Microstructure Characterization and Battery Performance Comparison of MOF-235 and TiO2-P25 Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Performance Test and Surface Characterization
3. Results and Discussion
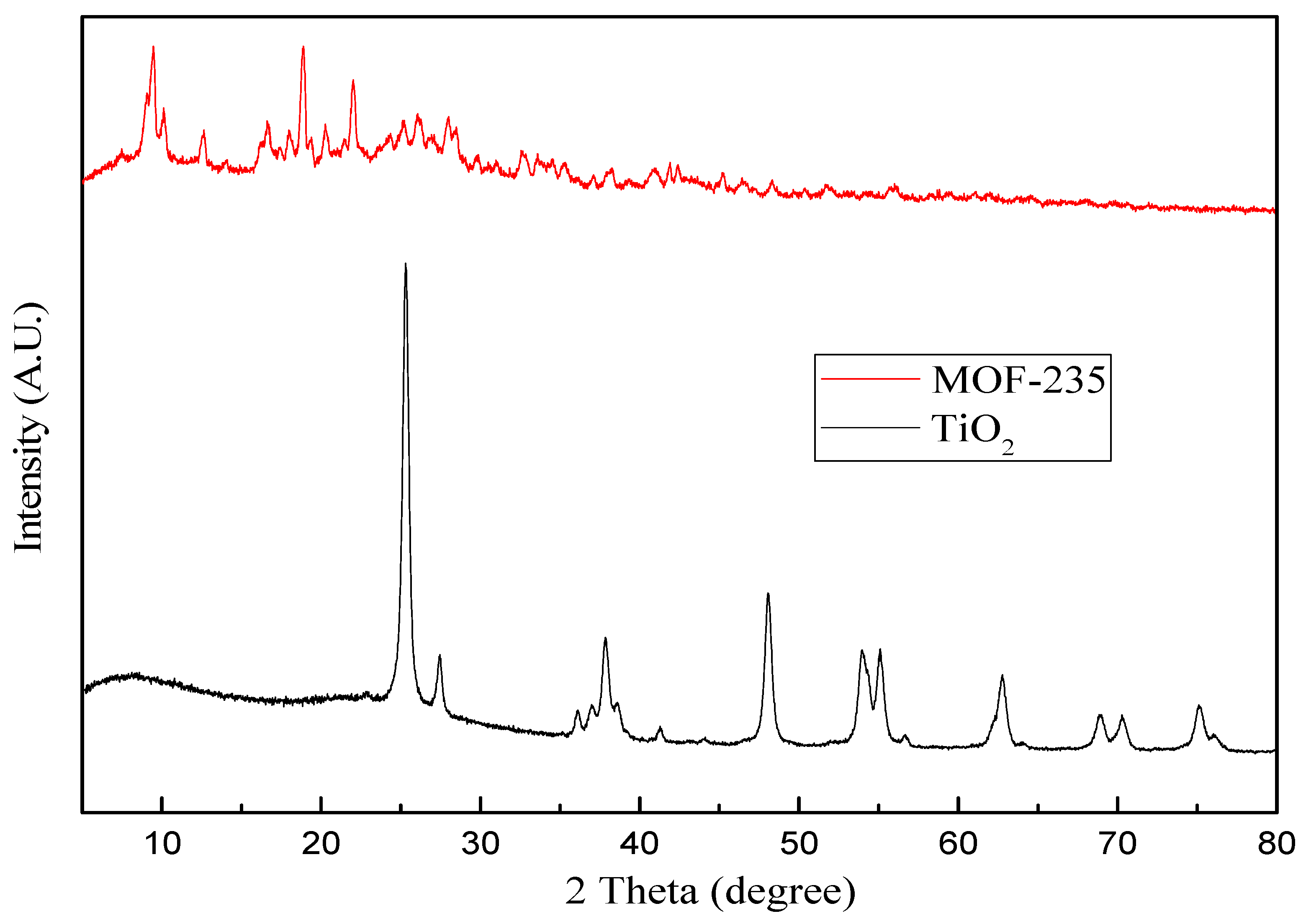
3.1. Surface Characterization via SEM and XRD
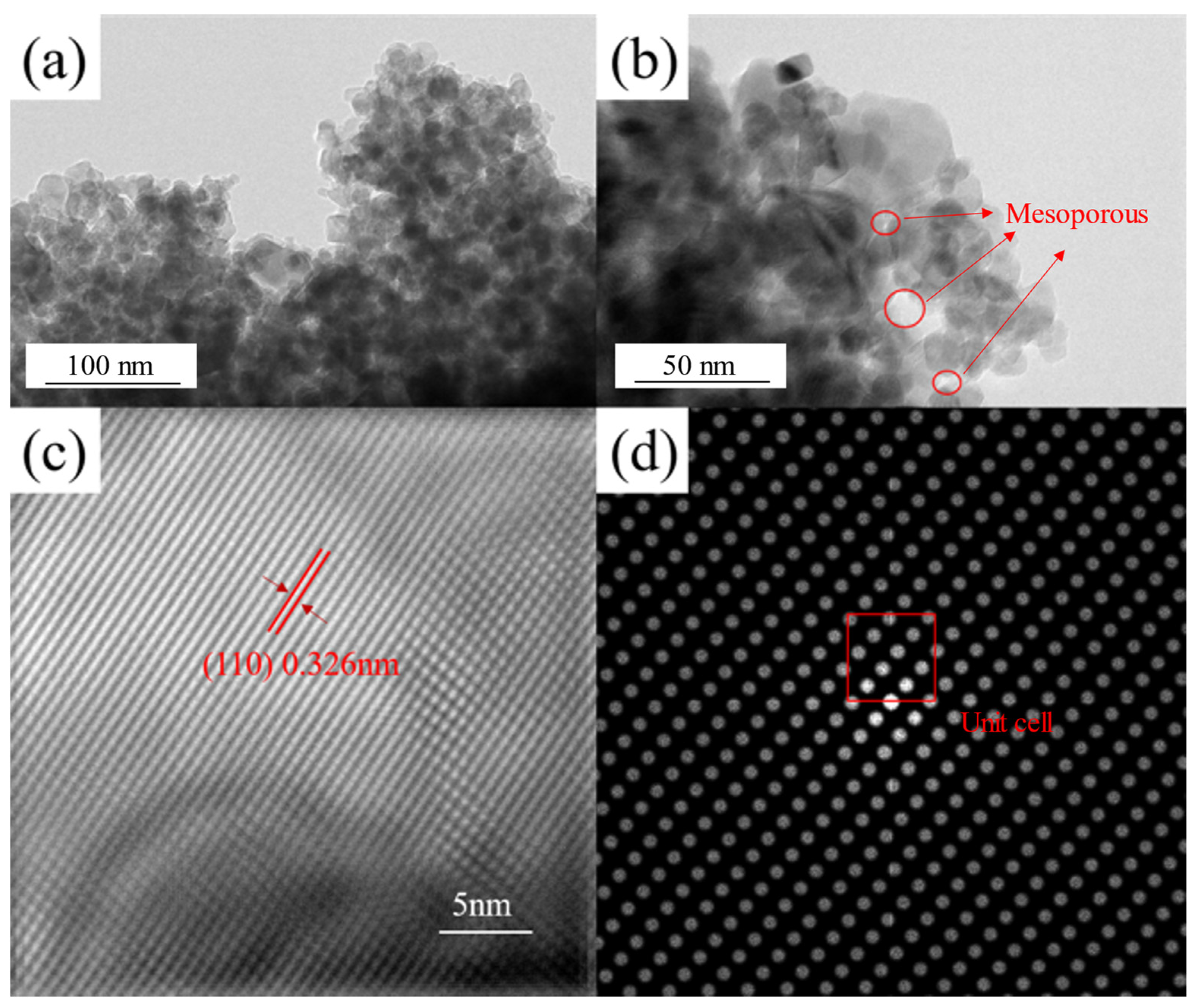
3.2. Battery Performance Analysis via Cyclic Voltammetric, Charge–Discharge, and TEM Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nong, S.; Dong, W.; Yin, J.; Dong, B.; Lu, Y.; Yuan, X.; Wang, X.; Bu, K.; Chen, M.; Jiang, S.; et al. Well-Dispersed Ruthenium in Mesoporous Crystal TiO2 as an Advanced Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 5719–5727. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, H.S. A reversible long-life lithium-air battery in ambient air. Nat. Commun. 2013, 4, 1817. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Liu, K.; Lang, J.L.; Zhuo, D.; Huang, Z.Y.; Wang, C.A.; Wu, H.; Cui, Y. An intermediate temperature garnet-type solid electrolyte-based molten lithium battery for grid energy storage. Nat. Energy 2018, 3, 732–738. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium-air batteries. Nat. Energy 2016, 1, 128. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Liu, L.; Ning, J.; Lei, K.; Lu, Y.; Li, F.; Chen, J. A Microporous Covalent-Organic Framework with Abundant Accessible Carbonyl Groups for Lithium-Ion Batteries. Angew. Chem.-Int. Ed. 2018, 57, 9443–9446. [Google Scholar] [CrossRef]
- Wang, K.L.; Jiang, K.; Chung, B.; Ouchi, T.; Burke, P.J.; Boysen, D.A.; Bradwell, D.J.; Kim, H.; Muecke, U.; Sadoway, D.R. Lithium-antimony-lead liquid metal battery for grid-level energy storage. Nature 2014, 514, 348. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Feng, S.; Hui, P.; Chen, H.; Tian, C.; Yang, R.; Xu, Y. Exploration of low-cost microporous Fe(III)-based organic framework as anode material for potassium-ion batteries. J. Alloys Compd. 2020, 830, 154714. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Wu, T.W.; Kong, W.H.; Zhang, Y.; Xing, Z.; Zhao, J.X.; Wang, T.; Shi, X.F.; Luo, Y.L.; Sun, X.P. Greatly Enhanced Electrocatalytic N-2 Reduction on TiO2 via V Doping. Small Methods 2019, 3, 8. [Google Scholar] [CrossRef]
- Li, Z.H.; Luo, L.; Li, M.; Chen, W.S.; Liu, Y.G.; Yang, J.R.; Xu, S.M.; Zhou, H.; Ma, L.N.; Xu, M.; et al. Photoelectrocatalytic C-H halogenation over an oxygen vacancy-rich TiO2 photoanode. Nat. Commun. 2021, 12, 6698. [Google Scholar] [CrossRef]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: A review. Innov. Food Sci. Emerg. 2016, 38, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Bai, H.; Liu, L.; Zan, X.; Gao, P.; Sun, D.D.; Yan, W. A green approach assembled multifunctional Ag/AgBr/TNF membrane for clean water production & disinfection of bacteria through utilizing visible light. Appl. Catal. B-Environ. 2016, 196, 57–67. [Google Scholar] [CrossRef]
- Rego, R.M.; Sriram, G.; Ajeya, K.V.; Jung, H.Y.; Kurkuri, M.D.; Kigga, M. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification. J. Hazard. Mater. 2021, 416, 125941. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, L.; Wang, Y.; Ji, Y.; Horvat, J.; Cheng, M.-L.; Jia, X.; Wang, G. Manganese-Based Layered Coordination Polymer: Synthesis, Structural Characterization, Magnetic Property, and Electrochemical Performance in Lithium-Ion Batteries. Inorg. Chem. 2013, 52, 2817–2822. [Google Scholar] [CrossRef]
- Dong, C.; Xu, L. Cobalt- and Cadmium-Based Metal-Organic Frameworks as High-Performance Anodes for Sodium Ion Batteries and Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 7160–7168. [Google Scholar] [CrossRef]
- Li, G.; Xia, L.; Dong, J.; Chen, Y.; Li, Y. 10 - Metal-organic frameworks. In Solid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Cambridge, MA, USA, 2020; pp. 285–309. [Google Scholar]
- Simonsson, I.; Gardhagen, P.; Andren, M.; Tam, P.L.; Abbas, Z. Experimental investigations into the irregular synthesis of iron(iii) terephthalate metal-organic frameworks MOF-235 and MIL-101. Dalton Trans. 2021, 50, 4976–4985. [Google Scholar] [CrossRef]
- Asheghhosseini, A.; Zolgharnein, J. Iron terephthalate metal-organic framework (MOF-235) as an efficient adsorbent for removal of toluidine blue dye from aqueous solution using Box-Behnken design as multivariate optimization approach. J. Iran. Chem. Soc. 2020, 17, 2663–2673. [Google Scholar] [CrossRef]
- Zhao, Z.; Lai, H.S.; Li, H.; Li, L. Preparation and Properties of Graphene Doped TiO2 Mesoporous Materials for Photocathode Protection. Int. J. Electrochem. Sci. 2021, 16, 210316. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, H.; Lin, D.D.; Pan, W. Preparation of Necklace-Structured TiO2/SnO2 Hybrid Nanofibers and Their Photocatalytic Activity. J. Am. Ceram. Soc. 2009, 92, 2463–2466. [Google Scholar] [CrossRef]
- Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribiere, P.; Poizot, P.; Tarascon, J.M. Conjugated dicarboxylate anodes for Li-ion batteries. Nat. Mater. 2009, 8, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lu, J.; Xu, T.; Gao, S.; Huang, B.; Dai, Y. I-2-Hydrosol-Seeded Growth of (I-2)(n)-C-Codoped Meso/Nanoporous TiO2 for Visible Light-Driven Photocatalysis. J. Phys. Chem. C 2010, 114, 9510–9517. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, W.; Xue, L.X.; Jiao, Y.; Lei, T.Y.; Chu, J.W.; Huang, J.W.; Gong, C.H.; Yan, C.Y.; Yan, Y.C.; et al. Adsorption-Catalysis Design in the Lithium-Sulfur Battery. Adv. Energy Mater. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Lyu, Y.H.; Wang, R.L.; Xie, C.; Zhou, H.J.; Jiang, S.P.; Wang, S.Y. Crystalline TiO2 protective layer with graded oxygen defects for efficient and stable silicon-based photocathode. Nat. Commun. 2018, 9, 3572. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Li, W.; Wang, J.-Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-Ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rao, L.; Wang, P.F.; Shi, Z.Y.; Zhang, L.X. Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr(VI) coexistence environment. Appl. Catal. B-Environ. 2020, 262, 12. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.M.; Cheng, H.M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem.-Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wang, J.; Duan, W.; Zhao, Z.; Li, L.; Zhang, Z. One-step preparation of C-doped TiO2 nanotubes with enhanced photocatalytic activity by a water-assisted method. Crystengcomm 2021, 23, 3015–3025. [Google Scholar] [CrossRef]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, D.P.; Li, M.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.L.; Li, D.; Wan, J.F.; Yu, X.J. Characterization and mechanism analysis of graphite/C-doped TiO2 composite for enhanced photocatalytic performance. J. Ind. Eng. Chem. 2016, 33, 162–169. [Google Scholar] [CrossRef]
- Ghicov, A.; Albu, S.P.; Hahn, R.; Kim, D.; Stergiopoulos, T.; Kunze, J.; Schiller, C.A.; Falaras, P.; Schmuki, P. TiO2 Nanotubes in Dye-Sensitized Solar Cells: Critical Factors for the Conversion Efficiency. Chem. Asian J. 2009, 4, 520–525. [Google Scholar] [CrossRef]
- Xiao, Y.-X.; Ying, J.; Tian, G.; Yang, X.; Zhang, Y.-X.; Chen, J.B.; Wang, Y.; Symes, M.D.; Ozoemena, K.I.; Wu, J.; et al. Hierarchically Fractal PtPdCu Sponges and their Directed Mass- and Electron-Transfer Effects. Nano. Lett. 2021, 21, 7870–7878. [Google Scholar] [CrossRef]
- Xiao, Y.-X.; Ying, J.; Tian, G.; Tao, Y.; Wei, H.; Fan, S.-Y.; Sun, Z.-H.; Zou, W.-J.; Hu, J.; Chang, G.-G.; et al. Highly dispersed PtPd on graphitic nanofibers and its heavy d-pi effect. Appl. Catal. B-Environ. 2019, 259, 118080. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Xue, Y.; Yang, Y.; Ao, X. Hybrid nanostructures of mixed-phase TiO2 for enhanced photoelectrochemical water splitting. RSC Adv. 2015, 5, 56098–56102. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Hurley, B.; Zhu, M.L.; Yang, Z.; Hwang, J.; Buchheit, R. Corrosion Inhibition of AZ31 Mg Alloy by Aqueous Selenite (SeO32−). J. Electrochem. Soc. 2019, 166, C520–C529. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Novel-structured electrospun TiO2/CuO composite nanofibers for high efficient photocatalytic cogeneration of clean water and energy from dye wastewater. Water Res. 2013, 47, 4059–4073. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, C.; Jang, J. SnO2 nanoparticle embedded TiO2 nanofibers-Highly efficient photocatalyst for the degradation of rhodamine B. Catal. Commun. 2011, 12, 1037–1041. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Jiang, X.; Li, S.; Li, L.; Feng, Z.; Lai, H. Microstructure Characterization and Battery Performance Comparison of MOF-235 and TiO2-P25 Materials. Crystals 2022, 12, 152. https://doi.org/10.3390/cryst12020152
Zhao Z, Jiang X, Li S, Li L, Feng Z, Lai H. Microstructure Characterization and Battery Performance Comparison of MOF-235 and TiO2-P25 Materials. Crystals. 2022; 12(2):152. https://doi.org/10.3390/cryst12020152
Chicago/Turabian StyleZhao, Zilong, Xiaowei Jiang, Sirui Li, Liang Li, Zhiyuan Feng, and Huansheng Lai. 2022. "Microstructure Characterization and Battery Performance Comparison of MOF-235 and TiO2-P25 Materials" Crystals 12, no. 2: 152. https://doi.org/10.3390/cryst12020152






