Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CuO/Functionalized Graphene Hybrid and Thin-Film Development
2.3. Characterization
3. Results and Discussion
3.1. FTIR Analysis
3.2. Raman Analysis
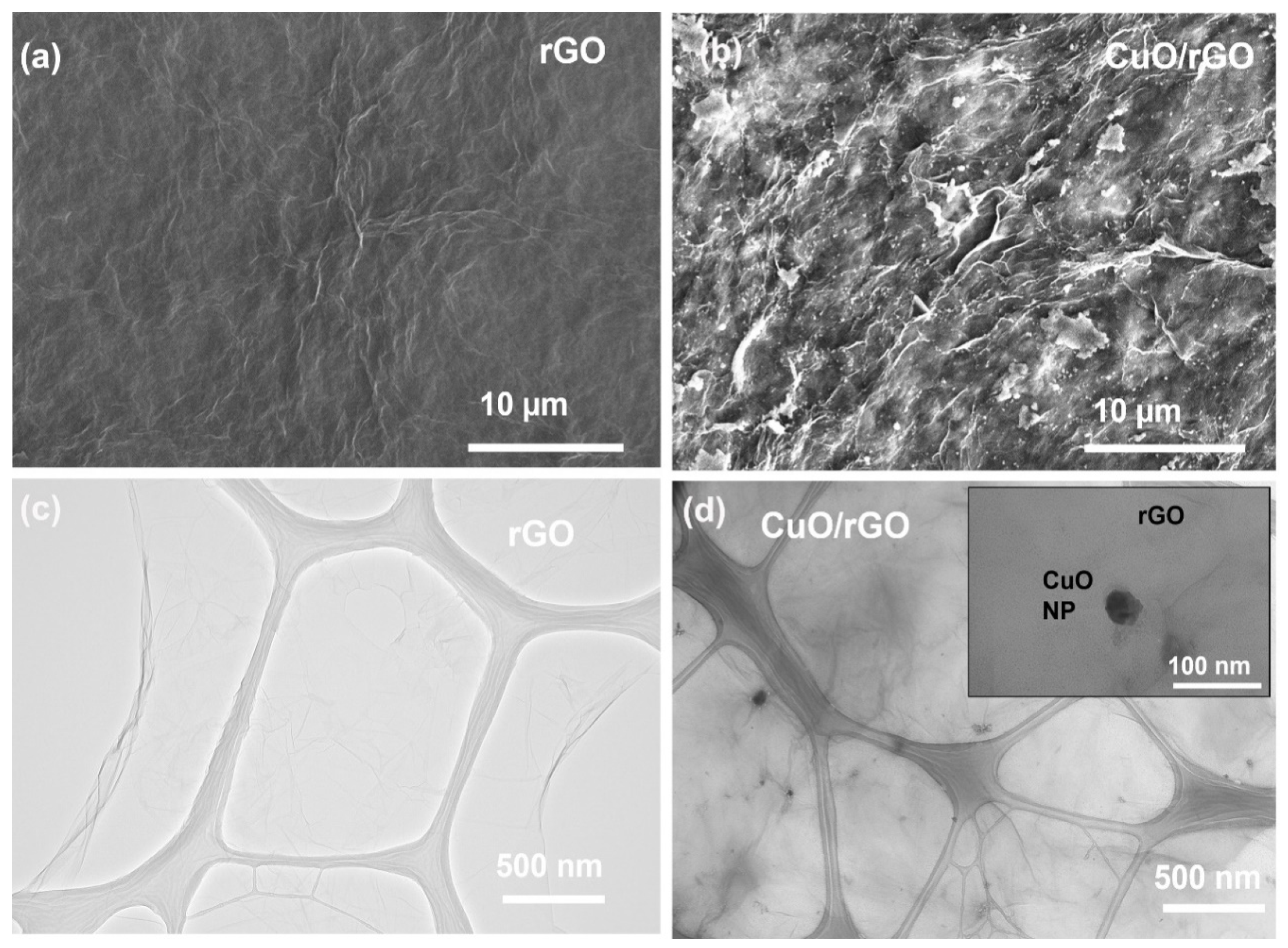
3.3. SEM and TEM Analysis
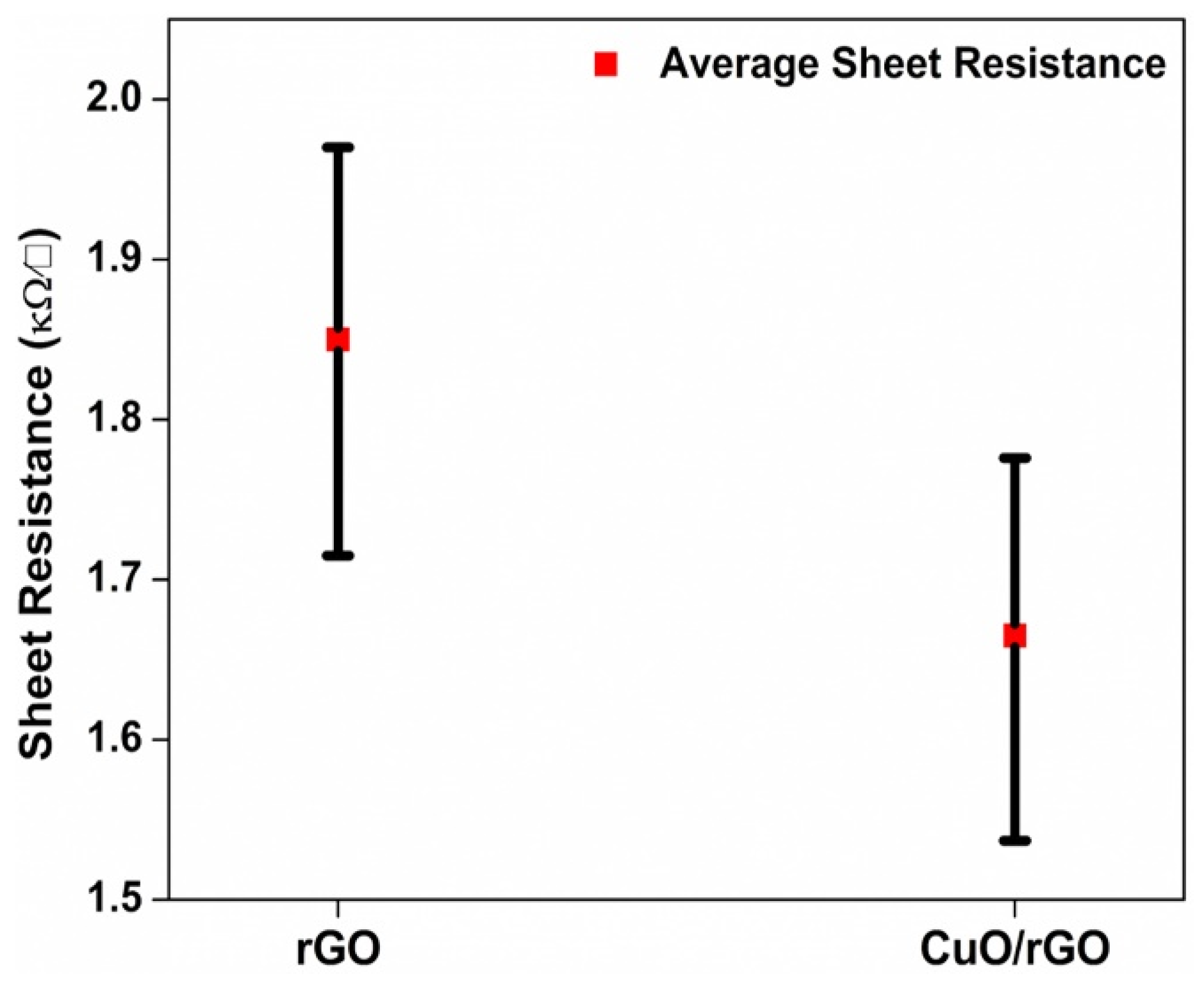
3.4. Electrical Analysis
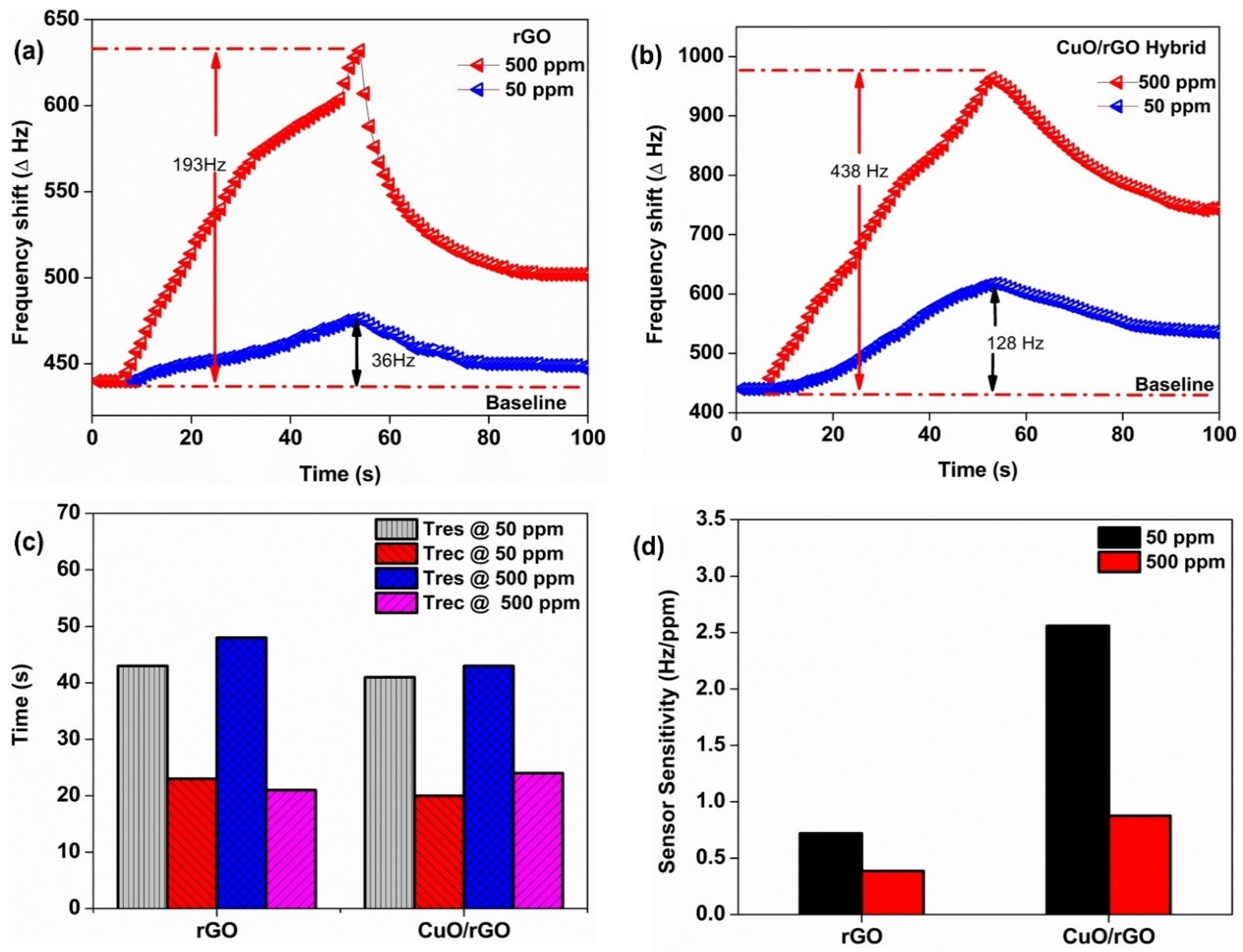
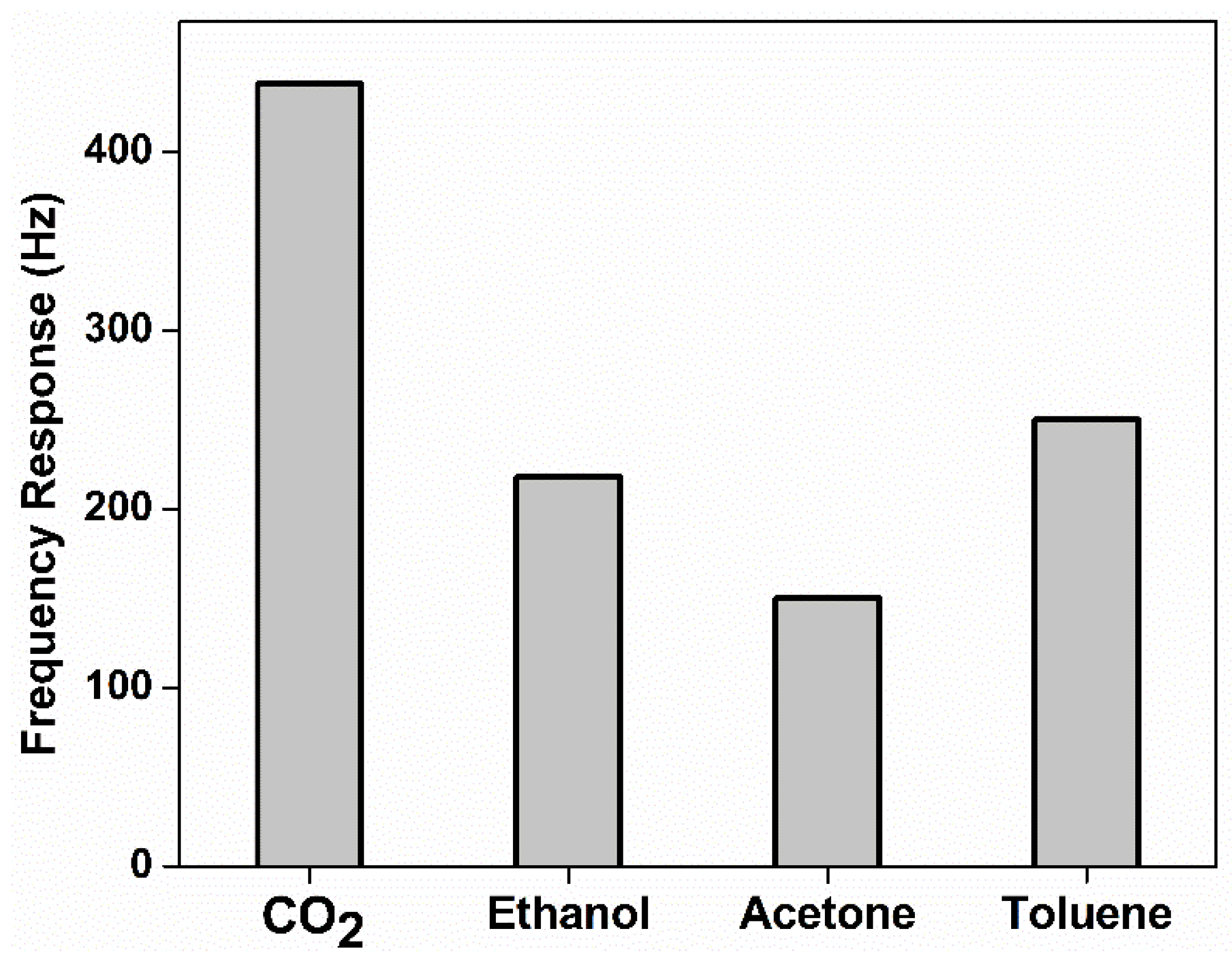
3.5. Gas Sensing Performance
| Materials | Synthesis Method | Detected Gas | Sensor Sensitivity (Hz/ppm) | Mass Sensitivity (Hz/µg) | Frequency Shift (Hz)/Tres (s)/Trec (s) | References |
|---|---|---|---|---|---|---|
| graphene oxide/TiO2 composite | liquid phase deposition | ethanol | ~0.925 | - | 370 Hz | [55] |
| citric acid monohydrate (CA) poly (ethylene glycol) diacrylate (CAPEGDA) | - | NH3 | ~0.85 | - | 18 Hz/~5 min/~10 min | [56] |
| polyvinyl acetate (PVAc) nanofibers | electrospinning method | safrole | ~1.866 | ~0.799 | 16.3 Hz/Tres = 171 s | [37] |
| vanadium Oxide | vacuum thermal evaporation | CO2 | - | - | 50 s/125 s | [12] |
| cryptophane-A-based QCM sensor | electrospray method | CH4, NO2 | 0.103, 0.032 | - | - | [57] |
| commercial CuO | hydrothermal treatment method | HCN | ~0.82 | ~0.31 | 41 Hz/~300 s/~350 s | [13] |
| CuO/rGO hybrid | chemical synthesis | CO2 | 2.56 | 15 | 438 Hz/~41 s/20 s | This work |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leghrib, R. Design, Fabrication and Characterisation of Gas Sensors Based on Nanohybrid Materials; Universitat Rovira i Virgili: Tarragona, Spain, 2011. [Google Scholar]
- Azuma, K.; Kagi, N.; Yanagi, U.; Osawa, H. Effects of low-level inhalation exposure to carbon dioxide in indoor environments: A short review on human health and psychomotor performance. Environ. Int. 2018, 121, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.F.; Hu, Z.B.; Liu, M.; Yang, H.L.; Kong, Q.X.; Liu, Y.H. Review of research on air-conditioning systems and indoor air quality control for human health. Int. J. Refrig. 2009, 32, 3–20. [Google Scholar] [CrossRef]
- Tanvir, N.B.; Yurchenko, O.; Wilbertz, C.; Urban, G. Investigation of CO2 reaction with copper oxide nanoparticles for room temperature gas sensing. J. Mater. Chem. A 2016, 4, 5294–5302. [Google Scholar] [CrossRef] [Green Version]
- Rathi, K.; Pal, K. Ruthenium decorated tungsten disulfide quantum dots for CO2 gas sensor. Nanot 2019, 31, 135502. [Google Scholar] [CrossRef]
- Daud, A.I.; Wahid, K.A.A.; Khairul, W.M. Room-temperature operated cyano-terminated ethynylated-thiourea as a resistive-type carbon dioxide (CO2) gas sensor. Org. Electron. 2019, 70, 32–41. [Google Scholar] [CrossRef]
- Young, S.-J.; Lin, Z.-D. Sensing Performance of Carbon Dioxide Gas Sensors with Carbon Nanotubes on Plastic Substrate. ECS J. Solid State Sci. Technol. 2017, 6, 72–74. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Brooks, L.L.; Chartuprayoon, N.; Bosze, W.; Choa, Y.H.; Myung, N.V. Palladium/single-walled carbon nanotube back-to-back schottky contact-based hydrogen sensors and their sensing mechanism. ACS Appl. Mater. Interfaces 2014, 6, 319–326. [Google Scholar] [CrossRef]
- Zia, A.I.; Syaifudin, A.R.M.; Mukhopadhyay, S.C.; Yu, P.L.; Al-Bahadly, I.H.; Gooneratne, C.P.; Kosel, J.; Liao, T.S. Electrochemical impedance spectroscopy based MEMS sensors for phthalates detection in water and juices. J. Phys. Conf. Ser. 2013, 439, 012026. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Ba Hashwan, S.S.; Junaid, M.M. A Review of Actuation and Sensing Mechanisms in MEMS-Based Sensor Devices. Nanoscale Res. Lett. 2021, 16, 16. [Google Scholar] [CrossRef]
- Berouaken, M.; Talbi, L.; Alkama, R.; Sam, S.; Menari, H.; Chebout, K.; Manseri, A.; Boucheham, A.; Gabouze, N. Quartz Crystal Microbalance Coated with Vanadium Oxide Thin Film for CO2 Gas Sensor at Room Temperature. Arab. J. Sci. Eng. 2018, 43, 5957–5963. [Google Scholar] [CrossRef]
- Yang, M.; He, J.; Hu, X.; Yan, C.; Cheng, Z. CuO nanostructures as quartz crystal microbalance sensing layers for detection of trace hydrogen cyanide gas. Environ. Sci. Technol. 2011, 45, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Vashist, P. Recent advances in quartz crystal microbalance-based sensors. J. Sens. 2011, 2011, 571405. [Google Scholar] [CrossRef]
- Varga, M.; Laposa, A.; Kulha, P.; Kroutil, J.; Husak, M.; Kromka, A. Quartz crystal microbalance gas sensor with nanocrystalline diamond sensitive layer. Phys. Status Solidi Basic Res. 2015, 252, 2591–2597. [Google Scholar] [CrossRef]
- Gupta, M.; Athirah, N.; Hawari, H.F. Graphene derivative coated QCM-based gas sensor for volatile organic compound (VOC) detection at room temperature. Indones. J. Electr. Eng. Comput. Sci. 2020, 18, 1279–1286. [Google Scholar] [CrossRef]
- Zhu, B.L.; Xie, C.S.; Wang, W.Y.; Huang, K.J.; Hu, J.H. Improvement in gas sensitivity of ZnO thick film to volatile organic compounds (VOCs) by adding TiO2. Mater. Lett. 2004, 58, 624–629. [Google Scholar] [CrossRef]
- Ren, H.; Gu, C.; Joo, S.W.; Zhao, J.; Sun, Y.; Huang, J. Effective hydrogen gas sensor based on NiO@rGO nanocomposite. Sens. Actuators B Chem. 2018, 266, 506–513. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Wan, F.; Gao, T. Gas sensing properties and mechanism of Nano-SnO2-based sensor for hydrogen and carbon monoxide. J. Nanomater. 2012, 2012, 612420. [Google Scholar] [CrossRef] [Green Version]
- Lee, Z.Y.; Hawari, H.F.B.; Djaswadi, G.W.B.; Kamarudin, K. A highly sensitive room temperature CO2 gas sensor based on sno2-rgo hybrid composite. Materials 2021, 14, 522. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, J.-J.; Yang, M.-P.; Meng, W.-J.; Wang, H.; Lu, J.-X. CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol. Catalysts 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Bhuvaneshwari, S.; Gopalakrishnan, N. Facile synthesis of low dimensional CuO nanostructures and their gas sensing applications. Cryst. Res. Technol. 2016, 51, 145–153. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Saravanakumar, B.; Babu, I.M.; Sethuraman, B.; Muralidharan, G. Nanostructured CuO/reduced graphene oxide composite for hybrid supercapacitors. RSC Adv. 2014, 4, 23485–23491. [Google Scholar] [CrossRef]
- Nagaraja, M.; Prashanth, S.; Pattar, J.; Mahesh, H.M.; Rajanna, K. Polyaniline-CuO nanocomposite: Electrical, structural and sensor properties. Mater. Today Proc. 2022, 49, 1989–1992. [Google Scholar] [CrossRef]
- Kumar, P.; Kang, C.H.; Burhanudin, Z.A.; Saheed, M.S.M.; Irshad, M.I.; Mohamed, N.M. Graphene-based hybrid thin films as transparent conductive electrode for optoelectronic devices. In Proceedings of the IEEE International Conference on Semiconductor Electronics, Kuala Lumpur, Malaysia, 17–19 August 2016; Volume 2016, pp. 216–219. [Google Scholar]
- Kondee, S.; Arayawut, O.; Pon-On, W.; Wongchoosuk, C. Nitrogen-doped carbon oxide quantum dots for flexible humidity sensor: Experimental and SCC-DFTB study. Vacuum 2022, 195, 110648. [Google Scholar] [CrossRef]
- Mandayo, G.G.; González, F.; Rivas, I.; Ayerdi, I.; Herrán, J. BaTiO3-CuO sputtered thin film for carbon dioxide detection. Sens. Actuators B Chem. 2006, 118, 305–310. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Macinnes, M.M.; Hlynchuk, S.; Acharya, S.; Lehnert, N.; Maldonado, S. Reduction of Graphene Oxide Thin Films by Cobaltocene and Decamethylcobaltocene. ACS Appl. Mater. Interfaces 2018, 10, 2004–2015. [Google Scholar] [CrossRef] [Green Version]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and structural properties of reduced graphene oxide—dependence on the reducing agent. J. Mater. Sci. 2021, 56, 3738–3754. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Ahmed, R.E.; Al-Rashid, M.A.; Alarrouqi, R.A.; Saleh, B.; Abdulrehman, T.; Haik, Y.; Al-Sulaiti, L.A. Selective gas sensors using graphene and CuO nanorods. Sens. Actuators A Phys. 2018, 283, 107–112. [Google Scholar] [CrossRef]
- Guo, L.; Hao, Y.W.; Li, P.L.; Song, J.F.; Yang, R.Z.; Fu, X.Y.; Xie, S.Y.; Zhao, J.; Zhang, Y.L. Improved NO2 Gas Sensing Properties of Graphene Oxide Reduced by Two-beam-laser Interference. Sci. Rep. 2018, 8, 4918. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.S. MEMS based highly sensitive dual FET gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H2 detection. Sci. Rep. 2018, 8, 5902. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, Z.; Wang, Y.; Zhang, L.; Wang, Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 2013, 25, 025502. [Google Scholar] [CrossRef] [PubMed]
- Chaloeipote, G.; Samarnwong, J.; Traiwatcharanon, P.; Kerdcharoen, T.; Wongchoosuk, C. High-performance resistive humidity sensor based on Ag nanoparticles decorated with graphene quantum dots. R. Soc. Open Sci. 2021, 8, 210407. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Hawari, H.F.; Kumar, P.; Burhanudin, Z.A.; Tansu, N. Functionalized reduced graphene oxide thin films for ultrahigh CO2 gas sensing performance at room temperature. Nanomaterials 2021, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Triyana, K.; Rianjanu, A.; Nugroho, D.B.; As’ari, A.H.; Kusumaatmaja, A.; Roto, R.; Suryana, R.; Wasisto, H.S. A highly sensitive safrole sensor based on polyvinyl acetate (PVAc) nanofiber-coated QCM. Sci. Rep. 2019, 9, 15407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Hu, R.; Fan, G.; Li, G. Graphene oxide/chitosan nanocomposite coated quartz crystal microbalance sensor for detection of amine vapors. Sens. Actuators B Chem. 2017, 243, 721–730. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.S.; Zeng, X. Correlation between molecular structure and interfacial properties of edge or basal plane modified graphene oxide. ACS Appl. Nano Mater. 2018, 1, 2763–2773. [Google Scholar] [CrossRef]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of functional groups and determination of carboxyl formation temperature in graphene oxide using the XPS O 1s spectrum. Thin Solid Films 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Liu, J.; Cao, Y. Investigation of CO2 reaction with copper oxide nanoparticles for room temperature gas sensing. Sens. Actuators B Chem. 2017, 247, 875–882. [Google Scholar] [CrossRef]
- Singh, P.; Nath, P.; Arun, R.K.; Mandal, S.; Chanda, N. Novel synthesis of a mixed Cu/CuO-reduced graphene oxide nanocomposite with enhanced peroxidase-like catalytic activity for easy detection of glutathione in solution and using a paper strip. RSC Adv. 2016, 6, 92729–92738. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalization. Nanoscale 2013, 5, 4541–4583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pang, X.; Zhao, Y. Effect of the external velocity on the exfoliation properties of graphene from amorphous SiO2 surface. Crystals 2021, 11, 454. [Google Scholar] [CrossRef]
- Bhaumik, A.; Haque, A.; Taufique, M.; Karnati, P.; Patel, R.; Nath, M.; Ghosh, K. Reduced Graphene Oxide Thin Films with Very Large Charge Carrier Mobility Using Pulsed Laser Deposition. J. Mater. Sci. Eng. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Guo, D.; Guo, J.; Su, Y. Room-temperature synthesis of CuO/reduced graphene oxide nanohybrids for high-performance NO2 gas sensor. Sens. Actuators B Chem. 2018, 271, 306–310. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Lin, K.; Seng, W.; Shuaib, M.; Saheed, M. Hybrid film of single-layer graphene and carbon nanotube as transparent conductive electrode for organic light emitting diode. Synth. Met. 2019, 257, 116186. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, C.; Gao, X.; Zhuang, Z.; Du, C.; Chen, W. 3D Network and 2D Paper of Reduced Graphene Oxide/Cu2O Composite for Electrochemical Sensing of Hydrogen Peroxide. Anal. Chem. 2018, 90, 1983–1991. [Google Scholar] [CrossRef] [Green Version]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wan, L.; Wei, L.; Talwar, D.N.; He, K.; Feng, Z. Temperature-dependent optical properties of graphene on Si and SiO2/Si substrates. Crystals 2021, 11, 358. [Google Scholar] [CrossRef]
- Zaki, S.E.; Basyooni, M.A.; Shaban, M.; Rabia, M.; Eker, Y.R.; Attia, G.F.; Yilmaz, M.; Ahmed, A.M. Role of oxygen vacancies in vanadium oxide and oxygen functional groups in graphene oxide for room temperature CO2 gas sensors. Sens. Actuators A Phys. 2019, 294, 17–24. [Google Scholar] [CrossRef]
- Rodríguez-García, S.; Santiago, R.; López-Díaz, D.; Merchán, M.D.; Velázquez, M.M.; Fierro, J.L.G.; Palomar, J. Role of the Structure of Graphene Oxide Sheets on the CO2 Adsorption Properties of Nanocomposites Based on Graphene Oxide and Polyaniline or Fe3O4-Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 12464–12473. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Jayawardena, S.; Siriwardena, H.D.; Rajapakse, R.M.G.; Kubono, A.; Shimomura, M. Fabrication of a quartz crystal microbalance sensor based on graphene oxide/TiO2 composite for the detection of chemical vapors at room temperature. Appl. Surf. Sci. 2019, 493, 250–260. [Google Scholar] [CrossRef]
- Liu, L.; Fei, T.; Guan, X.; Zhao, H.; Zhang, T. Highly sensitive and chemically stable NH3 sensors based on an organic acid-sensitized cross-linked hydrogel for exhaled breath analysis. Biosens. Bioelectron. 2021, 191, 113459. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Jiang, Y.; Xie, G.; Du, X.; Hu, J. Sensors and Actuators B: Chemical A room temperature supramolecular-based quartz crystal microbalance ( QCM ) methane gas sensor. Sens. Actuators B Chem. 2009, 141, 104–108. [Google Scholar] [CrossRef]


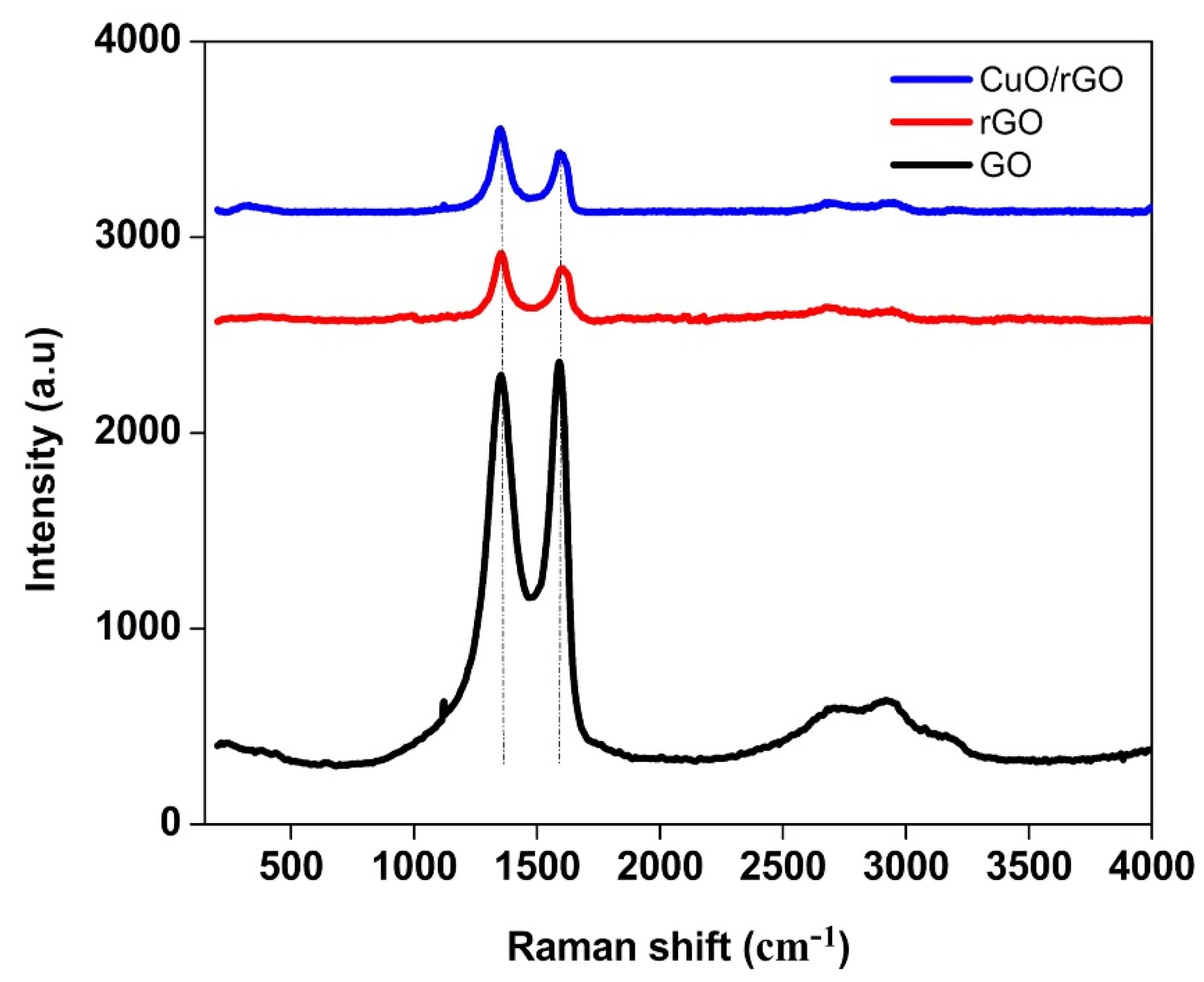
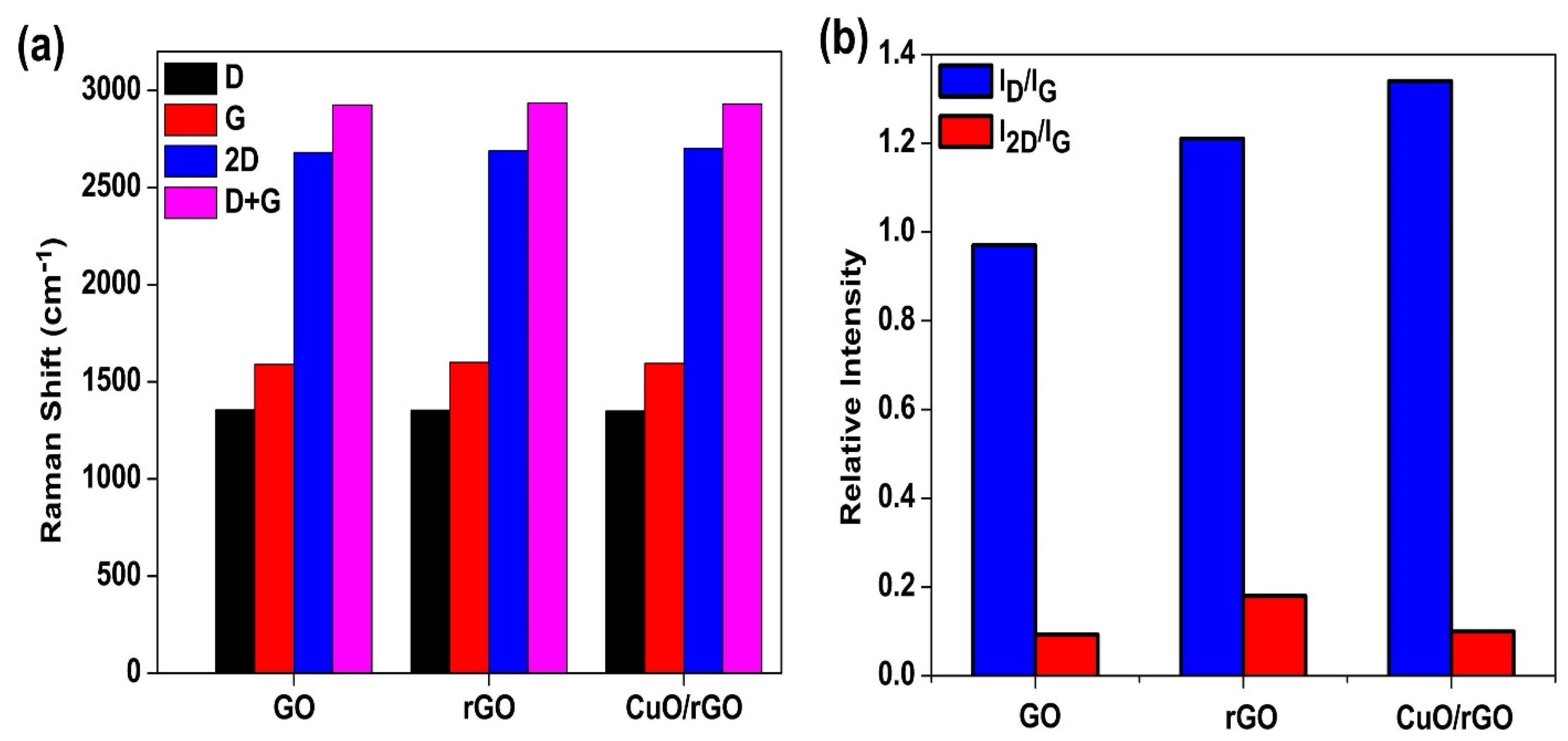
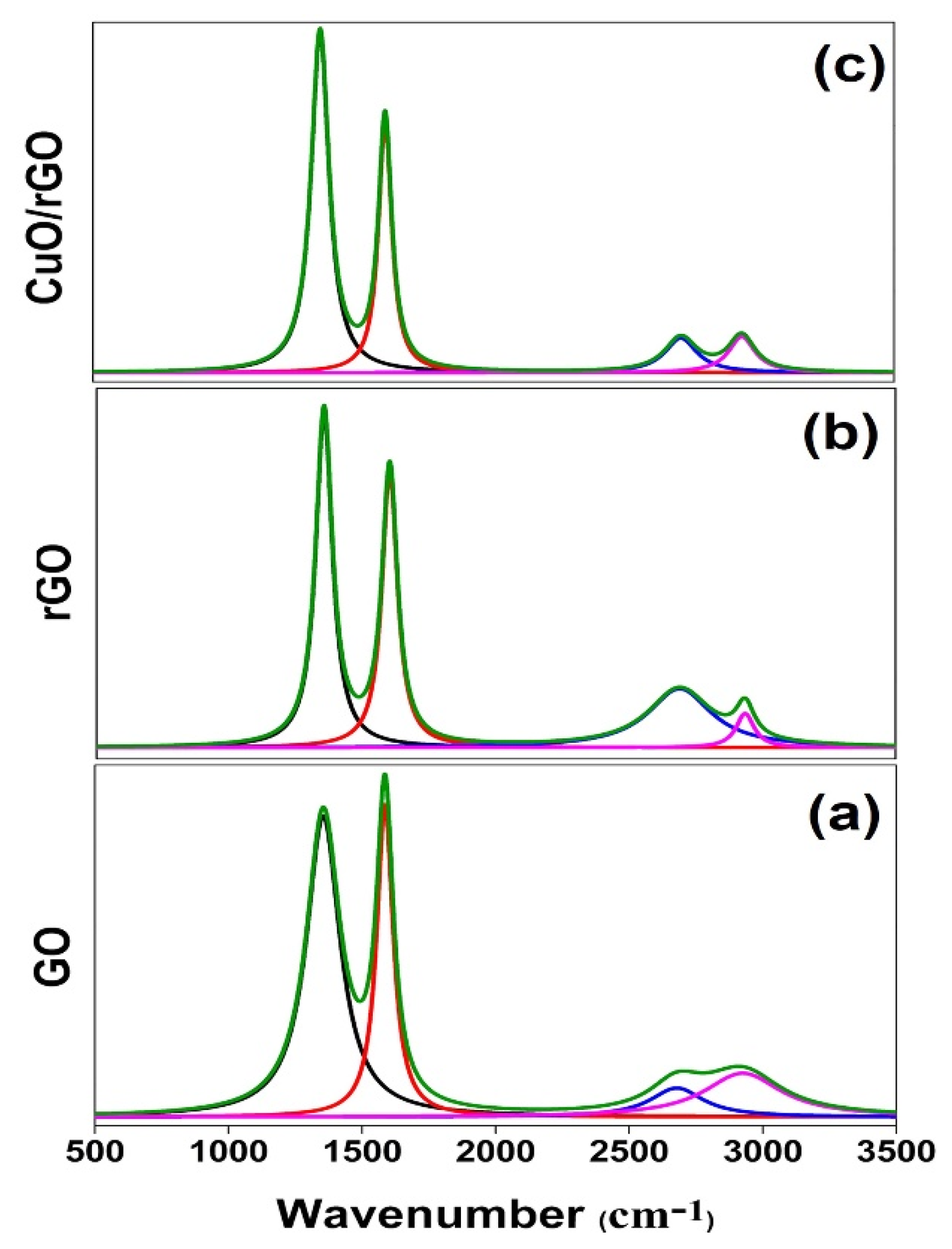

| Sample | D-Band | G-Band | 2D-Band | D + G |
|---|---|---|---|---|
| GO | 1877 | 1947 | 181 | 273 |
| rGO | 321 | 265 | 48 | 32 |
| CuO/rGO | 411 | 305 | 41 | 43 |
| Sample | D-Band | G-Band | 2D-Band | D + G |
|---|---|---|---|---|
| GO | 153 | 75 | 241 | 350 |
| rGO | 76 | 288 | 82 | 82 |
| CuO/rGO | 81 | 60 | 146 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, M.; Hawari, H.F.; Kumar, P.; Burhanudin, Z.A. Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications. Crystals 2022, 12, 264. https://doi.org/10.3390/cryst12020264
Gupta M, Hawari HF, Kumar P, Burhanudin ZA. Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications. Crystals. 2022; 12(2):264. https://doi.org/10.3390/cryst12020264
Chicago/Turabian StyleGupta, Monika, Huzein Fahmi Hawari, Pradeep Kumar, and Zainal Arif Burhanudin. 2022. "Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications" Crystals 12, no. 2: 264. https://doi.org/10.3390/cryst12020264
APA StyleGupta, M., Hawari, H. F., Kumar, P., & Burhanudin, Z. A. (2022). Copper Oxide/Functionalized Graphene Hybrid Nanostructures for Room Temperature Gas Sensing Applications. Crystals, 12(2), 264. https://doi.org/10.3390/cryst12020264








