Constructing Supramolecular Frameworks Based Imidazolate-Edge-Bridged Metallacalix[3]arenes via Hierarchical Self-Assemblies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.1.1. Synthesis of Complex [(dmbpy)3Pd3L3](NO3)3 (1•3NO3)
2.1.2. Synthesis of Complex [(phen)3Pd3L3](NO3)3 (2•3NO3)
2.2. X-ray Structure Determination and Structure Refinement
3. Results and Discussion
3.1. Ligand Synthesis and Structural Characterization
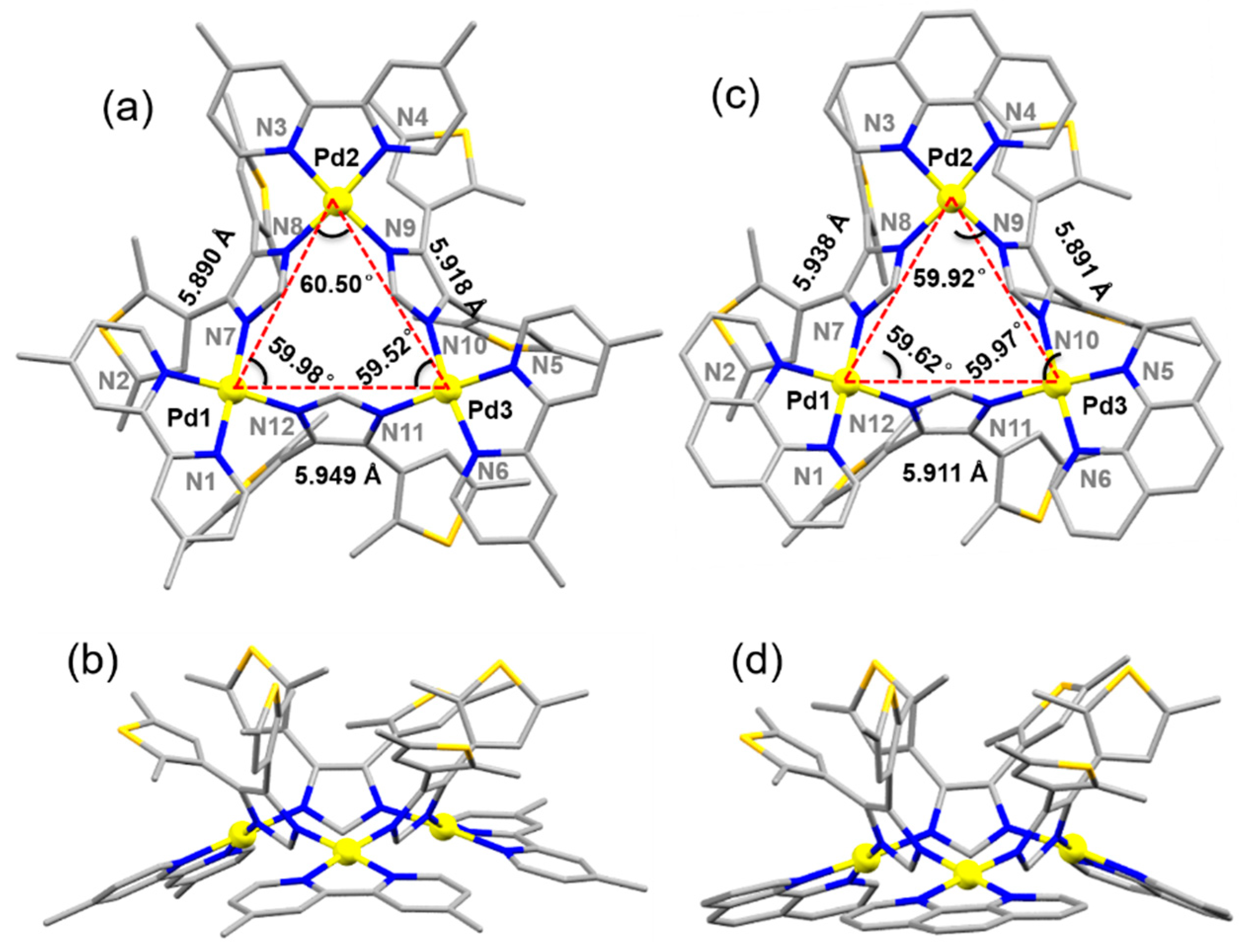
3.2. Synthesis and Structural Characterization of Metallacalix[3]arenes
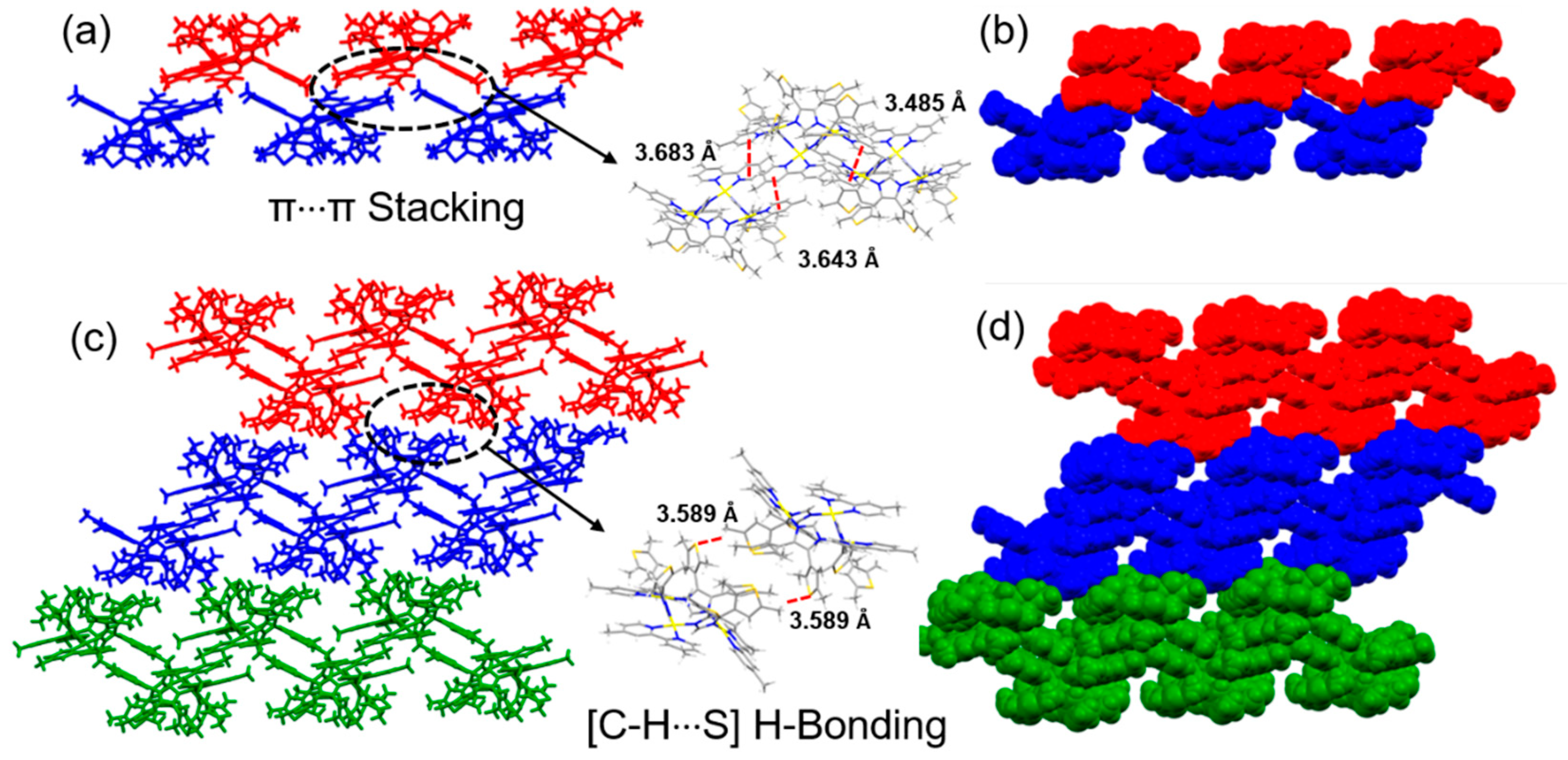
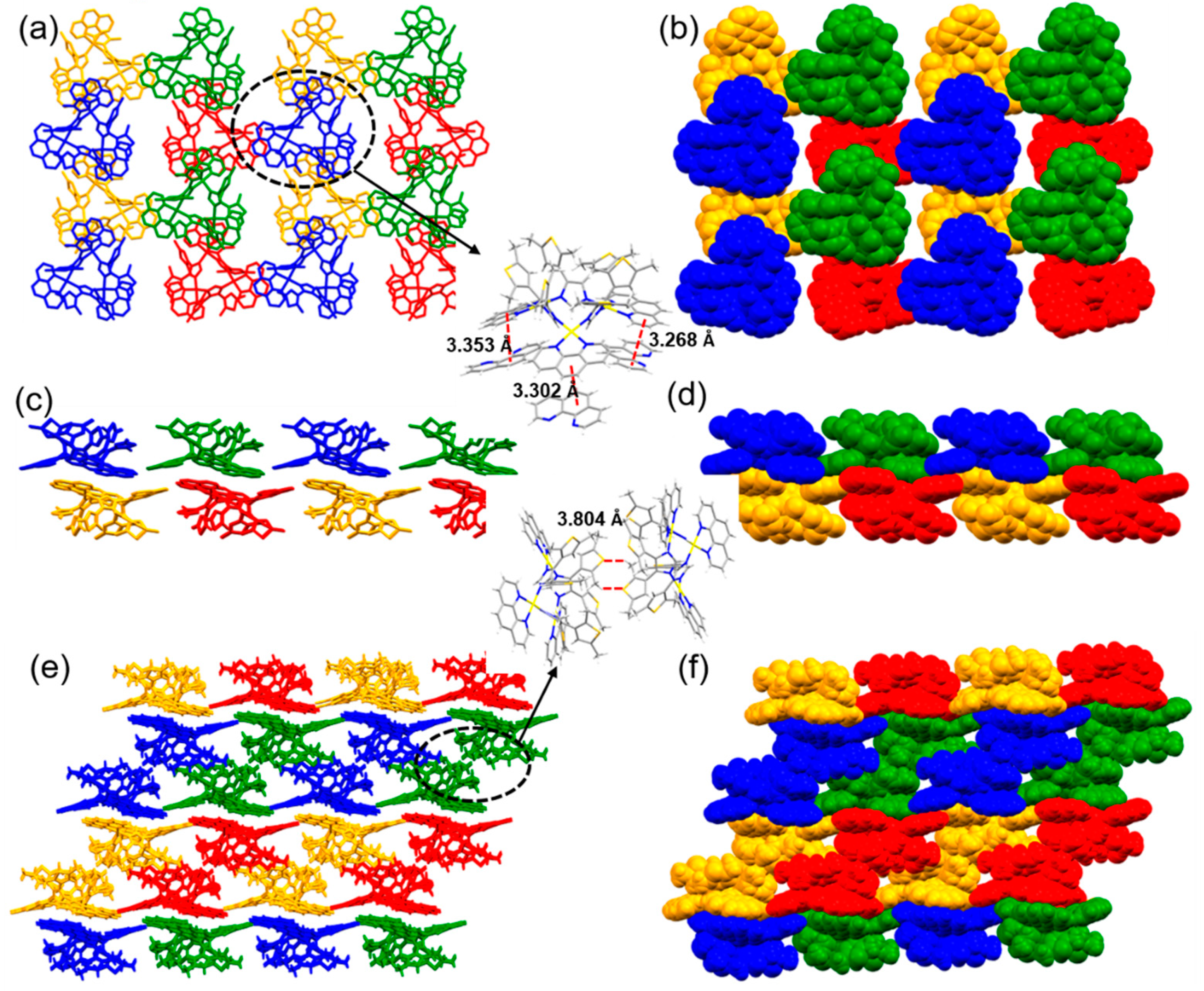
3.3. Influence of cis-Protecting Groups on the Crystal Engineering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D.; Voegtle, F.; Suslick, K.S. Comprehensive Supramolecular Chemistry; Elsevier: Oxford, UK, 1996. [Google Scholar]
- Grimes, J.M.; Burroughs, J.N.; Gouet, P.; Diprose, J.M.; Malby, R.; Ziéntara, S.; Mertens, P.P.C.; Stuart, D.I. The atomic structure of the blue tongueviruscore. Nature 1998, 395, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Yang, H.B. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef]
- Feng, L.; Wang, k.y.; Powell, J.; Zhou, H.C. Controllable Synthesis of Metal-Organic Frameworks and Their Hierarchical Assemblies. Matter 2019, 1, 801–824. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tang, D.; Zhang, J.; Ni, R.; Xu, L.; He, T.; Lin, X.; Li, X.; Qiu, H.; Yin, S.; et al. Self-Healing Heterometallic Supramolecular Polymers Constructed by Hierarchical Assembly of Triply Orthogonal Interactions with Tunable Photophysical Properties. J. Am. Chem. Soc. 2019, 141, 17909–17917. [Google Scholar] [CrossRef]
- Datta, S.; Saha, M.L.; Stang, P.J. Hierarchical Assemblies of Supramolecular Coordination Complexes. Acc. Chem. Res. 2018, 51, 2047–2063. [Google Scholar] [CrossRef]
- Guo, X.Q.; Zhou, L.P.; Hu, S.J.; Cai, L.X.; Cheng, P.M.; Sun, Q.F. Hexameric Lanthanide–Organic Capsules with Tertiary Structure and Emergent Functions. J. Am. Chem. Soc. 2021, 143, 6202–6210. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, Z.; Wang, B.; Cao, X.L.; Su, H.F.; Wang, W.; Huang, Y.G.; Hong, M.C. Constructing π-Stacked Supramolecular Cage Based Hierarchical Self-Assemblies via π···π Stacking and Hydrogen Bonding. J. Am. Chem. Soc. 2021, 143, 10920–10929. [Google Scholar] [CrossRef]
- Sun, Z.W.; Li, P.; Xu, S.J.; Li, Z.Y.; Nomura, Y.; Li, Z.M.; Liu, X.Y.; Zhang, S.D. Controlled Hierarchical Self-Assembly of Catenated Cages. J. Am. Chem. Soc. 2020, 142, 10833–10840. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal–Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for binding multiple guests in metal–organic cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Yan, X.; Wang, M.; Cook, T.R.; Zhang, M.; Saha, M.L.; Zhou, Z.; Li, X.; Huang, F.; Stang, P.J. Light-emitting superstructures with anion effect: Coordination-driven self-assembly of pure tetraphenylethylene metallacycles and metallacages. J. Am. Chem. Soc. 2016, 138, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, S.; Chand, D.K. cis-Protected palladium(II) based binuclear complexes as tectons in crystal engineering and the imperative role of the cis-protecting agent. CrystEngComm 2017, 19, 5157–5172. [Google Scholar] [CrossRef]
- Dai, D.-H.; Li, Z.; Yang, J.; Wang, C.-Y.; Wu, J.-R.; Wang, Y.; Zhang, D.-M.; Yang, Y.-W. Supramolecular Assembly-Induced Emission Enhancement for Efficient Mercury (II) Detection and Removal. J. Am. Chem. Soc. 2019, 141, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Yang, X.; Zhang, M.T.; Guo, J.; Parkin, S.; Li, T.; Yu, F.Q.; Long, S.H. Synthon Polymorphism and π-π Stacking in N-Phenyl-2-hydroxynicotinanilides. Cryst. Growth Des. 2021, 21, 6155–6165. [Google Scholar]
- Wang, D.; Guo, G.; Chen, R.; Gong, Y.; Sun, L.; Zhao, Y. Single-crystal structure of two-dimensional organic framework based on donor-acceptor interactions with charge-transfer effect. Sci. China Chem. 2021, 64, 1510–1514. [Google Scholar] [CrossRef]
- Beldjoudi, Y.; Narayanan, A.; Roy, I.; Pearson, T.J.; Mustafa, C.; Nguyen, M.T.; Krzyaniak, M.D.; Alsubaie, F.M.; Wasielewski, M.R.; Stupp, S.I.; et al. Supramolecular Tessellations by a Rigid Naphthalene Diimide Triangle. J. Am. Chem. Soc. 2019, 141, 17783–17795. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Iguchi, H.; Takaishi, S.; Habib, F.; Leong, C.F.; D’Alessandro, D.M.; Yoshida, T.; Abe, H.; Nishibori, E.; Yamashita, M. Porous Molecular Conductor: Electrochemical Fabrication of Through-Space Conduction Pathways among Linear Coordination Polymers. J. Am. Chem. Soc. 2019, 141, 6802–6806. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Zhang, P.; Huang, X.; Zheng, X.; Chen, M.; Feng, H.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Ionization and Anion–π+ Interaction: A New Strategy for Structural Design of Aggregation-Induced Emission Luminogens. J. Am. Chem. Soc. 2017, 139, 16974–16979. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A Healable Supramolecular Polymer Blend Based on Aromatic π–π Stacking and Hydrogen-Bonding Interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Wang, B.; Lin, R.B.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Hydrogen-Bonded Organic Frameworks as a Tunable Platform for Functional Materials. J. Am. Chem. Soc. 2020, 142, 14399–14416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Kan, L.; Li, J.Y.; Liu, Y.L.; Eddaoudi, M. Quest for Zeolite-like Supramolecular Assemblies: Self-Assembly of Metal Organic Squares via Directed Hydrogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 19659–19662. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Z.; Wang, H.Z.; Hauke, C.E.; Cook, T.R.; Wang, M.; Saha, M.L.; Zhou, Z.X.; Zhang, M.M.; Li, X.P.; Huang, F.; et al. A Suite of Tetraphenylethylene-Based Discrete Organoplatinum(II) Metallacycles: Controllable Structure and Stoichiometry, Aggregation-Induced Emission, and Nitroaromatics Sensing. J. Am. Chem. Soc. 2015, 137, 15276–15286. [Google Scholar] [CrossRef]
- Dong, J.Q.; Pan, Y.T.; Wang, H.; Yang, K.W.; Liu, L.M.; Qiao, Z.W.; Yuan, Y.D.; Peh, S.B.; Zhang, J.; Shi, L.L.; et al. Self-Assembly of Highly Stable Zirconium(IV) Coordination Cages with Aggregation Induced Emission Molecular Rotors for Live-Cell Imaging. Angew. Chem. Int. Ed. 2020, 59, 10151–10159. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Wu, L.; Lu, S.; Ling, S.; Li, G.; Xu, L.; Ma, L.; Hou, Y.; Wang, X.; et al. Emissive Platinum(II) Cages with Reverse Fluorescence Resonance Energy Transfer for Multiple Sensing. J. Am. Chem. Soc. 2020, 142, 2592–2600. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, Z.; Li, G.; Stang, P.J.; Yan, X. Light-Emitting Self-Assembled Metallacages. Nat. Sci. Rev. 2021, 8, nwab045. [Google Scholar] [CrossRef]
- Bhowal, R.; Chopra, D. Investigating the Role of Weak Interactions to Explore the Polymorphic Diversity in Difluorinated Isomeric N-Phenylcinnamamides. Cryst. Growth Des. 2021, 21, 4162–4177. [Google Scholar] [CrossRef]
- Deng, W.; Yu, Z.S.; Ma, H.W.; Yu, S.Y. Self-Assembly of Water-Soluble Platinum(II)-Based Metallacalixarenes and Tuning Their Conformational Interconversion via Synergistic Effects between Solvents and Anions. Chem. Asian J. 2018, 13, 2805–2811. [Google Scholar] [CrossRef]
- Ma, T.T.; Tong, J.; Sun, W.Q.; Ma, H.W.; Yu, S.Y. Self-assembly of a Pd-based molecular bowl as anion receptor featured by multiple C-H···anion hydrogen bonds. Inorg. Chem. Commun. 2018, 91, 24–28. [Google Scholar] [CrossRef]
- Yao, L.Y.; Qin, L.; Xie, T.Z.; Li, Y.Z.; Yu, S.Y. Synthesis and anion sensing of water-soluble metallomacrocycles. Inorg. Chem. 2011, 50, 6055–6062. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Huang, H.; Liu, H.B.; Chen, Z.N.; Zhang, R.; Fujita, M. Modular Cavity-Tunable Self-Assembly of Molecular Bowls and Crowns as Structural Analogues of Calix[3]arenes. Angew. Chem. Int. Ed. 2003, 42, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Kusukawa, T.; Biradha, K.; Fujita, M. Hydrophobic Assembling of a Coordination Nanobowl into a Dimeric Capsule Which Can Accommodate up to Six Large Organic Molecules. J. Am. Chem. Soc. 2000, 122, 2665–2666. [Google Scholar] [CrossRef]
- Fujita, M.; Yu, S.Y.; Kusukawa, T.; Funaki, H.; Ogura, K.; Yamaguchi, K. Self-Assembly of Nanometer-Sized Macrotricyclic Complexes from Ten Small Component Molecules. Angew. Chem. Int. Ed. 1998, 37, 2082–2085. [Google Scholar] [CrossRef]
- Liu, L.X.; Huang, H.P.; Li, X.; Sun, Q.F.; Sun, C.R.; Li, Y.Z.; Yu, S.Y. Coordination molecular hats binding acetonitrile via C-H…π interactions. Dalton Trans. 2008, 12, 1544–1546. [Google Scholar] [CrossRef]
- Mu, Y.J.; Yu, L.N.; Jiang, X.F.; Yu, S.Y.; Yamaguchi, K. Self-assembly of an organo-palladium molecular basket that encapsulates cobalticarborane anion in water. Inorg. Chem. Commun. 2014, 44, 119–123. [Google Scholar] [CrossRef]
- Yu, L.N.; Jiang, X.F.; Yu, S.Y. Self-Assembly of Chiral Nano-bowl from Chiral Pd-complex Building Corners and 4,7-Phenanthroline Linkers. Chin. J. Chem. 2015, 33, 152–155. [Google Scholar] [CrossRef]
- Xie, T.Z.; Guo, C.; Yu, S.Y.; Pan, Y.J. Fine-Tuning Conformational Motion of a Self-Assembled Metal–Organic Macrocycleby Multiple CH···Anion Hydrogen Bonds. Angew. Chem. Int. Ed. 2012, 51, 1177–1181. [Google Scholar] [CrossRef]
- Ning, G.H.; Xie, T.Z.; Pan, Y.J.; Li, Y.Z.; Yu, S.Y. Self-assembly of bowl-like trinuclearmetallo-macrocycles. Dalton Trans. 2010, 39, 3203–3211. [Google Scholar] [CrossRef]
- Zangrando, E.; Casanova, M.; Alessio, E. Trinuclear Metallacycles: Metallatriangles and Much More. Chem. Rev. 2008, 108, 4979–5013. [Google Scholar] [CrossRef]
- Hess, J.L.; Hsieh, C.H.; Brothers, S.M.; Hall, M.B.; Darensbourg, M.Y. Self-Assembly of Dinitrosyl Iron Units into Imidazolate-Edge-Bridged Molecular Squares: Characterization Including Mossbauer Spectroscopy. J. Am. Chem. Soc. 2011, 133, 20426–20434. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Ramírez, M.L.; Huggins, H.; Donnadieu, B.; Lopez, N.; Muñoz-Hernández, M.A. The quest for large Group 13 metallacalixarenes based onbenzymidazolyl ligands and Al and Ga alkyls. Eur. J. Inorg. Chem. 2021, 2021, 3896–3902. [Google Scholar] [CrossRef]
- Steffen, A.; Braun, T.; Neumann, B.; Stammler, H.G. Palladium Fluoro Complexes: Useful Tools to Access Organometallic Metallamacrocycles. Angew. Chem. Int. Ed. 2007, 46, 8674–8678. [Google Scholar] [CrossRef]
- Naranthatta, M.C.; Ramkumar, V.; Chand, D.K. Self-assembly of self-assembled molecular triangles. J. Chem. Sci. 2014, 126, 1493–1499. [Google Scholar] [CrossRef]
- Naranthatta, M.C.; Ramkumar, V.; Chand, D.K. Role of peripheral phenanthroline groups in the self-assembly of self-assembled molecular triangles. J. Chem. Sci. 2015, 127, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Yu, Z.S.; Liu, X.H.; Yu, S.Y. Self-Assembly and C@H···Anion Hydrogen Bonding of Palladium(II)-based Metallacalixarenes Using Pyridyl- or Phenyl-Bridged Di-Naphthoimidazoles. Chem. Asian J. 2018, 13, 3173–3179. [Google Scholar] [CrossRef] [PubMed]
- Du, W.T.; Tong, J.; Deng, W.; Wang, M.X.; Yu, S.Y. Coordination-driven self-assembly of palladium(II)-based metallacalixarenes as anion receptors using flexible pyridine-bridged diimidazole ligands. Chin. Chem. Lett. 2021, 32, 485–488. [Google Scholar]
- Neilson, B.M.; Lynch, V.M.; Bielawski, C.W. Photoswitchable N-Heterocyclic Carbenes: Using Light to Modulate Electron-Donating Properties. Angew. Chem. Int. Ed. 2011, 50, 10322–10326. [Google Scholar] [CrossRef]
- Gong, D.P.; Chen, J.F.; Zhao, Y.; Cao, D.K. Bisthienylethene Th2im and its complex (Th2imH)2[ReCl6]: Crystalline-phase photochromism, and photochemical regulation of luminescence and magnetic properties. Dalton Trans. 2016, 45, 3443–3449. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97; University of Göttingen: Göttingen, Germany, 1990. [Google Scholar]
- Sheldrick, G.M. SHELXL-97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Domagała, M.; Grabowski, S.J.; Urbaniak, G.; Mloston, G. Role of C-H···S and C-H···N Hydrogen Bonds in Organic Crystal Structuress The Crystal and Molecular Structure of 3-Methyl-2,4-diphenyl-(1,3)-thiazolidine-5-spiro-2′-adamantane and 3-Methyl-2,4,5,5-tetraphenyl-(1,3)-thiazolidine. J. Phys. Chem. A. 2003, 107, 2730–2736. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromaticnitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Naranthatta, M.C.; Das, D.; Tripathy, D. Consequence of Presence and Absence of π–Clouds at Strategic Locations of Designed Binuclear Pd(II) Complexes on Packing: Self Assembly of Self-Assembly by Intermolecular Locking and Packing. Cryst. Growth Des. 2012, 12, 6012–6022. [Google Scholar] [CrossRef]
- Ramkumar, V.; Chand, D.K. Toppled Molecular-Domino Sets by Self-Assembly of Self-assembly: The π-Polymers Debakanta Tripathy. Cryst. Growth Des. 2013, 13, 3763–3772. [Google Scholar]


| HL | 1•3PF6 | 2•3NO3 | |
|---|---|---|---|
| Formula | C32H32N4S4 | C81H81F18N12S6P3Pd3 | C81H69N15O9S6Pd3 |
| Formula weight | 600.85 | 2169.04 | 1908.07 |
| crystal system | triclinic | triclinic | monoclinic |
| space group | P-1 | P-1 | C2/c |
| a [Å] | 9.6510(7) | 16.8822(9) | 33.3674(14) |
| b [Å] | 12.1557(10) | 18.9348(7) | 20.8260(9) |
| c [Å] | 13.4203(11) | 18.9951(7) | 35.088(3) |
| α [°] | 98.101(3) | 60.273(3) | 90 |
| β [°] | 91.796(3) | 74.678(3) | 115.090(5) |
| γ [°] | 101.972(2) | 89.701(4) | 90 |
| V [Å3] | 1521.8(2) | 5027.8(4) | 22082(2) |
| Z | 2 | 2 | 8 |
| ρcalcd, [g/cm−3] | 1.311 | 1.433 | 1.148 |
| μ [mm−1] | 0.341 | 6.581 | 0.646 |
| F(000) | 632 | 2184 | 7728 |
| 2θmax [°] | 56.764 | 69.999 | 27.000 |
| no. unique data | 7567 | 18,840 | 24,101 |
| Parameters | 5616 | 1126 | 1135 |
| GOF [F2] | 1.049 | 1.050 | 1.056 |
| R[F2 > 2σ(F2)] | 0.0568 | 0.0404 | 0.0508 |
| wR[F2] | 0.1755 | 0.0977 | 0.1035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Zhao, L.; Tong, J.; Wang, X.; Yu, S. Constructing Supramolecular Frameworks Based Imidazolate-Edge-Bridged Metallacalix[3]arenes via Hierarchical Self-Assemblies. Crystals 2022, 12, 212. https://doi.org/10.3390/cryst12020212
Cui B, Zhao L, Tong J, Wang X, Yu S. Constructing Supramolecular Frameworks Based Imidazolate-Edge-Bridged Metallacalix[3]arenes via Hierarchical Self-Assemblies. Crystals. 2022; 12(2):212. https://doi.org/10.3390/cryst12020212
Chicago/Turabian StyleCui, Bo, Lirong Zhao, Jin Tong, Xiayan Wang, and Shuyan Yu. 2022. "Constructing Supramolecular Frameworks Based Imidazolate-Edge-Bridged Metallacalix[3]arenes via Hierarchical Self-Assemblies" Crystals 12, no. 2: 212. https://doi.org/10.3390/cryst12020212





