] | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steenweg, C.; Habicht, J.; Wohlgemuth, K. Continuous Isolation of Particles with Varying Aspect Ratios up to Thin Needles Achieving Free-Flowing Products. Crystals 2022, 12, 137. https://doi.org/10.3390/cryst12020137
Steenweg C, Habicht J, Wohlgemuth K. Continuous Isolation of Particles with Varying Aspect Ratios up to Thin Needles Achieving Free-Flowing Products. Crystals. 2022; 12(2):137. https://doi.org/10.3390/cryst12020137
Chicago/Turabian StyleSteenweg, Claas, Jonas Habicht, and Kerstin Wohlgemuth. 2022. "Continuous Isolation of Particles with Varying Aspect Ratios up to Thin Needles Achieving Free-Flowing Products" Crystals 12, no. 2: 137. https://doi.org/10.3390/cryst12020137