Effect of B2O3 on the Radiation Shielding Performance of Telluride Lead Glass System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structure Stability Experiment
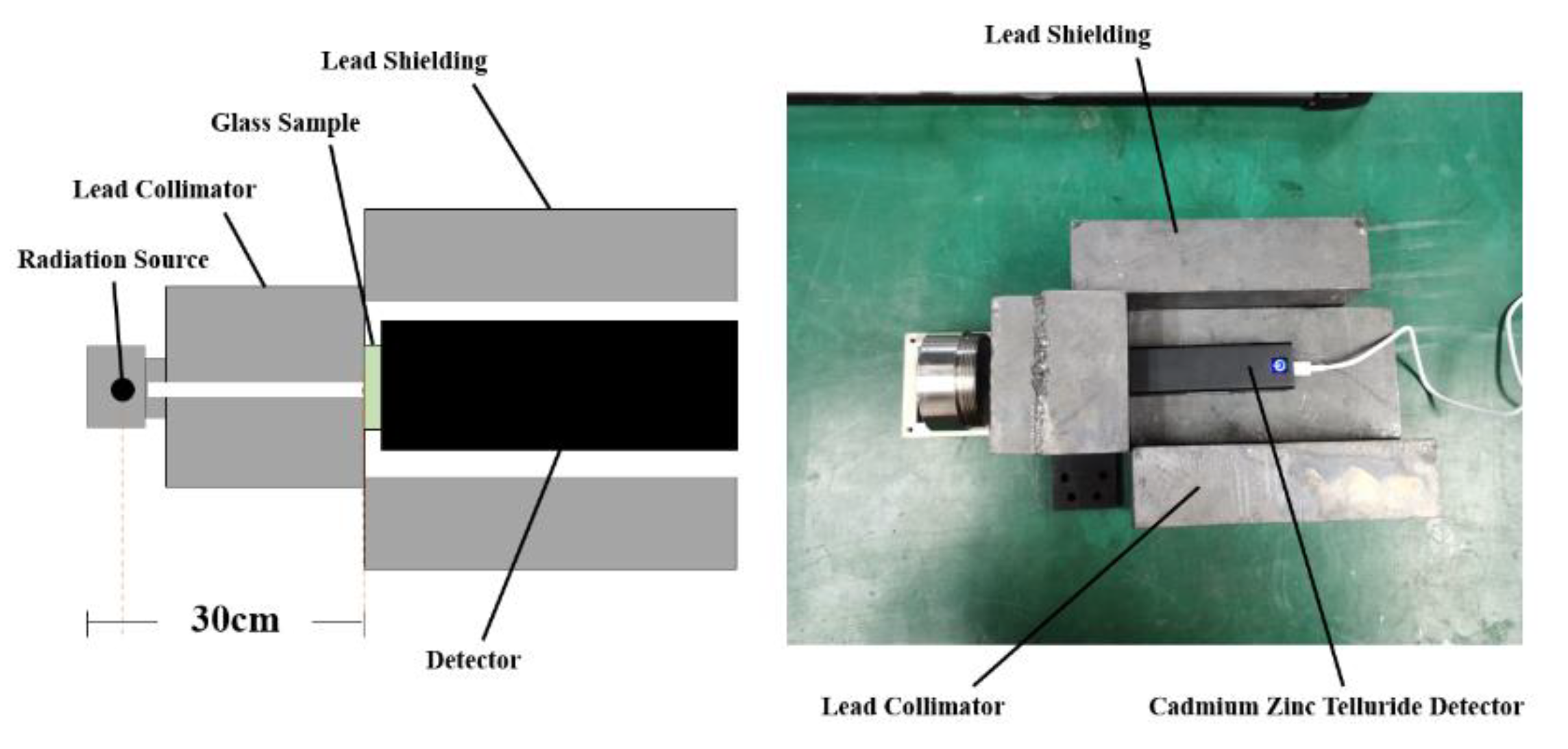
2.3. Radiation Shielding Experiment
3. Calculation
4. Results and Discussion
4.1. Structural Properties
4.2. Density
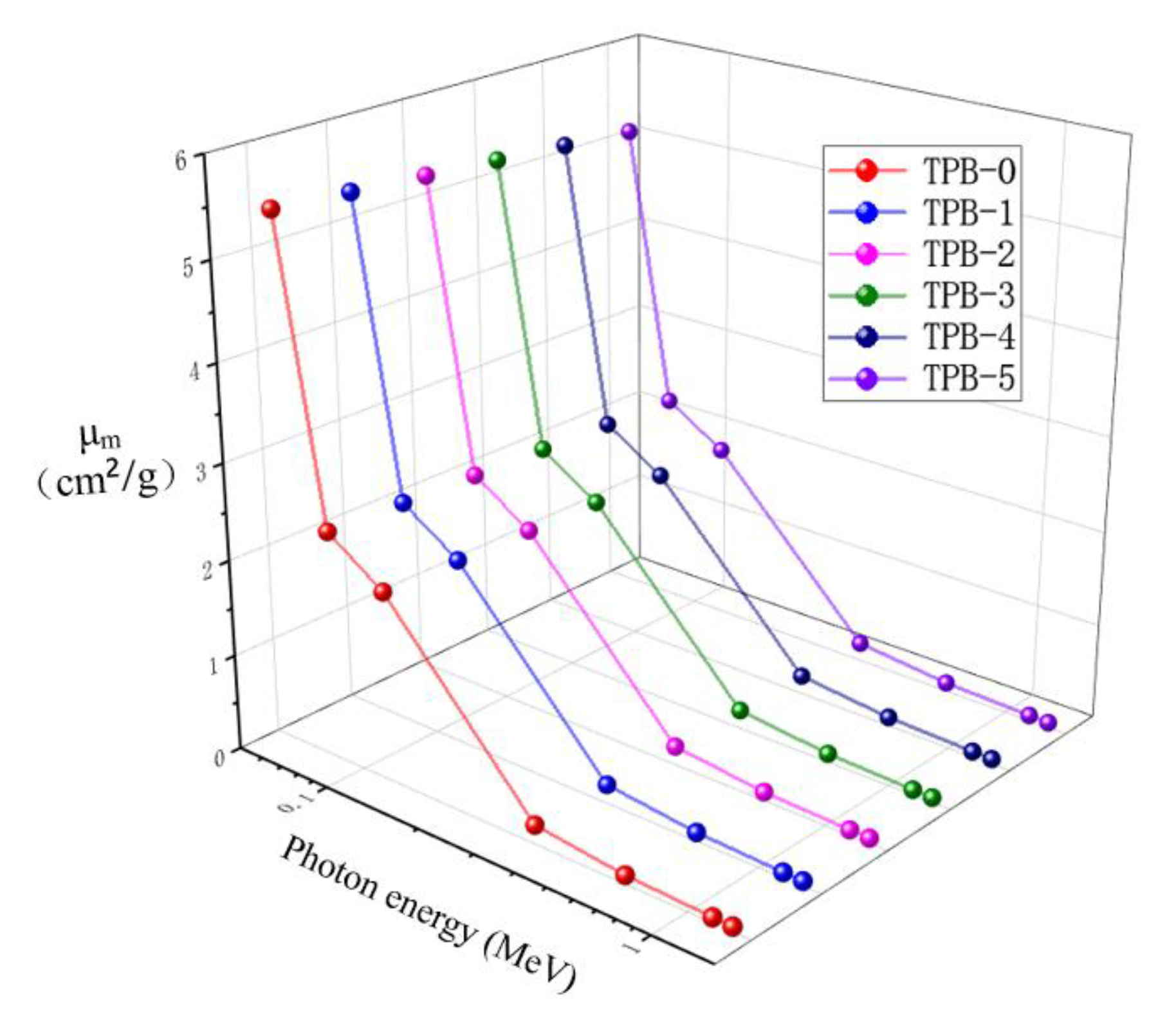
4.3. Mass Attenuation Coefficient
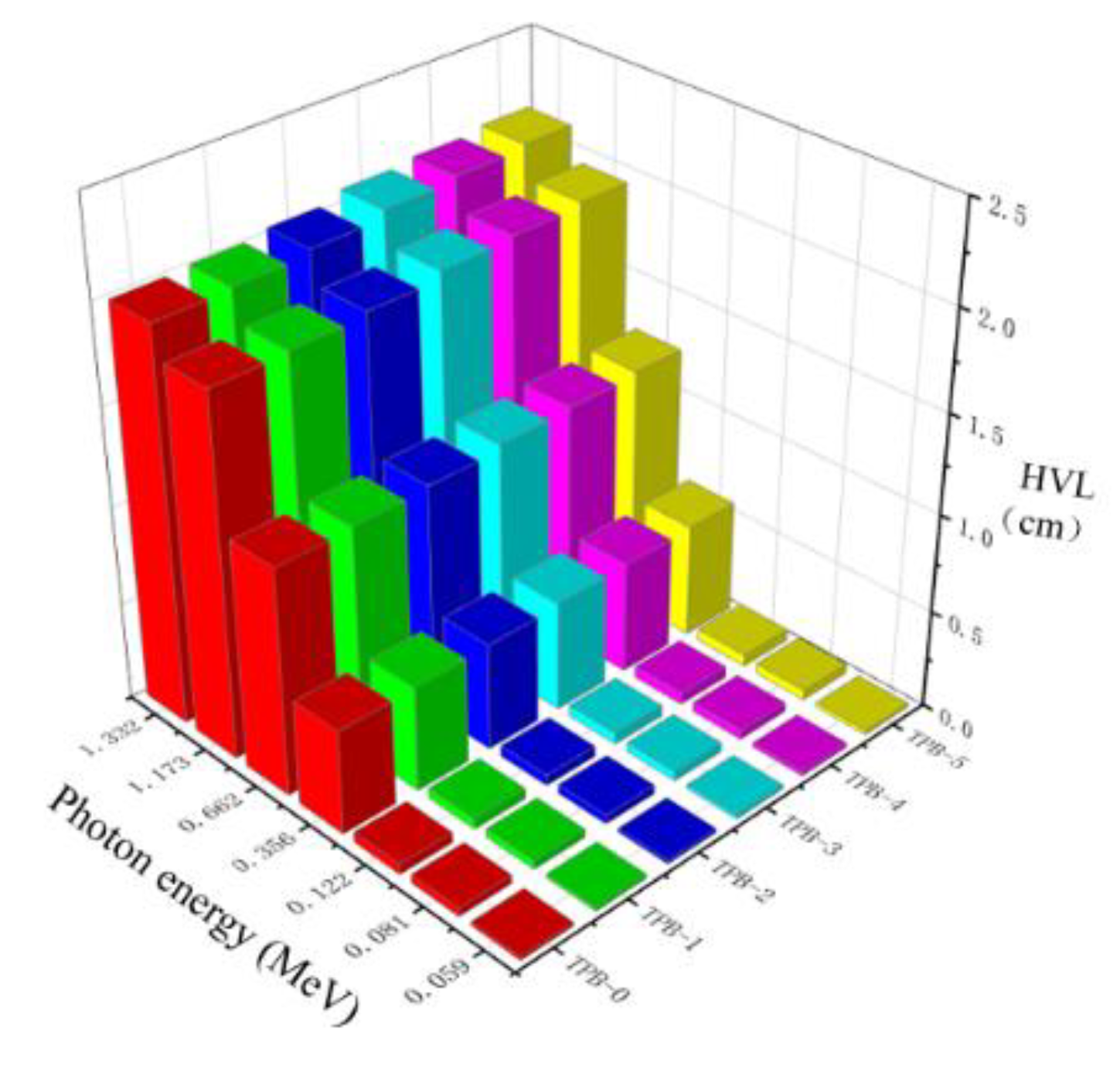
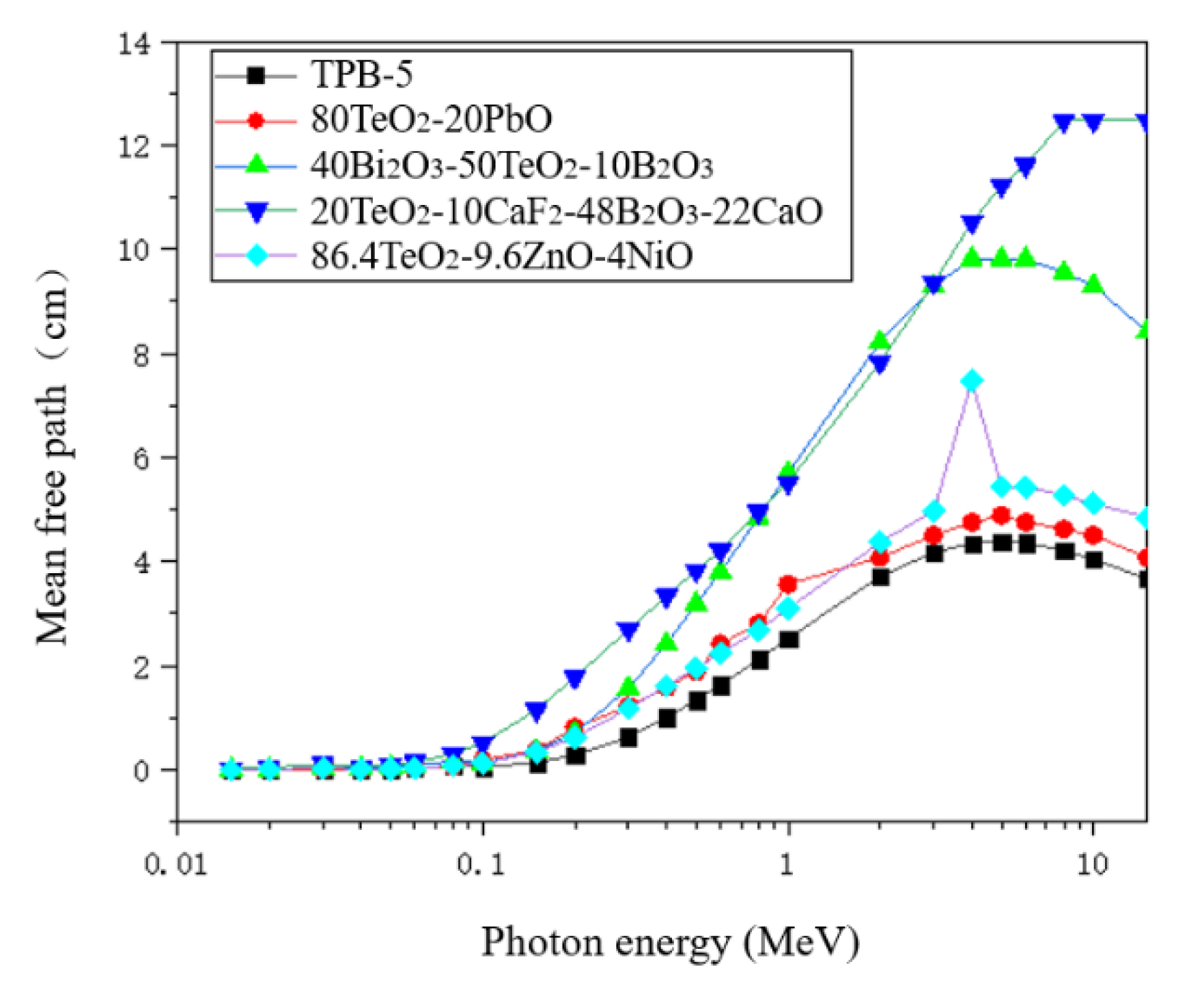
4.4. Half-Value Layer and Mean Free Path
4.5. Effective Atomic Number
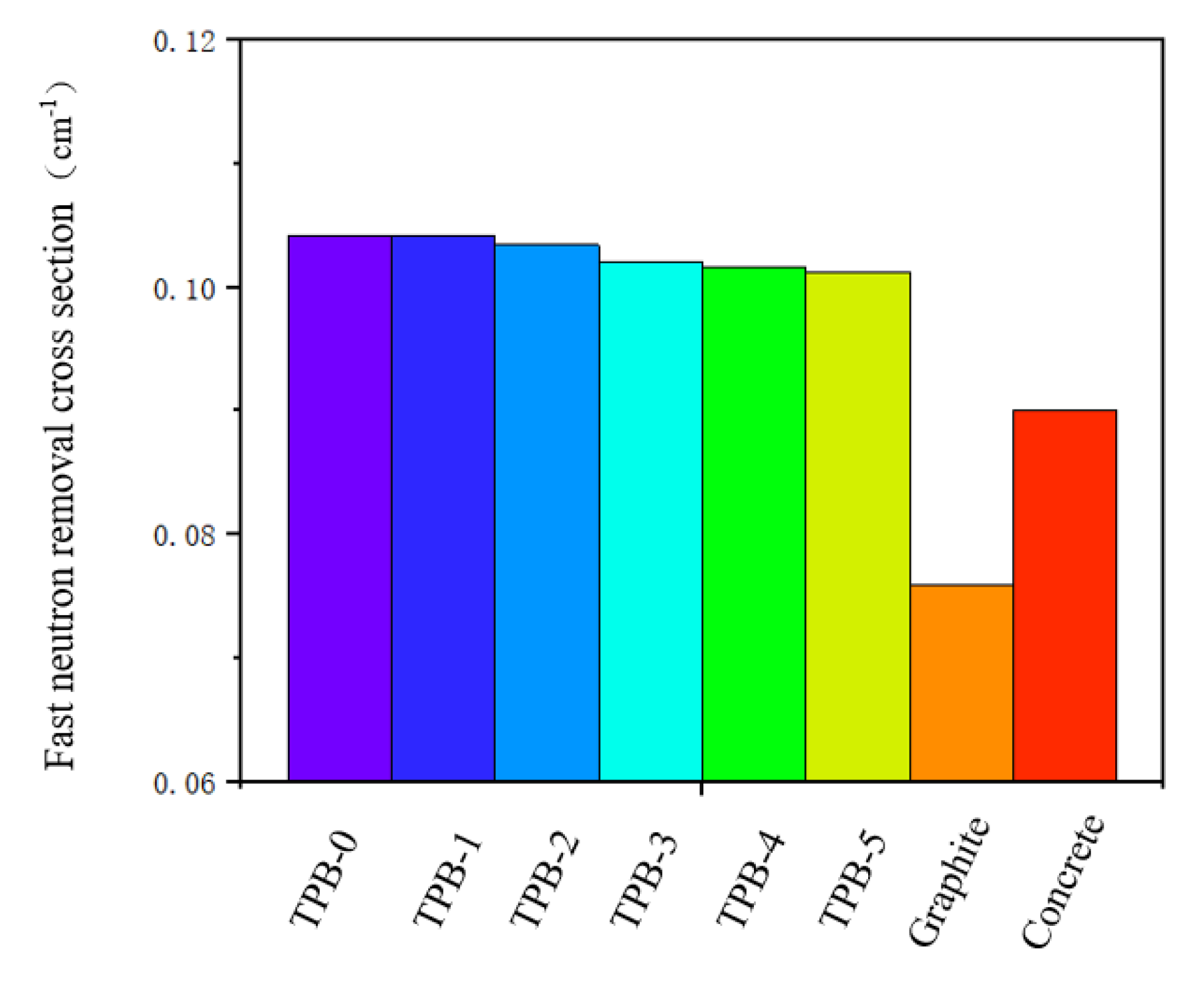
4.6. Removal Cross Section
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rammah, Y.; El-Agawany, F.; Abu El Soad, A.; Yousef, E.S.S.; El-Mesady, I. Ionizing Radiation Attenuation Competences of Gallium Germanate-Tellurite Glasses Utilizing MCNP5 Simulation Code and Phy-X/PSD Program. Ceram. Int. 2020, 46, 22766–22773. [Google Scholar] [CrossRef]
- Hamad, R.; Mhareb, M.; Alajerami, Y.; Sayyed, M.; Saleh, G.; Hamad, M.K.; Ziq, K. A Comprehensive Ionizing Radiation Shielding Study of FexSe0.5Te0.5 Alloys with Various Iron Concentrations. J. Alloy. Compd. 2021, 858, 157636. [Google Scholar] [CrossRef]
- Olarinoye, I.; Alomairy, S.; Sriwunkum, C.; Al-Buriahi, M.S. Effect of Ag2O/V2O5 Substitution on the Radiation Shielding Ability of Tellurite Glass System via XCOM Approach and FLUKA Simulations. Phys. Scr. 2021, 96, 065308. [Google Scholar] [CrossRef]
- Boonin, K.; Yasaka, P.; Limkitjaroenporn, P.; Rajaramakrishna, R.; Askin, A.; Sayyed, M.; Kothan, S.; Kaewkhao, J. Effect of BaO on Lead Free Zinc Barium Tellurite Glass for Radiation Shielding Materials in Nuclear Application. J. Non-Cryst. Solids 2020, 550, 120386. [Google Scholar] [CrossRef]
- Sayyed, M.; Issa, S.A.; Büyükyıldız, M.; Dong, M. Determination of Nuclear Radiation Shielding Properties of Some Tellurite Glasses Using MCNP5 Code. Radiat. Phys. Chem. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Sayyed, M.; Dong, M.; Tekin, H.; Lakshminarayana, G.; Mahdi, M. Comparative Investigations of Gamma and Neutron Radiation Shielding Parameters for Different Borate and Tellurite Glass Systems Using WinXCom Program and MCNPX Code. Mater. Chem. Phys. 2018, 215, 183–202. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.; Tijani, S. Gamma Radiation Attenuation Properties of Tellurite Glasses: A Comparative Study. Nucl. Eng. Technol. 2019, 51, 2005–2012. [Google Scholar] [CrossRef]
- Kavaz, E.; Tekin, H.; Kilic, G.; Susoy, G. Newly Developed Zinc-Tellurite Glass System: An Experimental Investigation on Impact of Ta2O5 on Nuclear Radiation Shielding Ability. J. Non-Cryst. Solids 2020, 544, 120169. [Google Scholar] [CrossRef]
- Tijani, S.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.; Wageh, S.; Dim, L. Radiation Shielding Properties of Transparent Erbium Zinc Tellurite Glass System Determined at Medical Diagnostic Energies. J. Alloy. Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- Rammah, Y.S. Evaluation of radiation shielding ability of boro-tellurite glasses: TeO2–B2O3–SrCl2–LiF–Bi2O3. Appl. Phys. A 2019, 125, 1–11. [Google Scholar] [CrossRef]
- Almasbek, A.; Kozlovskiy, A.; Zdorovets, M. The Effect of Doping With Gallium and Indium Oxides on the Optical and Shielding Characteristics of 0.5TeO2-(0.5-2x)MoO3-xGa2O3-xIn2O3 Glasses. Opt. Mater. 2021, 118, 111271. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Mann, K.S. Radiation Shielding Investigations for Selected Tellurite-Based Glasses Belonging to the TNW System. Mater. Res. Express 2019, 6, 105206. [Google Scholar] [CrossRef]
- Alalawi, A.; Al-Buriahi, M.; Sayyed, M.; Akyildirim, H.; Arslan, H.; Zaid, M.; Tonguc, B. Influence of Lead and Zinc Oxides on the Radiation Shielding Properties of Tellurite Glass Systems. Ceram. Int. 2020, 46, 17300–17306. [Google Scholar] [CrossRef]
- Mhareb, M.H.A.; Alajerami, Y.S.M.; Dwaikat, N.; Al-Buriahi, M.S.; Alqahtani, M.; Alshahri, F.; Saleh, N.; Alonizan, N.; Saleh, M.A.; Sayyed, M.I. Investigation of photon, neutron and proton shielding features of H3BO3–ZnO–Na2O–BaO glass system. Nucl. Eng. Technol. 2021, 53, 949–959. [Google Scholar] [CrossRef]
- Gaballah, M.; Issa, S.A.M.; Saddeek, Y.B.; Elsaman, R.; Susoy, G.; Erguzel, T.T.; Alharbi, T.; Tekin, H.O. Mechanical and Nuclear Radiation Shielding Properties of Different Boro-Tellurite Glasses: A Comprehensive Investigation on Large Bi2O3 Concentration. Phys. Scr. 2020, 95, 085701. [Google Scholar] [CrossRef]
- Issa, S.; Sayyed, M.; Kurudirek, M. Investigation of Gamma Radiation Shielding Properties of Some Zinc Tellurite Glasses. J. Phys. Sci. 2016, 27, 97–119. [Google Scholar] [CrossRef]
- Hanfi, M.; Sayyed, M.; Lacomme, E.; Akkurt, I.; Mahmoud, K. The Influence of MgO on the Radiation Protection and Mechanical Properties of Tellurite Glasses. Nucl. Eng. Technol. 2021, 53, 2000–2010. [Google Scholar] [CrossRef]
- Al-Buriahi, M.; Sayyed, M.; Al-Hadeethi, Y. Role of TeO2 in Radiation Shielding Characteristics of Calcium Boro-Tellurite Glasses. Ceram. Int. 2020, 46, 13622–13629. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Akyildirim, H.; Al-Buriahi, M.S.; Lacomme, E.; Ayad, R.; Bonvicini, G. Oxyfluoro-Tellurite-Zinc Glasses and the Nuclear-Shielding Ability under the Substitution of AlF3 by ZnO. Appl. Phys. A 2020, 126, 88. [Google Scholar] [CrossRef]
- Wood, J. Computational Methods in Reactor Shielding, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Chilton, A.B.; Shultis, J.K.; Faw, R.E. Principles of Radiation Shielding; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]



| Sample Code | Mole Fraction/% | Density | Thickness | Wt. Fraction of Elements in Each Sample | |||||
|---|---|---|---|---|---|---|---|---|---|
| TeO2 | PbO | B2O3 | d/(g·cm−3) | t(mm) | B | O | Te | Pb | |
| TPB-0 | 60.0 | 40.0 | 0.0 | 6.5917 | 0.756 | 0 | 0.138348 | 0.413748 | 0.447903 |
| TPB-1 | 59.4 | 39.6 | 1.0 | 6.5498 | 1.506 | 0.001176 | 0.140435 | 0.412182 | 0.446207 |
| TPB-2 | 58.8 | 39.2 | 2.0 | 6.4605 | 1.522 | 0.002366 | 0.142548 | 0.410596 | 0.444490 |
| TPB-3 | 58.2 | 38.8 | 3.0 | 6.3277 | 1.510 | 0.003572 | 0.144688 | 0.408989 | 0.442751 |
| TPB-4 | 57.6 | 38.4 | 4.0 | 6.2640 | 1.535 | 0.004793 | 0.146855 | 0.407362 | 0.440990 |
| TPB-5 | 57.0 | 38.0 | 5.0 | 6.1984 | 1.516 | 0.006030 | 0.149050 | 0.405714 | 0.439206 |
| Energy (MeV) | TPB-0 | TPB-1 | TPB-2 | TPB-3 | TPB-4 | TPB-5 | |
|---|---|---|---|---|---|---|---|
| XCOM | 5.4235 | 5.3583 | 5.2959 | 5.2330 | 5.1700 | 5.1080 | |
| 0.059 | Exp | 5.6363 | 5.4266 | 5.3587 | 5.3262 | 5.2015 | 5.1822 |
| Dev | 3.78% | 1.26% | 1.17% | 1.75% | 0.61% | 1.43% | |
| XCOM | 2.3770 | 2.3501 | 2.3236 | 2.2970 | 2.2700 | 2.2440 | |
| 0.081 | Exp | 2.4871 | 2.3890 | 2.3565 | 2.3075 | 2.2780 | 2.2832 |
| Dev | 4.43% | 1.63% | 1.40% | 0.45% | 0.35% | 1.72% | |
| XCOM | 1.9716 | 1.9525 | 1.9302 | 1.9080 | 1.8860 | 1.8650 | |
| 0.122 | Exp | 2.0041 | 1.9871 | 1.9394 | 1.9318 | 1.9029 | 1.8966 |
| Dev | 1.62% | 1.74% | 0.47% | 1.23% | 0.89% | 1.66% | |
| XCOM | 0.1975 | 0.1965 | 0.1953 | 0.1941 | 0.1929 | 0.1917 | |
| 0.356 | Exp | 0.2044 | 0.1991 | 0.1968 | 0.1979 | 0.1956 | 0.1939 |
| Dev | 3.40% | 1.32% | 0.77% | 1.94% | 1.39% | 1.13% | |
| XCOM | 0.0908 | 0.0907 | 0.0905 | 0.0903 | 0.0901 | 0.0899 | |
| 0.662 | Exp | 0.0947 | 0.0924 | 0.0915 | 0.0921 | 0.0912 | 0.0906 |
| Dev | 4.16% | 1.89% | 1.07% | 1.94% | 1.19% | 0.74% | |
| XCOM | 0.0571 | 0.0571 | 0.0571 | 0.0571 | 0.0571 | 0.0571 | |
| 1.173 | Exp | 0.0580 | 0.0581 | 0.0578 | 0.0581 | 0.0578 | 0.0578 |
| Dev | 1.50% | 1.76% | 1.12% | 1.65% | 1.20% | 1.24% | |
| XCOM | 0.0527 | 0.0527 | 0.0527 | 0.0527 | 0.0527 | 0.0526 | |
| 1.332 | Exp | 0.0538 | 0.0533 | 0.0529 | 0.0529 | 0.0537 | 0.0535 |
| Dev | 2.17% | 1.21% | 0.52% | 0.36% | 1.79% | 1.51% | |
| Ep (MeV) | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 5 | 15 |
|---|---|---|---|---|---|---|---|
| B | 1.2550 | 0.1665 | 0.1391 | 0.0806 | 0.0589 | 0.0247 | 0.0149 |
| Te | 146.4 | 11.38 | 1.7920 | 0.0931 | 0.0566 | 0.0349 | 0.0429 |
| Pb | 130.6 | 8.041 | 5.5500 | 0.1613 | 0.0710 | 0.0427 | 0.0565 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Wang, H.; Wang, S.; Zhang, J.; Zhu, Y. Effect of B2O3 on the Radiation Shielding Performance of Telluride Lead Glass System. Crystals 2022, 12, 178. https://doi.org/10.3390/cryst12020178
Yin S, Wang H, Wang S, Zhang J, Zhu Y. Effect of B2O3 on the Radiation Shielding Performance of Telluride Lead Glass System. Crystals. 2022; 12(2):178. https://doi.org/10.3390/cryst12020178
Chicago/Turabian StyleYin, Shiyu, Hao Wang, Shifeng Wang, Jing Zhang, and Yuanzhi Zhu. 2022. "Effect of B2O3 on the Radiation Shielding Performance of Telluride Lead Glass System" Crystals 12, no. 2: 178. https://doi.org/10.3390/cryst12020178






