Effect of Fineness and Heat Treatment on the Pozzolanic Activity of Natural Volcanic Ash for Its Utilization as Supplementary Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
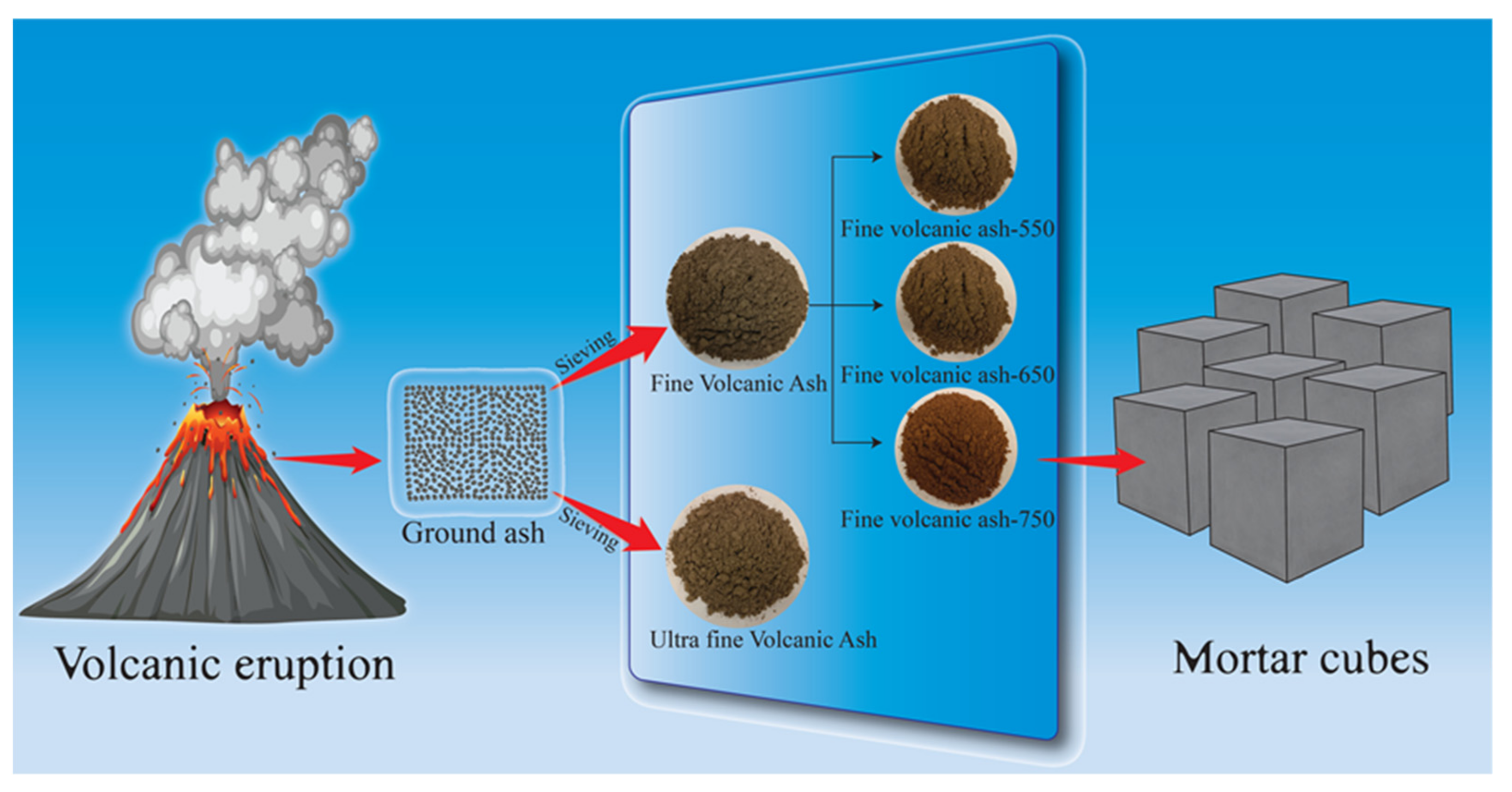
2.1. Materials
2.2. Mix Proportions and Test Methods
2.2.1. Mix Proportions
2.2.2. Mixing and Testing for Compressive Strength of Mortar Cubes
2.3. Modified Chappelle Test to Evalute the Reactivity of Pozzolanic Materials
2.4. Microstructure Analysis of Pastes Containing Pozzolanic Materials
2.4.1. X-ray Diffraction (XRD) Analysis
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3. Results and Discussions
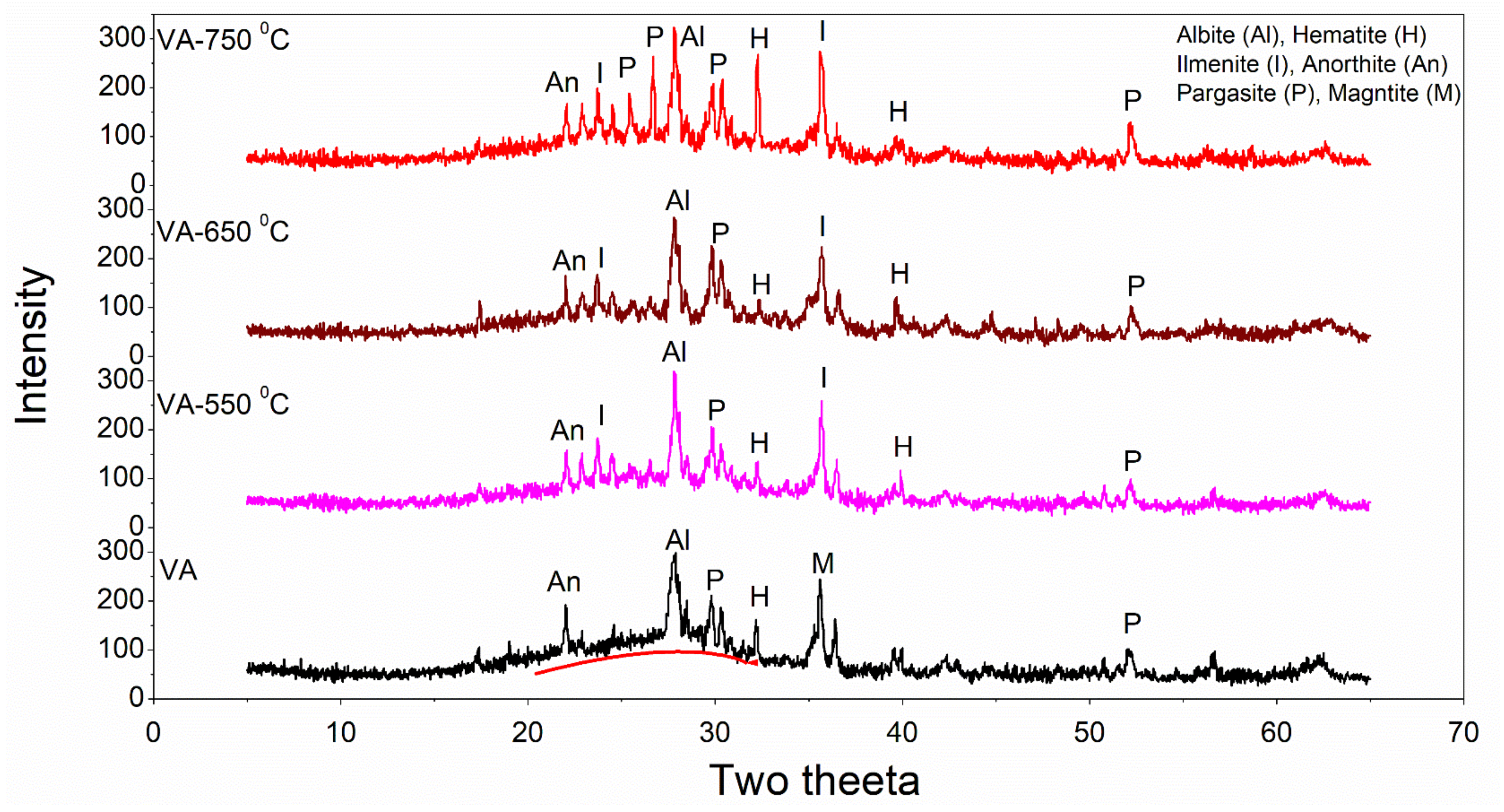
3.1. Material Characterisations by XRF and XRD Analysis
3.2. Chappelle Reactivity Test
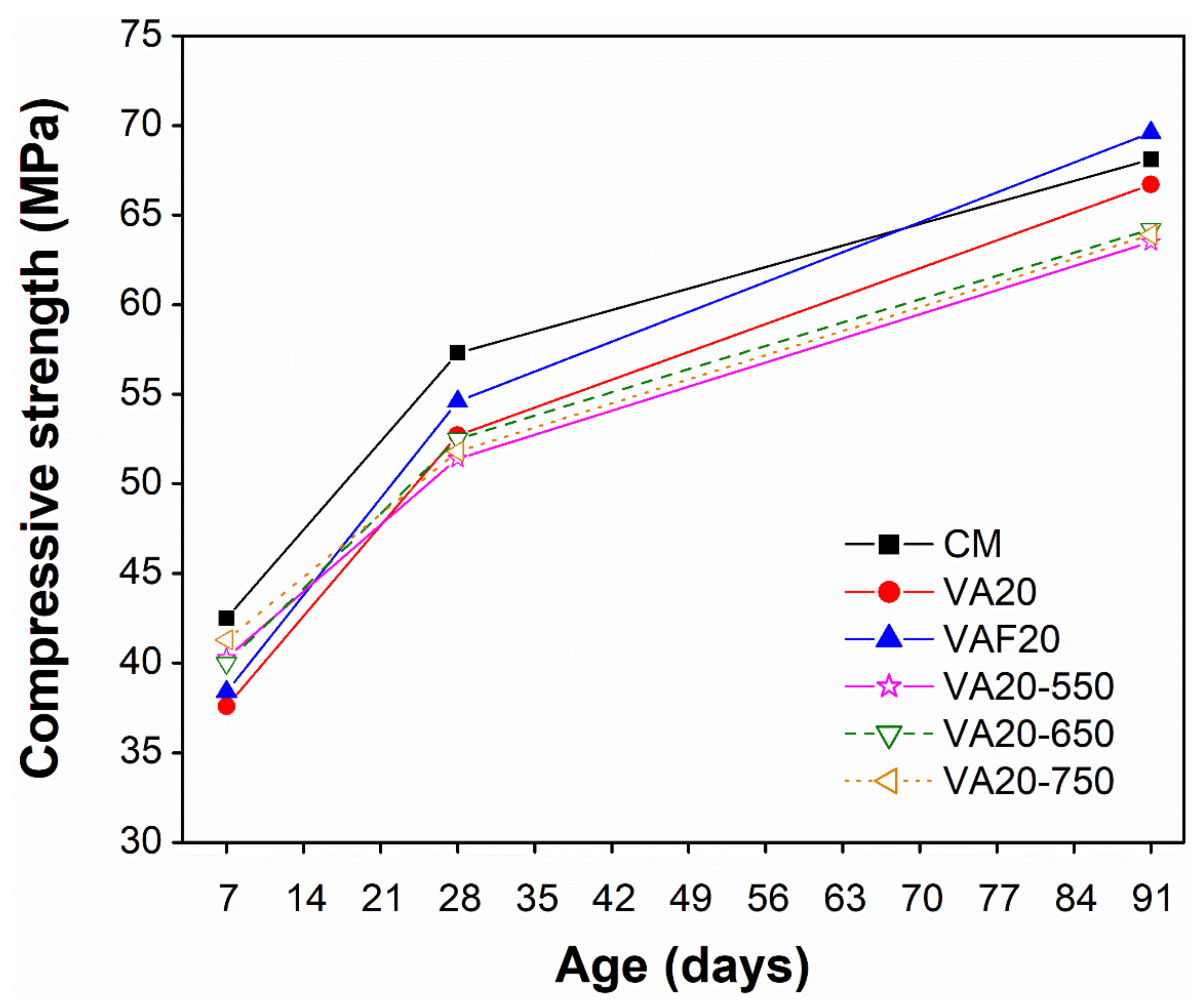
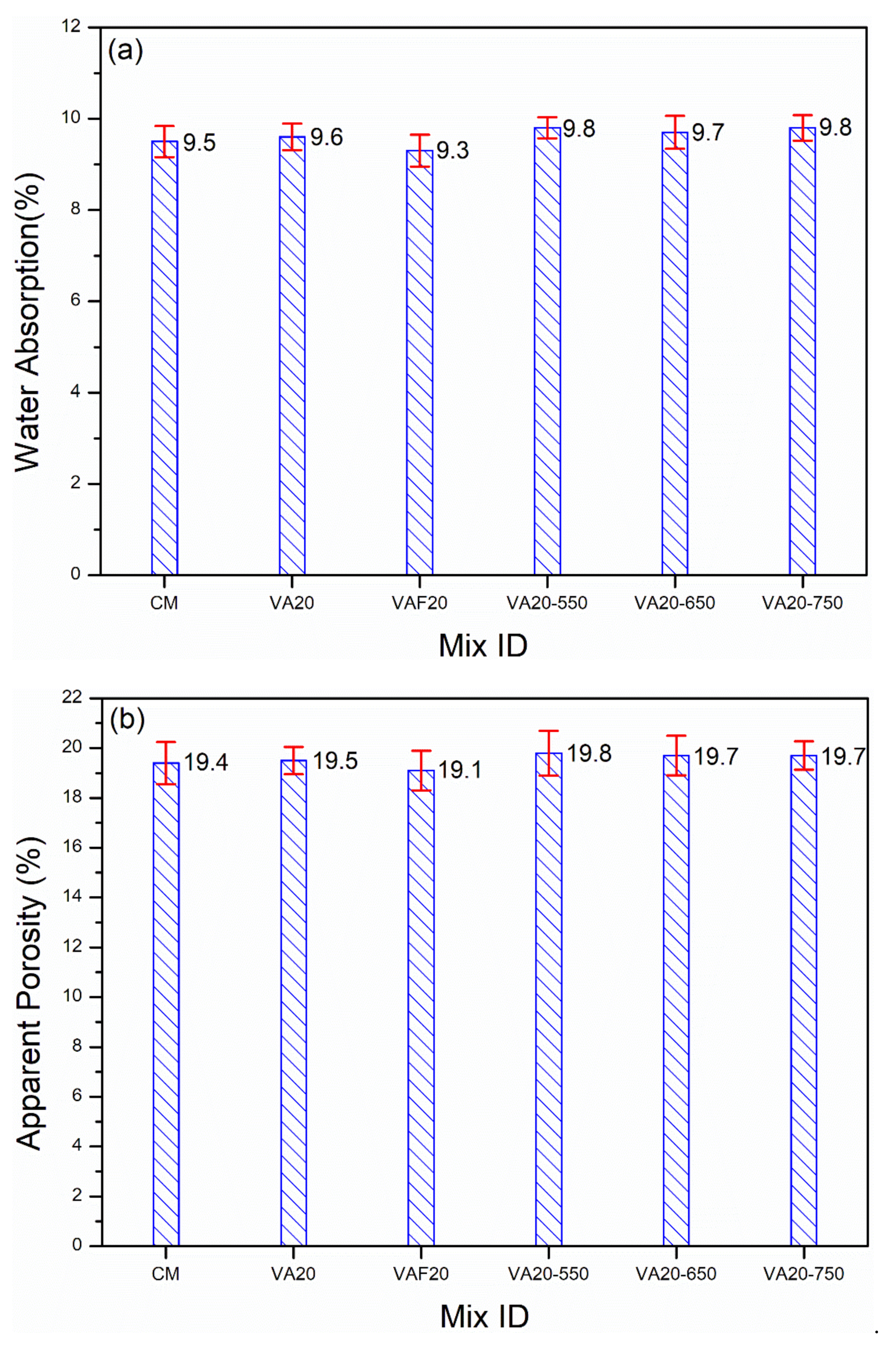
3.3. Influence of Fineness and Heat-Treatment on Strength, Porosity, and Water Absorption
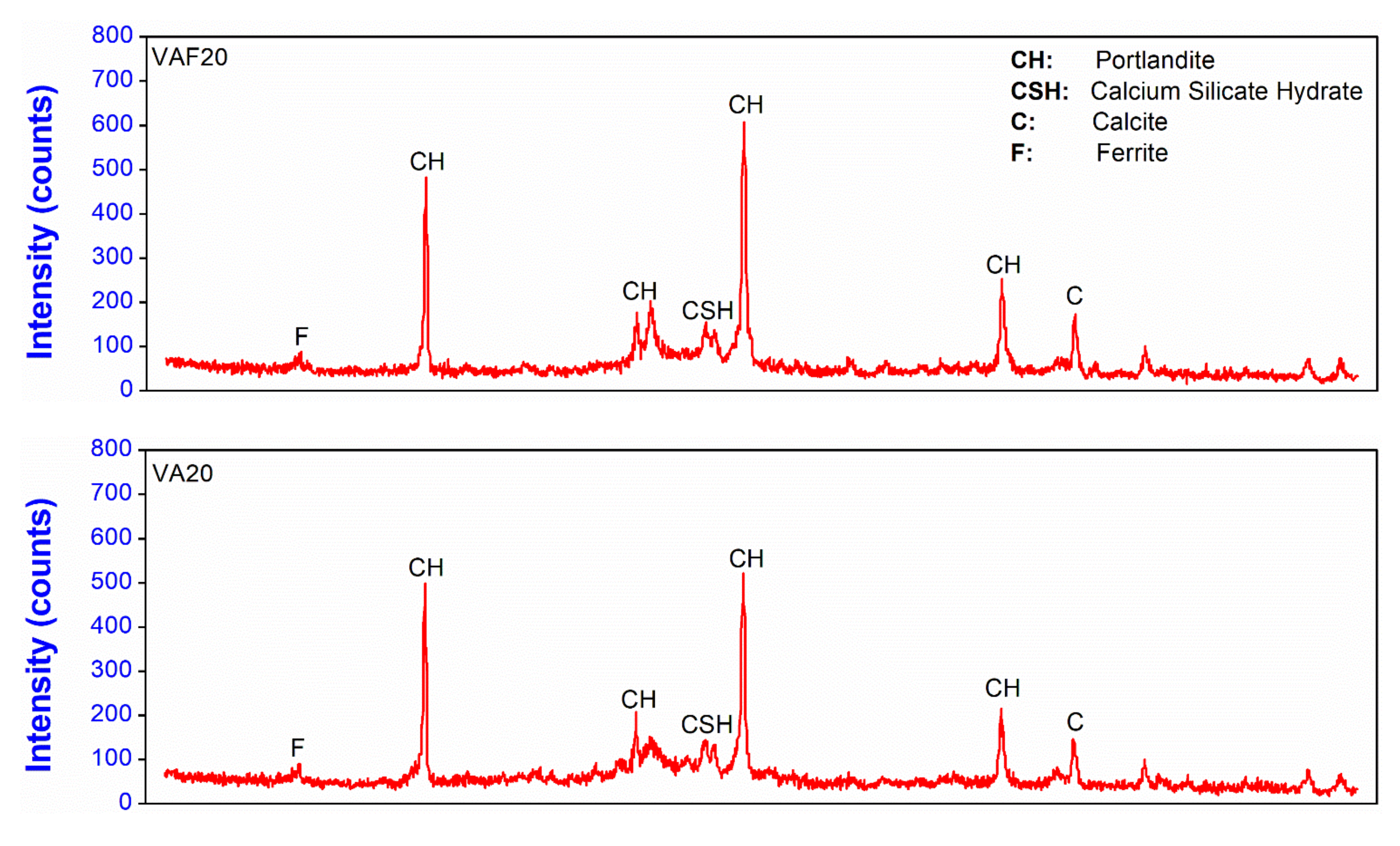
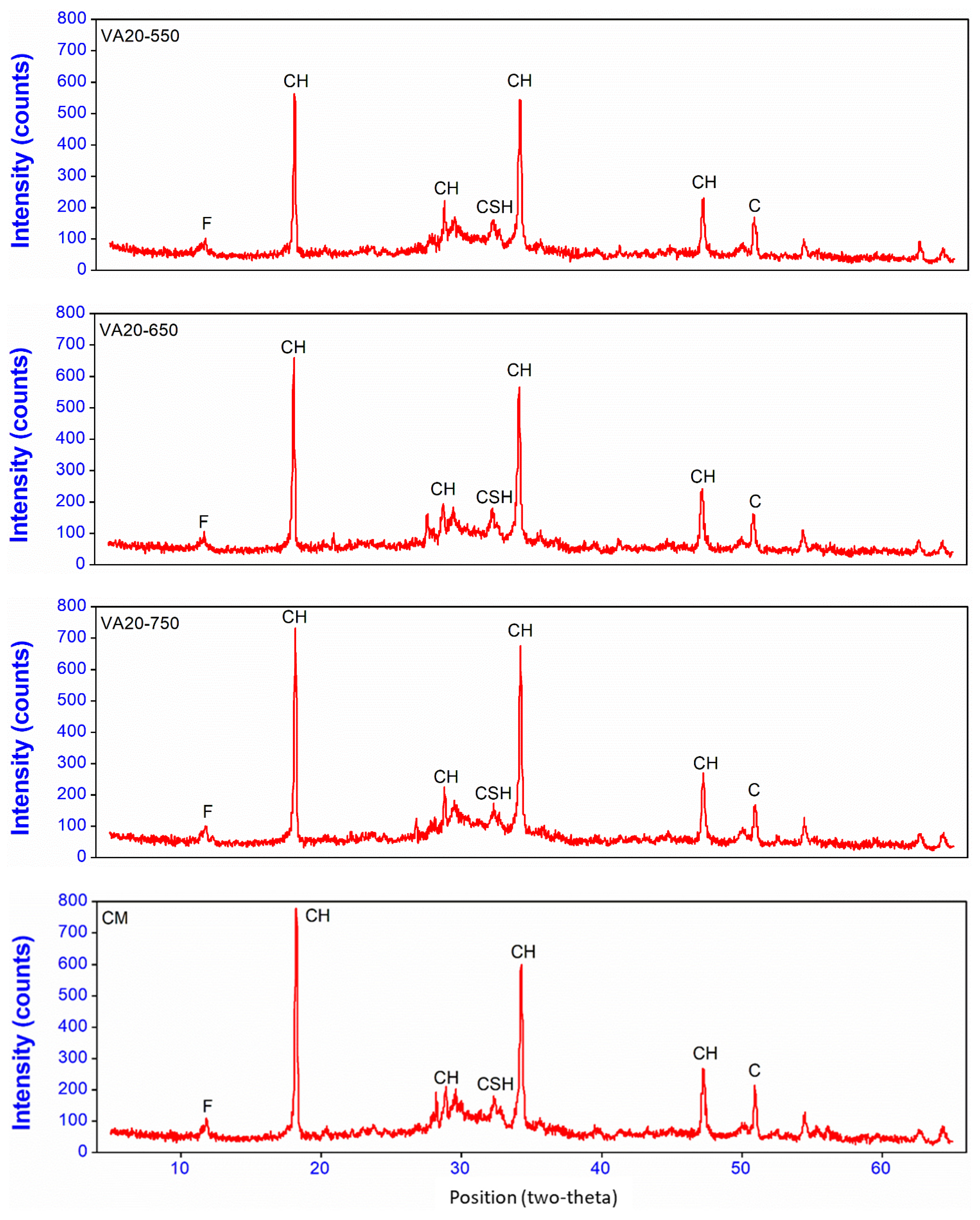
3.4. X-ray Diffraction of Cement Pastes Containing Pozzolanic Materials
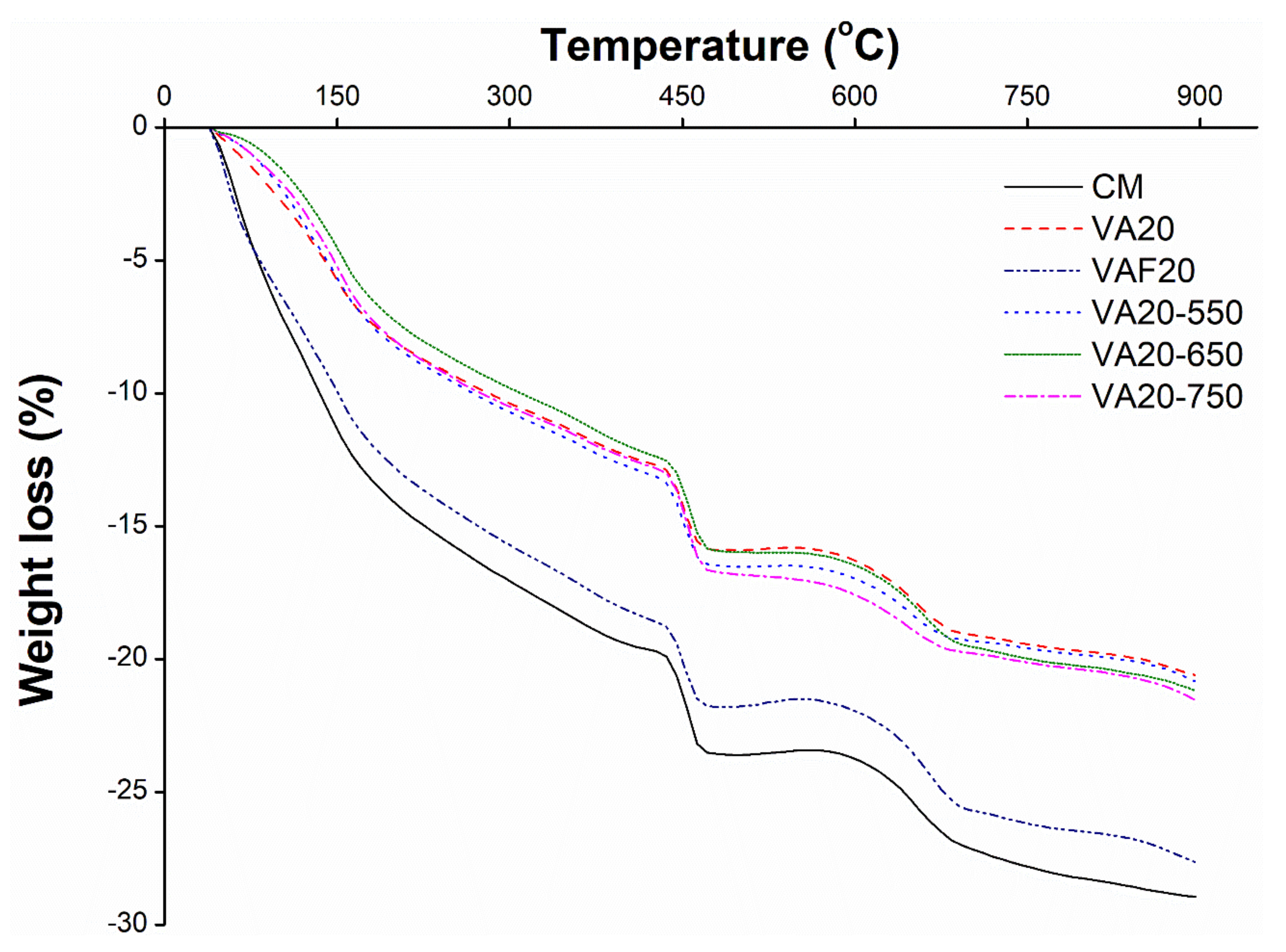
3.5. Thermo Gravimetric Analysis (TGA) of Cement Pastes Containing Pozzolanic Materials
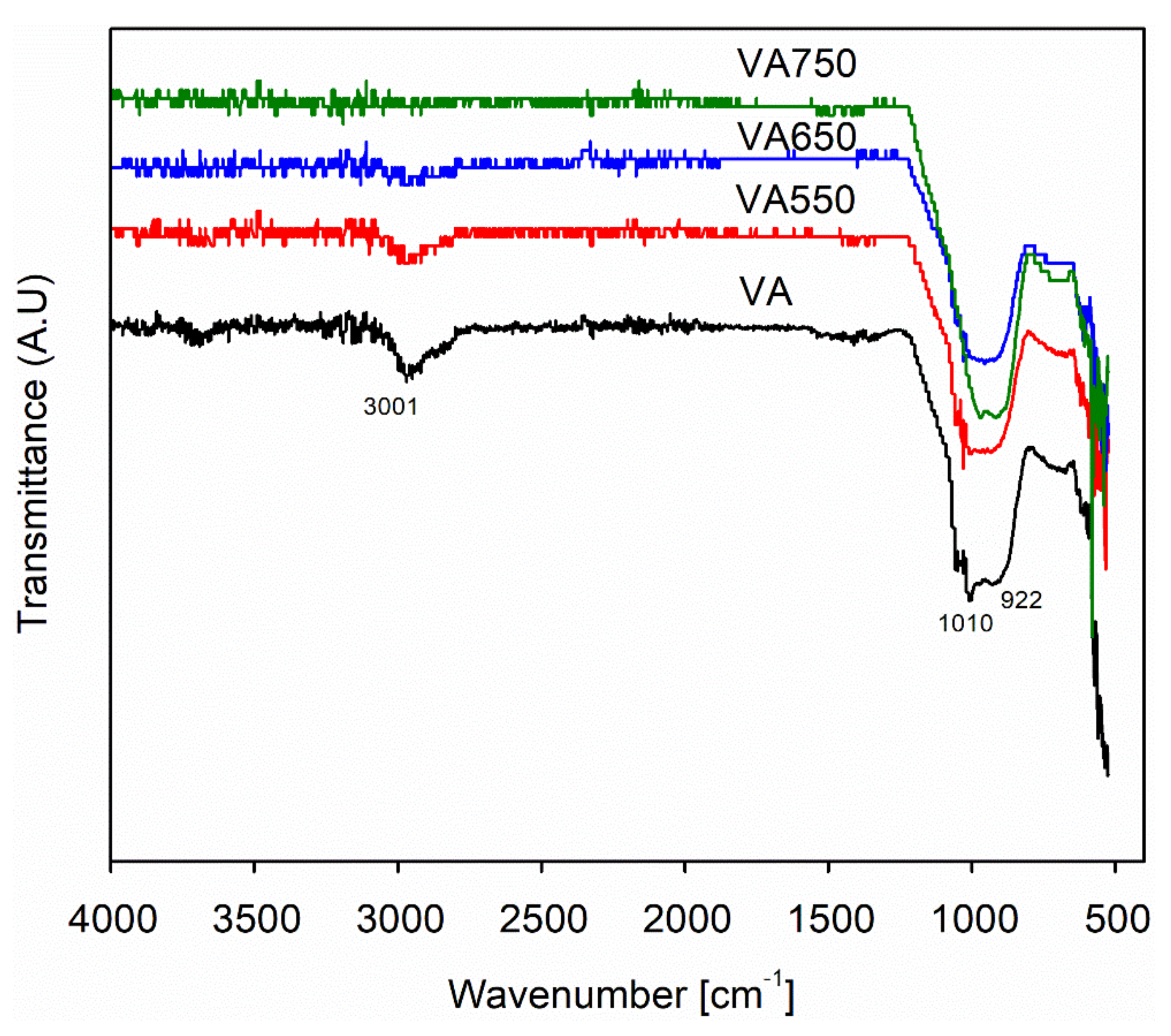
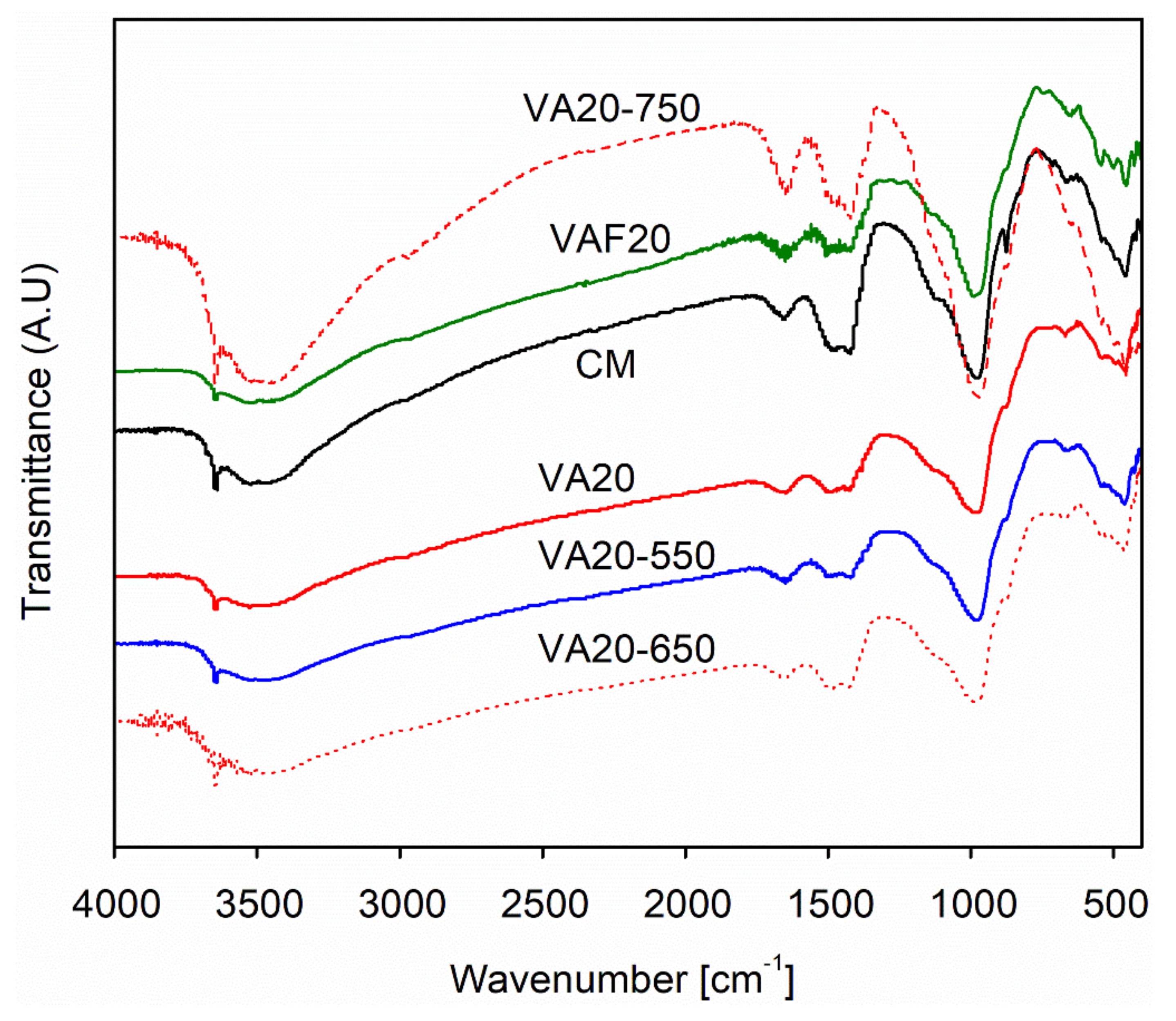
3.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Cement Pastes Containing Pozzolanic Materials
4. Conclusions
- The X-ray diffraction and Chapelle reactivity results indicate that pozzolanic reactivity increases with fineness. Therefore, ultra-fine volcanic ash (VAF) showed highest value of Chapelle reactivity due to the availability of very fine amorphous silica. Heat-treated volcanic ashes (VA-550, VA-650 and VA-750), on the other hand, had comparatively reduced pozzolanic reactivity due to the crystallization of the existing phases and the formation of new crystalline phases, when exposed to high temperatures.
- An improvement in compressive strength was observed in a mortar containing 20% VAF (69.6 MPa) due to increasing the fineness of VA, as compared to CM (68.1 MPa), especially at 91 days. The above results were validated by VAF20 being shown to have the lowest WA (9.3%) and AP (19.1%) capacity at 91 days among all mixes. Moreover, the negative impact of heat treatment on VA (at 550, 650, and 750 °C) was observed in terms of strength. The strength of the mortar containing heat-treated VA was slightly reduced (63.5, 64.2 and 63.9 MPa for VA20-550, VA20-650, and VA20-750, respectively) as compared to the control (68.1 MPa) or untreated VA (66.7 MPa). This is attributed to the change of the amorphous nature of the VA into crystalline, due to the heat treatment. The current findings suggest that grinding VA to increase its fineness is the most effective and viable approach to achieving optimal engineering performance.
- X-ray diffraction analysis results on the paste samples showed that the binary mixes with 20% VAF (VAF20) significantly reduced the intensity of calcium hydroxide due to its better pozzolanic behavior as compared to the other mixes. This resulted in the better pozzolanic reactivity of VAF in the paste matrix. The mixes having heat-treated volcanic ashes (VA20-550, VA20-650, and VA20-750) showed high intensities of portlandite peaks, which indicates that these volcanic ashes have low reactivity; therefore, they do not significantly contribute to the improvement of micro and pore structure. TGA analysis also showed that the portlandite phase is significantly reduced in binary mixes containing fine and ultra-fine volcanic ashes (VA20 and VAF20), as compared to the other mixes (including the control mix). This indicates the superior pozzolanic property of the fine amorphous silica present in these samples. On the other hand, heat-treated samples (VA20-550, VA20-650, and VA20-750) showed a high amount of portlandite phase among all binary mixes, due to its low reactivity, as was also shown by the XRD results and the results of the Chappelle reactivity test.
- FTIR analysis results showed a shift in the Si–O–Si band with the addition of fine and heat-treated volcanic ashes in the binary mixes. This shift (980 to 992 cm−1) was more pronounced in the binary mix containing VAF, which indicates the presence of a large number of high-density C–S–H phases, causing densification of the micro and pore structure of the paste mix. In addition, the portlandite peaks (3640 cm−1) are significantly reduced in VA20 and VAF20 mixes, as compared to other binary mixes, which shows the superior reactivity of fine VA, that ultimately results in the formation of more C–S–H phases, causing densification of paste matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, K.H.; Jung, Y.B.; Cho, M.S.; Tae, S.H. Effect of supplementary cementitious materials on reduction of CO2 emissions from Concrete. In Handbook of Low Carbon Concrete, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 89–110. [Google Scholar]
- Klee, H. The Cement Sustainability Initiative: Recycling Concrete-Summary; World Business Council for Sustainable Development (WBCSD): Geneva, Switzerland, 2009. [Google Scholar]
- Abergel, T.; Dean, B.; Dulac, J. Global status report 2017: Towards a zero-emission, efficient, and resilient buildings and construction sector. U. N. Environ. Program. 2017, 48, 1–48. [Google Scholar]
- IEA-WBCSD, Cement Technology Roadmap 2009—Carbon Emissions Reductions up to 2050. Available online: https://www.iea.org/publications/freepublications/publication/Cement.pdf (accessed on 25 January 2022).
- Coffetti, D.; Crotti, E.; Gazzaniga, G.; Carrara, M.; Pastore, T.; Coppola, L. Pathways towards sustainable concrete. Cem. Concr. Res. 2022, 154, 106718. [Google Scholar] [CrossRef]
- Emmanuel, H.; Marine, S.; Gondia, S.S.; Clément, B.; Aymen, J.; Samuel, C. The impact of future power generation on cement demand: An international and regional assessment based on climate scenarios. Int. Econ. 2020, 163, 114–133. [Google Scholar]
- Ozbay, E.; Erdemir, M.; Durmuş, H.I. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Abolhasani, A.; Samali, B.; Dehestani, M.; Libre, N.A. Effect of rice husk ash on mechanical properties, fracture energy, brittleness and aging of calcium aluminate cement concrete. Structures 2021, 36, 140–152. [Google Scholar] [CrossRef]
- Qudoos, A.; Kim, H.G.; Atta-ur-Rehman; Jeon, I.K.; Ryou, J.-S. Influence of the particle size of wheat straw ash on the microstructure of the interfacial transition zone. Powder Technol. 2019, 352, 453–461. [Google Scholar] [CrossRef]
- Rahla, K.M.; Mateus, R.; Bragança, L. Comparative sustainability assessment of binary blended concretes using Supplementary Cementitious Materials (SCMs) and Ordinary Portland Cement (OPC). J. Clean. Prod. 2019, 220, 445–459. [Google Scholar] [CrossRef]
- Amin, M.N.; Khan, K.; Saleem, M.U.; Khurram, N.; Niazi, M.U.K. Aging and Curing Temperature Effects on Compressive Strength of Mortar Containing Lime Stone Quarry Dust and Industrial Granite Sludge. Materials 2017, 10, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756. [Google Scholar] [CrossRef]
- Muhammad, U.; Naseem, I.; Tayyaba, N.; Neelam, Z.; Aisha, A.; Mahmoud, M.A.; Ahmad, G.; Aasif, H. Advanced strategies in Metal-Organic Frameworks for CO2 Capture and Separation. Chem. Rec. 2021. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Mohseni, E.; Yazdi, M.A.; Sarker, P.; Ranjbar, M.M. Effect of nano-CuO and fly ash on the properties of self-compacting mortar. Constr. Build. Mater. 2015, 94, 758–766. [Google Scholar] [CrossRef]
- Mazloom, M.; Ramezanianpour, A.A.; Brooks, J.J. Effect of silica fume on mechanical properties of high-strength concrete. Cem. Concr. Compos. 2004, 26, 347–357. [Google Scholar] [CrossRef]
- Amin, M.N.; Khan, K.; Saleem, M.U.; Khurram, N.; Niazi, M.U.K. Influence of mechanically activated electric arc furnace slag on compressive strength of mortars incorporating curing moisture and temperature effects. Sustainability 2017, 9, 1178. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Ullah, M.F.; Shahzada, K.; Amin, M.N.; Bibi, T.; Wahab, N.; Aljaafari, A. Effective use of micro-silica extracted from rice husk ash for the production of high-performance and sustainable cement mortar. Constr. Build. Mater. 2020, 258, 119589. [Google Scholar] [CrossRef]
- Amin, M.N.; Murtaza, T.; Shahzada, K.; Khan, K.; Adil, M. Pozzolanic Potential and Mechanical Performance of Wheat Straw Ash Incorporated Sustainable Concrete. Sustainability 2019, 11, 519. [Google Scholar] [CrossRef] [Green Version]
- Játiva, A.; Ruales, E.; Etxeberria, M. Volcanic Ash as a Sustainable Binder Material: An Extensive Review. Materials 2021, 14, 1302. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, Z.; Wang, D.; An, M. Review on the Application of Supplementary Cementitious Materials in Self-Compacting Concrete. Crystals 2022, 12, 180. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Wang, F.; Shi, Z.; You, H.; Tian, Y.; Liu, Y.; Hu, Z.; Song, H.; Wang, D.; et al. Experimental Study on Carbonation Durability of Kaolin Strengthened with Slag Portland Cement. Materials 2022, 15, 1240. [Google Scholar] [CrossRef]
- Mehta, P.K. Sustainable cements and concrete for the climate change era—A review. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies; Università Politecnica delle Marche: Ancona, Italy, 2010. [Google Scholar]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential, economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2016, 114, 2–26. [Google Scholar] [CrossRef]
- Cembureau. Activity Report 2020 of The European Cement Association; Cembureau: Bruxelles, Belgium, 2020. [Google Scholar]
- Camp, V.E.; Roobol, M.J. The Arabian continental alkali basalt province: Part 1. Evolution of Harrat Rahat, Kingdom of Saudi Arabia. Bull. Geol. Soc. Am. 1989, 101, 71–95. [Google Scholar] [CrossRef]
- Roobol, M.J.; Pint, J.J.; Al-Shanti, M.A.; Al-Juaid, A.J.; Al-Amoudi, S.A.; Pint, S.; Al-Eisa, A.M.; Allam, F.; Al-Sulaimani, G.S.; Banakhar, A.S. Preliminary Survey for Lavatube Caves on Harrat Kishb. In Open-File Report SGS-OF-2002-3; Saudi Geological Survey: Jeddah, Saudi Arabia, 2002. [Google Scholar]
- Camp, V.E.; Roobol, M.J.; Hooper, P.R. The Arabian continental alkali basalt province: Part III, Evolution of Harrat Kishb, Kingdom of Saudi Arabia. Bull. Geol. Soc. Am. 1992, 104, 379–396. [Google Scholar] [CrossRef]
- Camp, V.E.; Roobol, M.J.; Hooper, P.R. The Arabian continental alkali basalt province: Part II. Evolution of Harrats Khaybar, Ithnayn and Kura, Kingdom of Saudi Arabia. Bull. Geol. Soc. Am. 1991, 103, 363–391. [Google Scholar] [CrossRef]
- Al-Nakhebi, Z.; Laurent, D. Prospecting for Pozzolan in Midwest Harrat Rahat. In Open-File Report BRGM-OF-05-15; Saudi Arabian Deputy Ministry for Mineral Resources: Jeddah, Saudi Arabia, 1985. [Google Scholar]
- Sabbahi, A.A. Geology and Resources of Basalt Quarry Site Kishb-12, Southern Harrat Kishb. In Open File Report SGS-OF-2002-1; Saudi Geological Survey: Jeddah, Saudi Arabia, 2002. [Google Scholar]
- Kurdi, H.H. Geology and Resources of Basalt Quarry site Kishb-13, Southern Harrat Kishb. In Open File Report SGSOF-2002-4; Saudi Geological Survey: Jeddah, Saudi Arabia, 2002. [Google Scholar]
- Khan, K.; Amin, M.N.; Saleem, M.U.; Qureshi, H.J.; Al-Faiad, M.A.; Qadir, M.G. Effect of Fineness of Basaltic Volcanic Ash on Pozzolanic Reactivity, ASR Expansion and Drying Shrinkage of Blended Cement Mortars. Materials 2019, 12, 2603. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Amin, M.N. Influence of fineness of volcanic ash and its blends with quarry dust and slag on compressive strength of mortar under different curing temperatures. Constr. Build. Mater. 2017, 154, 514–528. [Google Scholar] [CrossRef]
- Moufti, M.R.; Sabtan, A.A.; El-Mahdy, O.R.; Shehata, W.M. Assessment of the industrial utilization of scoria materials in central Harrat Rahat, Kingdom of Saudi Arabia. Eng. Geol. 2000, 57, 155–162. [Google Scholar] [CrossRef]
- Sabtan, A.A.; Shehata, W.M. Evaluation of engineering properties of scoria in central Harrat Rahat, Kingdom of Saudi Arabia. Bull. Eng. Geol. Env. 2000, 59, 219–225. [Google Scholar] [CrossRef]
- Khan, M.I.; Alhozaimy, A.M. Properties of natural pozzolan and its potential utilization in environmental friendly concrete. Can. J. Civ. Eng. 2011, 38, 71–78. [Google Scholar] [CrossRef]
- Al-Chaar, G.K.; Alkadi, M.; Asteris, P.G. Natural pozzolan as a partial substitute for cement in concrete. Open Constr. Build. Technol. J. 2013, 7, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Celik, K.; Jackson, M.D.; Mancio, M.; Meral, C.; Emwas, A.-H.; Mehta, P.K.; Monteiro, P.J.M. High-volume natural volcanic pozzolan and limestone powder as partial replacements for portland cement in self-compacting and sustainable concrete. Cem. Concr. Compos. 2014, 45, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Kupwade-Patil, K.; Al-Aibani, A.F.; Abdulsalam, M.F.; Mao, C.; Bumajdad, A.; Palkovic, S.D.; Buyukozturk, O. Microstructure of cement paste with natural pozzolanic volcanic ash and Portland cement at different stages of curing. Constr. Build. Mater. 2016, 113, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Letelier, V.; Ortega, J.M.; Tremiño, R.M.; Henriquéz-Jara, B.I.; Fustos, I.; Real-Herraiz, T.; Moriconi, G.; Climent, M.Á.; Sánchez, I. The Use of Volcanic Powder as a Cement Replacement for the Development of Sustainable Mortars. Appl. Sci. 2020, 10, 1460. [Google Scholar] [CrossRef] [Green Version]
- Al-Fadala, J.S.; Chakkamalayath, S.; Al-Bahar, A.; Al-Aibani, S. Ahmed. Significance of performance based specifications in the qualification and characterization of blended cement using volcanic ash. Constr. Build. Mater. 2017, 144, 532–540. [Google Scholar] [CrossRef]
- Charkhi, A.; Kazemian, H.; Kazemeini, M. Optimized experimental design for natural clinoptilolite zeolite ball milling to produce nano powders. Powder Technol. 2010, 203, 389–396. [Google Scholar] [CrossRef]
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O. Propene Adsorption-Chemisorption Behaviors on H-SAPO-34 Zeolite Catalysts at Different Temperatures. Catalysts 2019, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Saamiya, S.; Raissa, D.F.; Maria, C.G.J. Calcining natural zeolites to improve their effect on cementitious mixture workability. Cem. Concr. Res. 2016, 85, 102–110. [Google Scholar]
- Moussa, H.; Larbi, K.; Martin, C.; Pierre, C. Evaluation and improvement of pozzolanic activity of andesite for its use in eco-efficient cement. Constr. Build. Mater. 2013, 47, 1268–1277. [Google Scholar]
- Kucukyıldırım, E.; Uzal, B. Characteristics of calcined natural zeolites for use in high-performance pozzolan blended cements. Constr. Build. Mater. 2014, 73, 229–234. [Google Scholar] [CrossRef]
- Alaettin, K.; Zeynep, S. Effect of heat treatment on pozzolanic activity of volcanic pumice used as cementitious material. Cem. Concr. Compos. 2015, 57, 128–132. [Google Scholar]
- Burris, L.E.; Juenger, M.C.G. Milling as a pretreatment method for increasing the reactivity of natural zeolites for use as supplementary cementitious materials. Cem. Concr. Compos. 2016, 65, 163–170. [Google Scholar] [CrossRef] [Green Version]
- American Society for Testing and Materials. Standard Specification for Portland Cement. Available online: www.astm.org (accessed on 10 January 2022).
- Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization. Available online: https://standards.cen.eu. (accessed on 10 January 2022).
- Cement—Test Methods—Determination of Strength. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/45568.html. (accessed on 10 January 2022).
- American Society for Testing and Materials. Standard Terminology Relating to Concrete and Concrete Aggregates. Available online: www.astm.org (accessed on 10 January 2022).
- American Society for Testing and Materials. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in or 50-mm. Cube Specimens). Available online: www.astm.org (accessed on 10 January 2022).
- Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2014. Available online: www.astm.org (accessed on 10 January 2022).
- Standard Test Method for Dry and Wet Bulk Density, Water Absorption, and Apparent Porosity of Thin Sections of Glass-Fiber Reinforced Concrete. Available online: www.astm.org (accessed on 10 January 2022). [CrossRef]
- Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. Available online: http://www.astm.org (accessed on 10 January 2022).
- Beleuk à Moungam, L.M.; Lemougna, P.N.; Kaze, R.C.; Mohamed, H.; Deutou Nemaleu, J.G.; Billong, N.; Kamseu, E.; Mvondo-Ze, A.D.; Kenfack, I.T. Synthesis of Volcanic Ash-based Porous Inorganic Polymers Using Biomass as Pore Inducing Agent: Phase Evolution and Descriptive Microstructure. Silicon 2021, 1–14. [Google Scholar] [CrossRef]
- Metekong, J.V.S.; Kaze, C.R.; Deutou, J.G.; Venyite, P.; Nana, A.; Kamseu, E.; Melo, U.C.; Tatietse, T.T. Evaluation of performances of volcanic-ash-laterite based blended geopolymer concretes: Mechanical properties and durability. J. Build. Eng. 2020, 34, 101935. [Google Scholar] [CrossRef]
- Kim, J.; Yi, C.; Zi, G. Waste glass sludge as a partial cement replacement in mortar. Constr. Build. Mater. 2015, 75, 242–246. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Sinsiri, T. Effect of fly ash fineness on microstructure of blended cement paste. Constr. Build. Mater. 2007, 21, 1534–1541. [Google Scholar] [CrossRef]
- Altwair, N.M.; Azmi, M.; Johari, M.; Fuad, S.; Hashim, S. Strength activity index and microstructural characteristics of treated palm oil fuel ash. Int. J. Civil Environ. Eng. 2011, 11, 100–107. [Google Scholar]
- Helmi, M.; Hall, M.R.; Stevens, L.A.; Rigby, S.P. Effects of high-pressure/temperature curing on reactive powder concrete microstructure formation. Constr. Build. Mater. 2016, 105, 554–562. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C–S–H): Near-, Mid-, and Far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Hughes, T.L.; Methven, C.M.; Jones, T.G.; Pelham, S.E.; Fletcher, P.; Hall, C. Determining cement composition by Fourier transform infrared spectroscopy. Adv. Cem. Based Mater. 1995, 2, 91–104. [Google Scholar] [CrossRef]


| C | VA | VAF | |
|---|---|---|---|
| Physical Properties | |||
| Specific gravity (g/cm3) | 3.15 | 2.64 | |
| Fineness (m2/kg) (Blain) | 344 | - | - |
| Fineness (m2/cc) (Microtrac S3500) | 0.5670 | 0.816 (<38 µ) | 1.194 (<20 µ) |
| Chemical properties (oxides, % by weight) | |||
| SiO2 | 20.9 | 46.4 | |
| Al2O3 | 5.18 | 14.4 | |
| Fe2O3 | 3.04 | 12.8 | |
| (SiO2 + Al2O3 + Fe2O3) * | - | 73.6 | |
| CaO | 63.9 | 8.80 | |
| MgO | 1.65 | 8.30 | |
| Na2O | 0.10 | 3.80 | |
| K2O | 0.52 | 1.90 | |
| SO3 | 2.61 | 0.80 | |
| LOI ** | 2.51 | 2.80 | |
| Compounds (%) | |||
| C2S | 52.1 | - | |
| C3S | 19.6 | - | |
| C3A | 8.17 | - | |
| C4AF | 8.81 | - | |
| Batch Quantities (g) for Nine 50-mm3 Mortar Specimens | |||||
|---|---|---|---|---|---|
| Mix ID | Water (w) | Cement (c) | VA | VAF | Sand (s) |
| Control Mortar (CM) | 440 | 1100 | 0 | 0 | 1500 |
| 20% VA (VA20) | 440 | 880 | 220 | 0 | 1500 |
| 20% VAF (VAF20) | 440 | 880 | 0 | 220 | 1500 |
| 20% VA550 (VA20-550) | 440 | 880 | 220 | 0 | 1500 |
| 20% VA650 (VA20-650) | 440 | 880 | 220 | 0 | 1500 |
| 20% VA750 (VA20-750) | 440 | 880 | 220 | 0 | 1500 |
| Materials | Chappelle Activity (mg CaO/g Sample) |
|---|---|
| VA | 821.51 |
| VAF | 844.32 |
| VA550 | 808.75 |
| VA650 | 792.80 |
| VA750 | 800.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, K.; Amin, M.N.; Usman, M.; Imran, M.; Al-Faiad, M.A.; Shalabi, F.I. Effect of Fineness and Heat Treatment on the Pozzolanic Activity of Natural Volcanic Ash for Its Utilization as Supplementary Cementitious Materials. Crystals 2022, 12, 302. https://doi.org/10.3390/cryst12020302
Khan K, Amin MN, Usman M, Imran M, Al-Faiad MA, Shalabi FI. Effect of Fineness and Heat Treatment on the Pozzolanic Activity of Natural Volcanic Ash for Its Utilization as Supplementary Cementitious Materials. Crystals. 2022; 12(2):302. https://doi.org/10.3390/cryst12020302
Chicago/Turabian StyleKhan, Kaffayatullah, Muhammad Nasir Amin, Muhammad Usman, Muhammad Imran, Majdi Adel Al-Faiad, and Faisal I. Shalabi. 2022. "Effect of Fineness and Heat Treatment on the Pozzolanic Activity of Natural Volcanic Ash for Its Utilization as Supplementary Cementitious Materials" Crystals 12, no. 2: 302. https://doi.org/10.3390/cryst12020302
APA StyleKhan, K., Amin, M. N., Usman, M., Imran, M., Al-Faiad, M. A., & Shalabi, F. I. (2022). Effect of Fineness and Heat Treatment on the Pozzolanic Activity of Natural Volcanic Ash for Its Utilization as Supplementary Cementitious Materials. Crystals, 12(2), 302. https://doi.org/10.3390/cryst12020302







