Abstract
Bioinformatics as a newly emerging discipline is considered nowadays a reference to characterize the physicochemical and pharmacological properties of the actual biocompounds contained in plants, which has helped the pharmaceutical industry a lot in the drug development process. In this study, a bioinformatics approach known as in silico was performed to predict, for the first time, the physicochemical properties, ADMET profile, pharmacological capacities, cytotoxicity, and nervous system macromolecular targets, as well as the gene expression profiles, of four compounds recently identified from Centaurea tougourensis via the gas chromatography–mass spectrometry (GC–MS) approach. Thus, four compounds were tested from the n-butanol (n-BuOH) extract of this plant, named, respectively, Acridin-9-amine, 1,2,3,4-tetrahydro-5,7-dimethyl- (compound 1), 3-[2,3-Dihydro-2,2-dimethylbenzofuran-7-yl]-5-methoxy-1,3,4-oxadiazol-2(3H)-one (compound 2), 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione (compound 3), and 3-[3-Bromophenyl]-7-chloro-3,4-dihydro-10-hydroxy-1,9(2H,10H)-acridinedione (compound 4). The insilico investigation revealed that the four tested compounds could be a good candidate to regulate the expression of key genes and may also exert significant cytotoxic effects against several tumor celllines. In addition, these compounds could also be effective in the treatment of some diseases related to diabetes, skin pathologies, cardiovascular, and central nervous system disorders. The bioactive compounds of plant remain the best alternative in the context of the drug discovery and development process.
1. Introduction
Bioinformatics, as a new emerging discipline, has evolved considerably over the past few decades and now represents an important field of study, allowing researchers to store, retrieve, organize, and analyze biological data [1]. Among these developments, a modern computational approach, named in silico, has allowed the prediction of the chemical and biological properties of thousands of natural and synthetic compounds, due to high algorithms accuracy and constantly updated databases [2]. Indeed, this approach allowed the scientific community to gain considerable time and decrease the cost of studies related to laboratory experiments on animals.
The in silico approach also allowed the scientific community to understand and overcome several ambiguities related to the cerebral and cognitive aspects [3], but also to explain some mechanisms linked to blood–brain barrier permeability and hence answer some fundamental questions related to some pathologies that affect the brain, such as epileptic encephalopathy, Alzheimer’s and Huntington’s diseases [4], which gives unprecedented credibility to this methodology, since the nervous system is well known for its complexity. This recent methodology has also provided mandatory data for the scientific community, helping them to understand more some phenomena related to oxidative stress, inflammation, and even cancer [5,6].
Phytotherapy is a newly emerging discipline, considered nowadays a reference for the elaboration process of new drugs, due in part to its accessibility, affordability, and efficacy, especially in developing countries [7]. This natural resource has a long history and large scale of applications, used in the past by many civilizations as a quick remedy to heal wounds and treat various inflammatory conditions, including fevers, arthritis [8]. However, plants are also known for their relaxation property and their ability to reduce stress and anxiety [9]. This helps to explain why it is currently being used and recommended by health care professionals.
In many countries, Centaurea species are prepared as an infusion due to their relaxing property, helping people reduce stress and anxiety [10]. These plants may also improve the digestion process and possibly support enzymatic reactions in the liver [11]. The genus Centaurea is also known for its antioxidant, immunomodulatory, anti-inflammatory, antidiabetic, and antimicrobial potential [12,13,14,15]. Recent studies [16,17] showed that Centaurea species could be able to inhibit the proliferation of several tumor celllines, which opens the door to many therapeutic avenues. It was also reported that Centaurea species could be useful as insecticides [18].
This study aims to predict, for the first time via in silico approach, the physicochemical and biological properties of four compounds recently identified in the n-BuOH extract of C. tougourensis by GC–MS analysis.
2. Materials and Methods
2.1. The Plant Material
C. tougourensis was collected at the Belezma national park in the municipality of Fesdis (Algeria) (GPS coordinates: latitude 35.621975; longitude 6.241327) and was identified by experts in the field from the Agronomic department of the Batna-1 University (Algeria). A voucher specimen under the code (CT/2019/LPTPCMB) was deposited at the Laboratory of Improvement of the Phytosanitary Protection Techniques in Mountainous Agrosystems, Agronomy Department, Institute of Veterinary and Agricultural Sciences, University of Batna-1-, Batna, Algeria.
2.2. Preparation of Plant Extract
The aerial parts of C. tougourensis were dried in a dry, ventilated place away from the sun rays and then ground to obtain a fine powder. Maceration was carried out three times with 3L EtOH–H20 (70:30) at room temperature for 3 days followed by liquid–liquid extraction using solvents of gradual polarity (hexane, ethyl acetate, and n-butanol).
2.3. In Silico Study
2.3.1. Determination of Canonical SMILES
To predict the possible biological properties of four phytocompounds recently identified in the n-BuOH extract of C. tougourensis, two bioinformatics tools were used: Open Babel (version 2.0.2) [19] and InDraw webserver (http://in.indraw.integle.com/, accessed on 8 September 2021), respectively, to generate the canonical smile of each compound by inputting their respective structures. These four compounds were, respectively, Acridin-9-amine, 1,2,3,4-tetrahydro-5,7-dimethyl- (compound 1), 3-[2,3-Dihydro-2,2-dimethylbenzofuran-7-yl]-5-methoxy-1,3,4-oxadiazol-2(3H)-one (compound 2), 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione (compound 3), and 3-[3-Bromophenyl]-7-chloro-3,4-dihydro-10-hydroxy-1,9(2H,10H)-acridinedione (compound 4).
2.3.2. Physicochemical Properties
In this test, six physicochemical parameters of the four compounds were predicted using the online platform of SwissADME (http://www.swissadme.ch/, accessed on 13 September 2021). These tested properties were lipophility, size, polarity, insolubility, unsaturation, and flexibility [20].
2.3.3. ADMET Profile
The pharmacokineticsand toxicity properties of the four compounds were estimated using the new online platform of admetSAR 2.0 (http://lmmd.ecust.edu.cn/admetsar2, accessed on 15 September 2021) [21]. The possible influences of these compounds were tested on different parameters, principally, human intestinal absorption (HIA),human oral bioavailability (HOB),blood–brain barrier (BBB) penetration, Caco-2 cells permeability, subcellular localization, interaction with P-glycoprotein and Cytochrome P450 (CYP450), mutagenicity, carcinogenicity, eye corrosion, eye irritation, hepatotoxicity, binding to receptors of estrogen, androgen, thyroid hormones, glucocorticoid, toxicity to the honey bee, fish, and aquatic crustaceans, biodegradation, water-solubility, plasma protein binding, and acute oral toxicity, as well as the median growth inhibition concentration (pIGC50) of Tetrahymena pyriformis.
2.3.4. Pharmacological Properties
The four compounds’ potential biological activities were predicted using the PASS online web server (http://way2drug.com/passonline/, accessed on 13 September 2021) [22]. This server is based on two probabilities, Pa and Pi, in which Pa represents the probability of the compound being active and Pi represents the probability of the compound being inactive, with values ranging from zero to one. The model type Pa > Pi was used by default because it is the most widely accepted model for studying activities.
2.3.5. Cytotoxicity Prediction
Based on the structural formula of each compound previously obtained [23], the possible cytotoxic effect of the four compounds on human tumor celllineswasinvestigated using CLC-Pred (Cell Line Cytotoxicity Predictor) web services (http://www.way2drug.com/cell-line/, accessed on 19 September 2021).
2.3.6. Gene Expression Profiles
The DIGEP Pred online server (http://www.way2drug.com/ge/, accessed on 5 October 2021) was used to make an insilico prediction of the four compounds’ potential impacts on gene expression, which is primarily based on upregulation and downregulation processes retrieved from the Comparative Toxicogenomics Database (CTD) [24]. An upregulation is a cell’s ability to increase the expression of one of its constituents in response to a stimulus, whereas a downregulation is the opposite [25].
2.3.7. Macromolecular Targets Prediction
SwissTargetPrediction was used to estimate the most probable macromolecular targets of a small molecule, assumed as bioactive. The prediction is founded on a combination of 2D and 3D similarity with a library of 370,000 known actives molecules on more than 3000 proteins from different species. In this study, the macromolecules of human nervous system were preferentially targeted in order to predict to neuroprotective effect of these four tested compounds [26].
3. Results and Discussion
3.1. Canonical SMILES Generation
Table 1 shows the corresponding molecular formula, structure, and canonical smile of each compound.

Table 1.
Generation of the canonical smile of the four tested compounds.
3.2. Physicochemical Properties
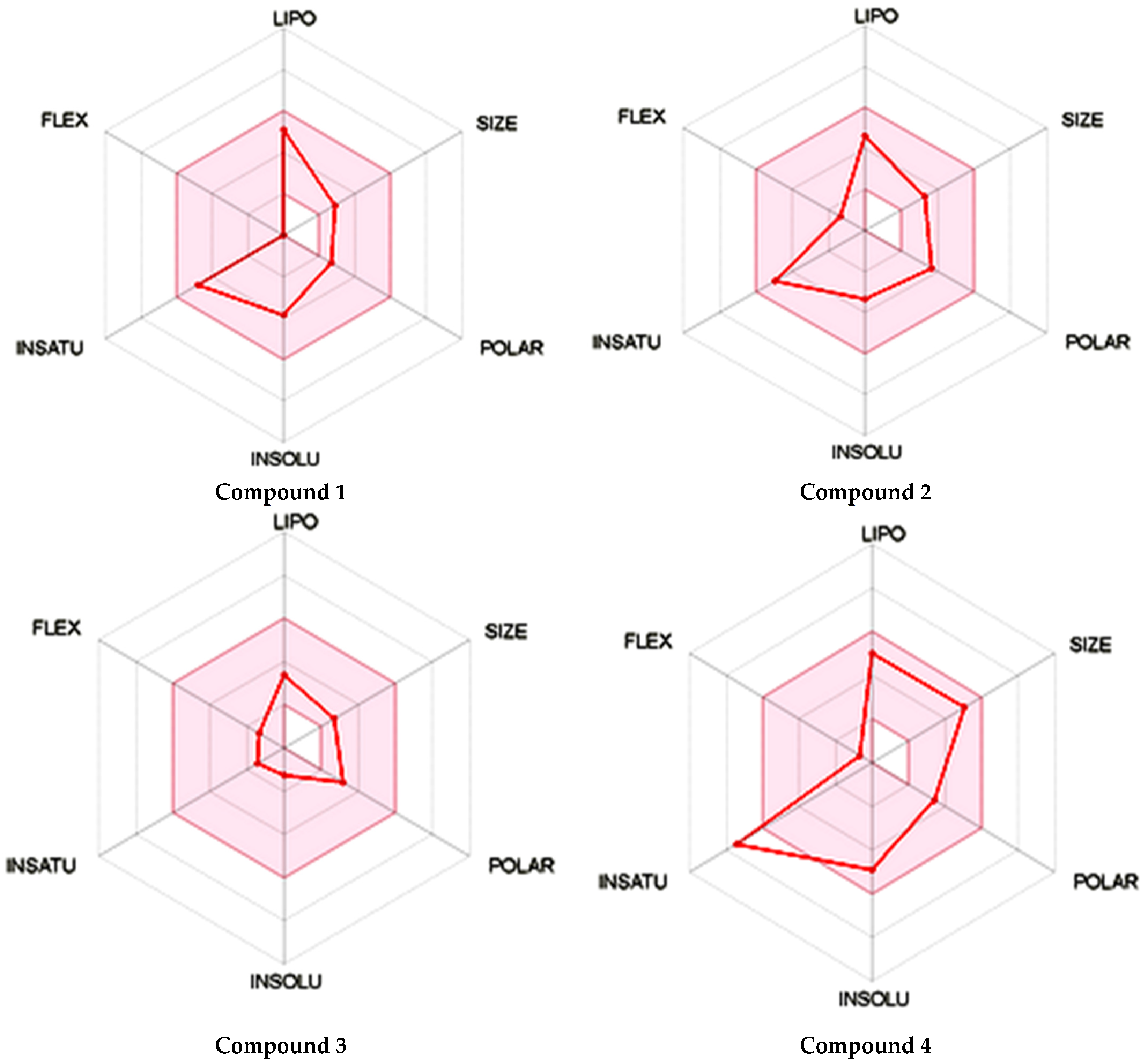
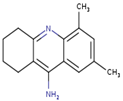
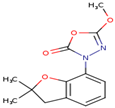
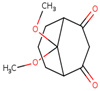
As shown in Figure 1, the unsaturation and insolubility of compound 4 were considered the best among the four tested compounds. The lipophilicity of this compound was also considerable, noting that compound 1 showed almost the same result. This information is crucial since high lipophilicity means that these compounds could easily cross biological membranes to bind with the corresponding receptor in order to generate the desired pharmacological effect [27] but will also have a positive repercussion on the pharmacokinetics and metabolism process of these compounds.
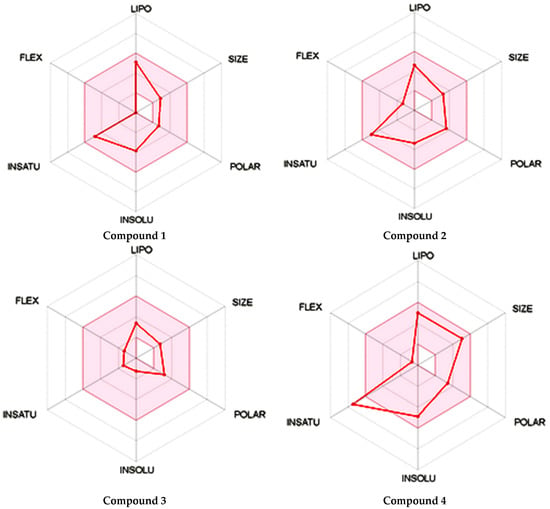
Figure 1.
Prediction of the physicochemical properties of the four tested compounds. LIPO: lipophilicity, SIZE: size, POLAR: polarity, INSOLU: insolubility, INSATU: unsaturation, FLEX: flexibility.
The polarity of compound 4 was the highest but in terms of flexibility; compound 2 exhibited the best results. However, a comparable flexibility was observed in compound 3. These pieces of information are crucial, since a high polarity and flexibility properties may increase the oral bioavailability of the compound, helping it to reach the therapeutic site of action due to a high molecular dynamics phenomenon, which also contributes to a nonnegligible increase of renal clearance process, and this was proved using rats and molecular docking approach [28].
3.3. ADMET Profile
The four compounds were evaluated for their absorption, distribution, metabolism, excretion, and toxicity (ADMET) profile using AdmetSAR server. As shown in Table 2, the results concerning the absorption process suggest that all compounds may be permeable to blood–brain barrier (BBB) and most of them to Caco-2 cell. The results indicated that these compounds could also be absorbed by the human intestine with a possible human oral bioavailability, suggesting that the four compounds may reach their site of action, which is beneficial in clinical trials, especially in the drug development process [29].The permeability of these compounds to the BBB membrane can make them good candidates for the elaboration of more effective central nervous system drugs(CNS) [30], but not good for non-CNS drugs due to their possible side effects [31]. On the other hand, the data showed that the four compounds could not be substrates for the P-glycoprotein (P-gp), also excluding a possible inhibition ofP-gp activity which could mean that the compounds will not interfere with the metabolism, half-lifetime, and clearance of drugs [32].

Table 2.
Prediction of the ADMET properties and toxicity of the four tested compounds.
In terms of distribution, the mitochondria appear to be the preferred organelles for compounds 2, 3 and 4, but not compound 1, for which distribution seems to be more oriented to lysosomes. This allows the emergence of the hypothesis that almost all compounds could be involved in the energy metabolism process of the cells [33], helping aerobic organisms to produce ATP by accelerating oxidative phosphorylation phenomenon [34].
Concerning the metabolism part, all tested compounds may be considered as non-substrate for cytochrome P450 2C9, 2D6 isoforms, while compounds 2 and 4 could be potential substrates for CYP450 3A4. It means that the influence of the compounds on the activity of CYP450 depends on the type of isoforms tested. It was also observed that almost all compounds cannot inhibit CYP450 3A4, 2C9, 2C19, and 2D6 isoforms, which suggest that our compounds may not interfere with the drug biotransformation made by CYP450 isoforms [35].
The results concerning the Ames mutagenicity suggest that compound 1 may be the only mutagenic compound, but none of the four compounds were considered carcinogenic. The lowest rate of acute oral toxicity was observed by compounds 1 and 2, while this parameter was very high for compounds 3 and 4. Almost all tested compounds seem to be hepatotoxic except compound 3. The four compounds appear to be non-corrosive to eyes, but concerning eye irritation, only compound 1 could be considered as an irritant agent, which suggests that these compounds could be good candidates for the elaboration of artificial tears for dry eyes by limiting the possible corrosive and irritant effects of other constituents of eye drops [36]. The toxicity prediction revealed that all compounds expressed high toxicity on fish and Crustacea, while compounds 1 and 4 were considered nontoxic to honey bees. This could mean that these compounds may be helpful for the development process of new drugs that may effectively limit the reproduction procedure of some harmful variety of fish such as pufferfish, considered deadly for humans due to their potent venomous effect generated by tetrodotoxin (TTX) [37] neurotoxin. These four tested compounds could be also effective against Xanthidaes, which is a family of Crustacea well known for their venomous effect, generally fatal for humans due to the presence of two neurotoxins, namely, saxitoxin and tetrodotoxin [38].
The data also revealed that compounds 1, 2, and 4 may have a high interaction with receptors of estrogen, androgen, thyroid hormones, and glucocorticoid, which suggests that these compounds may mimic the activity of the original hormones of these receptors [39]: this may be a key for the elaboration of new drugs with a great affinity for receptors already mentioned [40], or even to facilitate the interaction of drugs with these receptors via a synergistic effect [41].
The water solubility of compound 4 was considered the lowest (−3.987) among the tested compounds, while compound 1 exhibited the best plasma-protein-binding percentage (1.074%). This information isvery important since water solubility is considered as a key parameter to achieve desired concentration of drug in systemic circulation in order to generate the desired pharmacological response [42]. In this case, the low aqueous solubility of compound 4 could be a major obstacle in the development of highly potent pharmaceutics, since poorly water-soluble drugs often require high doses in order to reach therapeutic plasma concentrations after oral administration [43]. On the other hand, compound 1 may positively influence the pharmacokinetic properties and pharmacodynamics of drugs since the diffusion of drugs in tissues depends on the binding to plasma proteins [44]. The acute oral toxicity of compound 2 was considered the highest (3.176 kg/mol), while the median population growth inhibition of Tetrahymena pyriformis was expressed by compound 4 (1.172 μg/L) since the toxicity of this unicellular protozoa is well known and mostly found in polluted water, which partially explains why scientists use it as a toxic endpoint during experiments [45]. Noting that, acute oral toxicity is considered as a crucial parameter to determine the short-term adverse effects of a drug when administered in a single dose [46].
3.4. Pharmacological Properties
The possible biological score of each compound was investigated using the PASS online web server. A study conducted by Druzhilovskiy et al. [47] demonstrated the predictive effectiveness of PASS online of 32 from 33 compounds, which explains the choice of this server. As shown in Table 3, compound 1 could be useful as calcium channel (voltage-sensitive) activator but also as nootropic agent, with respective values of Pa = 0.703, Pa = 0.679. It is well known that voltage-gated calcium channels are mandatory for the initiation of many physiological events such as the secretion of hormones or the release of neurotransmitters, by maintaining a coherent synaptic transmission [48]. A nootropic effect could mean that compound 1 can boost brain performance, especially memory capacity, an important parameter of the learning process [49].

Table 3.
Bioactivity prediction of the four tested compounds.
It is also interesting to underline that compound 2 could be a potential antidiabetic agent, especially against Type 2 diabetes (Pa = 0.965), to ameliorate pancreatic beta cells function in order to reduce the abnormal elevation of blood glucose level and ameliorate insulin sensitivity [50]. This compound may also exert a great inhibition on lipase activity (Pa = 0.906), which may help scientists to develop new drugs to treat obesity since an excessive activity of lipase will significantly increase gastrointestinal absorption of fats and, in the long term, an abnormal increase in weight [51]. Compound 3 could be also a good candidate for the treatment of phobic disorders (Pa = 0.864), making it a good candidate in psychotherapy and in other cognitive behavioral therapy [52]. Compound 3 may also be effective against eczema (Pa = 0.730) but also be a great cardiovascular analeptic agent (Pa = 0.806) in order to boost heart activity to increase the blood pumping process in order to ensure an optimal distribution of oxygen and nutrients to all parts of the body [53]. This may also help scientists to develop a more effective treatment, especially for people suffering from valvular heart disease, cardiomyopathy, and heart failure [54]. These data could explain the antidiabetic and neuroprotective activities of the n-BuOH extract of C. tougourensis reported in the previous study [55,56].
The data also revealed that compound 4 may exert an important stimulation on membrane polarization (Pa = 0.926), which is mandatory to maintain an asymmetric organization of proteins and lipids in the plasma membrane [57]. This compound could also exert great antiprotozoal activity against plasmodium (Pa = 0.754), since the proliferation of this parasite is responsible for malaria, a disease widely present in the tropics regions of Africa and Southeast Asia [58].
3.5. Cytotoxicity Prediction
Plants have demonstrated their ability to inhibit the proliferation of several tumor cell lines over time due to their abundance of bioactive compounds, particularly flavonoids, terpenoids, and saponins, which have a potent cytotoxic effect [59,60]. These classes of secondary metabolites have already been identified in C. tougourensis [61]. The ability of each compound to induce cytotoxicity on tumor cell line was investigated using CLC-Pred server and, as shown in Table 4, data clearly indicated that the four tested compounds expressed a moderate cytotoxic effect on the majority of cell lines evaluated in the present study. Indeed, three compounds exerted a non-negligible cytotoxic effect against melanoma, in which compound 2 (Pa = 0.496) and compound 3 (Pa = 0.430) were active against (M19-MEL) cell line, while compound 4 (Pa = 0.548) was active against (SK-MEL-28) cell line. These details are crucial because melanoma is the most dangerous type of skin cancer [62], and the ozone layer’s depletion makes people more vulnerable to harmful UVB sun rays [63]. Note that the actual data reported in this study could partially explain the remarkable photoprotective effect of the n-BuOH extract of C. tougourensis reported in the previous study [64].

Table 4.
Cytotoxicity probabilities of the four tested compounds on some tumor celllines.
Compound 1 exhibited a modest cytotoxic effect against acute leukemic T-cells (Jurkat) cell line (Pa = 0.456), which may show promise in the treatment of diseases related to hematopoiesis since, in a leukemia situation, bone marrow is replaced with tumors, which prevents the regular producing of B or T lymphocytes and may cause long-term anemia [65,66]. Note that a negligible effect (Pa = 0.351) was exerted by compound 1 against breast adenocarcinoma (MDA-MB-453) cell line.
Compound 3 exerted a modest cytotoxic effect against oligodendroglioma (Hs 683) and non-small-cell lung carcinoma (HOP-18) cell lines with a respective activity values of Pa = 0.559 and Pa = 0.505. Despite the fact that non-small-cell lung carcinoma is classified as the tenth most common cancer in the world [67], its number has increased considerably over the last years, for example by 25% between 2014 and 2016 in Canada [68]. Recent statistics reported that this type of cancer accounts for 80% to 85% of all lung cancers [69], in which women have the highest rates for this type of cancer [70]. These facts highlight the critical need for a new treatment option for this type of cancer. It is worth noting that compound 2 was active on brain tissue, particularly against the glioblastoma (SF-295) cell line, with a cytotoxic effect of Pa = 0.593.
3.6. Gene Expression Profiles
As shown in Table 5, the results suggest that compound 1 may exert a moderate upregulation process on the mRNA expression of FOXO1 and ADIPOQ genes with respective values of Pa = 0.708 and Pa = 0.596. These two genes are mainly involved in the anti-diabetic process [71,72]. Indeed, the expression of the FOXO1 gene, which is mainly orchestrated by MAP kinase pathway, will significantly contribute to the maintenance of energy homeostasis, especially those in relation to carbohydrate metabolism, but may also strengthen insulin sensitivity, glucose uptake, and also promote the formation of adipose tissue and skeletal muscle [72,73,74]. This gene also seems to protect the keratinocytes of skin and mucous membranes by enhancing immune system functions when promoting the maturation and production of lymphocytes, especially type B, as well as the activation of macrophages, neutrophils, and dendritic cells [75]. The ADIPOQ gene encodes a protein called adiponectin, and it was reported that low plasma levels of this protein may lead to an abnormal increase of insulin resistance, as well as lipid metabolism imbalance [76]. The expression of this gene could also stimulate the sensitivity of insulin to the variation of blood sugar level, and thus to prevent the development of possible diabetes [77]. Compound 1 may also moderately (Pa = 0.597) stimulate the expression of LST1 gene, which may possibly modulate immune responses, especially against rubella [78,79]. On the other hand, compound 1 showed a moderate downregulation process on the expression of CCR6 (Pa = 0.573), DHFR (Pa = 0.474), and BCAS3 (Pa = 0.451) genes, and this information is very important since the overexpression of these genes is associated with several illnesses; CCR6 with psoriasis, systemic sclerosis, rheumatoid arthritis, lupus nephritis [80], while BCAS3 may alter neural tissue development [81] and DHFR with cerebral folate deficiency and megaloblastic anemia that lead in the long term to the development of severe neurologic diseases, especially brain tumors [82].

Table 5.
Probable effects of the four tested compounds on the expression of some genes: mRNA-based prediction result.
Among the four tested compounds, results revealed that compound 2 could be a potential molecule to upregulate the mRNA expression of CYP3A4 (Pa = 0.908) and CYP2B6 (Pa = 0.778) genes. These two genes are members of the cytochrome P450 superfamily of enzymes and play important roles. A study carried outby Chen et al. [83] indicates that CYP3A4 can detoxify bile acids, which gives a promising expectation for the treatment of cholestasis. CYP2B6 is mainly expressed in the liver, and it was reported that this enzyme can regulate the mRNA splicing and expression processes to form protein [84]. Clinically, data collected about CYP2B6 could be considered relevant for the treatment of HIV-infected patients [85]. A moderate downregulation process was exerted by compound 2 on themRNA expression of BGLAP (Pa = 0.627), MSH5 (Pa = 0.502), and TREX1 (Pa = 0.502) genes. The overexpression of the BGLAP gene is associated with the development of pancreatic ductal adenocarcinoma and chronic pancreatitis, and several assays, such as quantitative RT-PCR, have already proven it [86]. The mutation of the MSH5 gene could be responsible for premature ovarian failure [87], while mutations in the TREX1 gene are strongly linked with Aicardi–Goutières syndrome, which is a severe encephalopathy [88].
The results also indicated that compound 3 may possibly enhance the mRNA expression of PBK (Pa = 0.812), RACGAP1 (Pa = 0.781), and BRMS1 (Pa = 0.779) genes. These genes are very important in human physiology. Indeed, the protein encoded by the PBK gene serves as novel target for development of new cancer immunotherapy and diagnostic biomarker, especially to treat human bladder cancer [89], while the RACGAP1 gene is considered as an important controller of cellular phenomena related to hematopoietic cells, especially their growth and differentiation [90]. A recent study indicated that the BRMS1 gene is a potent metastasis suppressor, especially against breast cancer, via the regulation of NF-κB and epidermal growth factor receptor (EGFR)-signaling pathways [91]. Note that compound 2 showed also a non-negligible upregulation effect on the expression of the BRMS1 gene (Pa = 0.639). On the other hand, compound 3 showed almost the same probability to downregulate the expression of PLCG2 (Pa = 0.735), SUV39H2 (Pa = 0.732), and TREX1 (Pa = 0.725) genes. A recent study suggests that mutations in the PLCG2 gene can generate an autoimmune pathology condition characterized by an abnormal inflammation throughout the body and the incapacity of the body to correctly fight infections [92]. An overexpression of this gene is associated with osteosarcoma, considered as the most common primary bone cancer in children [93]. It was also reported that mutations in the TREX1 gene could generate an autosomal-dominant disorder called retinal vasculopathy with cerebral leukodystrophy (RVCL) [94].
In this study, compound 4 showed a modest upregulation on the mRNA expression process of REL (Pa = 0.557), TRIOBP (Pa = 0.411), and ADAMTS9 (Pa = 0.400) genes. The protein encoded by the REL gene called c-REL plays a key role in hemopoietic cells growth, differentiation, and function, especially those of lymphocytes B and T, which may help scientists treating certain autoimmune diseases such as psoriasis, arthritis, and celiac disease [95,96]. On the other hand, the TRIOBP gene is mandatory to regulate cell spreading and contraction by directly binding and stabilizing filamentous F-actin [97], while the ADAMTS9 gene helps transport a variety of secretion from the endoplasmic reticulum into Golgi apparatus via its own protease-independent function [98].A moderate downregulation process was also exerted by compound 4 on the mRNA expression of FABP4 (Pa = 0.567), while non-negligible values were recorded for TAGLN (Pa = 0.395) and NCOA1 (Pa = 0.335) genes. Note that the FABP4 gene activity is associated with the development of coronary restenosis [99] and liposarcoma of bone diseases [100], while the overexpression of the TAGLN gene is associated with the development of non-small-cell lung cancer, which is considered nowadays as the main cause of tumor mortality in the world [101]. Finally, a study showed that there could be a link between a mutation in NCOA1 gene and the development of rhabdomyosarcoma [102], which is a type of tumor that affects more neonatal and young children.
3.7. Macromolecular Targets Prediction
As shown in Table 6, compound 1 seems to be the most active on the nervous system by targeting key enzymes named acetylcholinesterase (Pa = 0.292) and butyrylcholinesterase (Pa = 0.219) in a non-negligible way, which suggests that this compound could actively participate in the regulation of the activity of these two enzymes known for their important roles in cholinergic transmission by facilitating the hydrolysis of acetylcholine (ACh) neurotransmitter [103]. On the other hand, compound 1 may also participate with other compounds ina synergistic way to limit the activity of these enzymes, since an excessive activity of acetylcholinesterase may lead in the long term to neurodegenerative illnesses such as Alzheimer’s and Parkinson’s diseases [104]. A key receptor called muscarinic acetylcholine receptor (M1) could be also targeted by this compound (Pa = 0.175), and several studies [105,106] reported the crucial role of this receptor during learning and memory processes, but it could also be a promising therapeutic target for the improvement of cognitive decline in Alzheimer’s disease (AD).

Table 6.
Nervous system macromolecular targets prediction of the four tested compounds.
Both beta amyloid A4 protein and serine/threonine protein kinase AKT may be targeted by compound 2 but in anonsignificant manner (Pa = 0.112), while compound 3 seems to be inactive on nervous system molecules. However, it is important to underline that compound 2 may be considered to have noticeable contribution in the elaboration process of anti-Alzheimer’s drugs, since Beta-amyloid protein is the main component of amyloid plaques, a protein aggregate found in neurons in certain neurodegenerative diseases [107]. This protein would notably decrease communication between neurons due tofilamentous aggregation in the extracellular space [108]. On the other hand, compound 2 may promote neuronal survival of the cerebellum through serine-threonine protein kinase Akt, and researchers underlined that this enzyme cangenerate this effect via a possible activation of insulin-like growth factor 1 (IGF-1)-signaling pathway [109].
Furthermore, the probability of compound 4 to target matrix metalloproteinase 16, matrix metalloproteinase 12, and nitric-oxide synthase in the brain is negligible (Pa = 0.097). Note that matrix metalloproteinase 16 is responsible for neural crest cell migration and the emergence of multiple cell lineages in the developing avian embryo [110]. However, matrix metalloproteinase 12 activity may significantly compromise the integrity of the blood–brain barrier after ischemic stroke in rats [111]. Finally, nitric-oxide synthase is mandatory to catalyze the production of nitric oxide (NO) considered as a major component of the signaling pathways that operate between cerebral blood vessels, neurons, and glial cells [112].
4. Conclusions
Various insilico tests conducted in this study revealed that certain compounds recently identified in the n-BuOH extract of this plant could be effective in the treatment of diabetes, cardiovascular disease, and central nervous system disorders. These compounds may also be used to regulate the expression of certain genes in order to better prevent and treat diseases related to immunity, cancer, and diabetes. These compounds may also have potent cytotoxic effects on several tumor cell lines.
Author Contributions
Methodology and writing, S.D.; software and writing M.S.B.; review and editing, L.H., A.H.A., M.A.M., R.S., A.A.M.A.-M. and L.M.A.M.; visualization, L.H., A.H.A. and L.M.A.M.; funding acquisition, R.S., L.M.A.M. and A.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request from the corresponding author.
Acknowledgments
The authors wish to express thanks to the Algerian Ministry of Higher Education and Scientific Research (MESRS, DGRSDT). Taif University Researchers Supporting Project Number (TURSP-2020/140), Taif University, Taif, Saudi Arabia. Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R249), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors also thank Prince Sattam Bin Abdulaziz University, Al-Kharj for their scientific contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.; Tian, S.; Li, Y.; Fang, Q.; Tan, R.; Pan, Y.; Huang, C.; Xu, Y.; Gao, X. Modern deep learning in bioinformatics. J. Mol. Cell Biol. 2020, 12, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Echigoya, Y.; Mouly, V.; Garcia, L.; Yokota, T.; Duddy, W. In silico screening based on predictive algorithms as a design tool for exon skipping oligonucleotides in Duchenne muscular dystrophy. PLoS ONE 2015, 10, e0120058. [Google Scholar]
- Zhang, X.; Liu, T.; Fan, X.; Ai, N. In silico modeling on ADME properties of natural products: Classification models for blood-brain barrier permeability, its application to traditional Chinese medicine and in vitro experimental validation. J. Mol. Graph. 2017, 75, 347–354. [Google Scholar] [CrossRef]
- Inui, T.; Kobayashi, S.; Haginoya, K. Predicting epileptic encephalopathy using mutation site analysis and in silico algorithms. Epilepsy Behav. 2020, 109, 107085–107086. [Google Scholar] [CrossRef]
- Falahi, S.; Karaji, A.G.; Koohyanizadeh, F.; Rezaiemanesh, A.; Salari, F. A comprehensive in silico analysis of the functional and structural impact of single nucleotide polymorphisms (SNPs) in the human IL-33 gene. Comput. Biol. Chem. 2021, 94, 107560. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yuan, B.; Lu, Y.; Zhao, X.; Shen, C.; Ji, J.; Lin, L.; Xu, J.; Xie, T.; Shan, J. In-silico-library-based method enables rapid and comprehensive annotation of cardiolipins and cardiolipin oxidation products using high resolution tandem mass spectrometer. Anal. Chim. Acta 2021, 1180, 338879–338888. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dzhambov, A.M.; Lercher, P.; Browning, M.; Stoyanov, D.; Petrova, N.; Novakov, S.; Dimitrova, D.D. Does greenery experienced indoors and outdoors provide an escape and support mental health during the COVID-19 quarantine? Environ. Res. 2021, 196, 110420–110431. [Google Scholar] [CrossRef]
- Yeung, W.F.; Chung, K.F.; Poon, M.M.; Ho, F.Y.; Zhang, S.P.; Zhang, Z.J.; Ziea, E.T.; Wong, V.T. Chinese herbal medicine for insomnia: A systematic review of randomized controlled trials. Sleep Med. Rev. 2012, 16, 497–507. [Google Scholar] [CrossRef]
- Köse, Y.B.; İşcan, G.; Göger, F.; Akalın, G.; Demirci, B.; Başer, K.H. Chemical Composition and Biological Activity of Centaurea baseri: New Species from Turkey. Chem. Biodivers. 2016, 13, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Chuclá, M.T.; Lamela, M.; Gato, A.; Cadavid, I. Centaurea corcubionensis: A study of its hypoglycemic activity in rats. Planta Med. 1988, 54, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Koca, U.; Süntar, I.P.; Keles, H.; Yesilada, E.; Akkol, E.K. In vivo anti-inflammatory and wound healing activities of Centaurea iberica Trev. ex Spreng. J. Ethnopharmacol. 2009, 126, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Csupor, D.; Peták, Z.; Hohmann, J. Medicinal perspective of Hungarian Centaurea species in the light of scientific evidence. Acta Pharm. Hung. 2011, 81, 63–75. [Google Scholar]
- Naeim, H.; El-Hawiet, A.; Abdel Rahman, R.A.; Hussein, A.; El Demellawy, M.A.; Embaby, A.M. Antibacterial activi-ty of Centaurea pumilio L. root and aerial part extracts against some multidrug resistant bacteria. BMC Complement. Med. Ther. 2020, 20, 79. [Google Scholar] [CrossRef] [Green Version]
- Dimkić, I.; Petrović, M.; Gavrilović, M.; Gašić, U.; Ristivojević, P.; Stanković, S.; Janaćković, P. New perspectives of purple starthistle (Centaurea calcitrapa) leaf extracts: Phytochemical analysis, cytotoxicity and antimicrobial activity. AMB Express 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Nasr, F.A.; Shahat, A.A.; Alqahtani, A.S.; Ahmed, M.Z.; Qamar, W.; Al-Mishari, A.A.; Almoqbil, A.N. Centaurea bruguierana inhibits cell proliferation, causes cell cycle arrest, and induces apoptosis in human MCF-7 breast carcinoma cells. Mol. Biol. Rep. 2020, 47, 6043–6051. [Google Scholar] [CrossRef]
- Khanavi, M.; Rajabi, A.; Behzad, M.; Hadjiakhoondi, A.; Vatandoost, H.; Abaee, M.R. Larvicidal Activity of Centaurea bruguierana ssp. belangerana Against Anopheles stephensi Larvae. Iran. J. Pharm. Sci. 2011, 10, 829–833. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.A.; Dubovskaja, V.I.; Rudik, A.V.; Pogodin, P.V.; Druzhilovskiy, D.S.; Gloriozova, T.A.; Filimonov, D.A.; Sastry, N.G.; Poroikov, V.V. CLC-Pred: A freely available web-service for in silico prediction of human cell line cytotoxicity for drug-like compounds. PLoS ONE 2018, 13, e0191838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagunin, A.; Ivanov, S.; Rudik, A.; Filimonov, D.; Poroikov, V. DIGEP-Pred: Web service for in silico prediction of drug-induced gene expression profiles based on structural formula. Bioinformatics 2013, 29, 2062–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, S.; Lokki, A.I.; Hanttu, A.; Nissilä, E.; Heinonen, S.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Saarinen, L.; Tynninen, O.; et al. Upregulation of Early and Downregulation of Terminal Pathway Complement Genes in Subcutaneous Adipose Tissue and Adipocytes in Acquired Obesity. Front. Immunol. 2017, 8, 545. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Tamaian, R.; Moţ, A.; Silaghi-Dumitrescu, R.; Ionuţ, I.; Stana, A.; Oniga, O.; Nastasă, C.; Benedec, D.; Tiperciuc, B. Study of the Relationships between the Structure, Lipophilicity and Biological Activity of Some Thiazolyl-carbonyl-thiosemicarbazides and Thiazolyl-azoles. Molecules 2015, 20, 22188–22201. [Google Scholar] [CrossRef]
- Kim, M.T.; Sedykh, A.; Chakravarti, S.K.; Saiakhov, R.D.; Zhu, H. Critical evaluation of human oral bioavailability for pharmaceutical drugs by using various cheminformatics approaches. Pharm. Res. 2014, 31, 1002–1014. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.H.; Lee, Y.J.; Lee, E.S.; Geng, Y.; Wang, X.S.; Cleeland, C.S. Current use of drugs affecting the central nervous system for chemotherapy-induced peripheral neuropathy in cancer patients: A systematic review. Support. Care Cancer 2015, 23, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zolkipli-Cunningham, Z.; Falk, M.J. Clinical effects of chemical exposures on mitochondrial function. Toxicology 2017, 391, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Naven, R.T.; Swiss, R.; Klug-McLeod, J.; Will, Y.; Greene, N. The development of structure-activity relationships for mitochondrial dysfunction: Uncoupling of oxidative phosphorylation. Toxicol. Sci. 2013, 131, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Haji-Ali-Nili, N.; Khoshzaban, F.; Karimi, M.; Roja, R.; Ashrafi, E.; Ghaffari, R.; Ghobadi, A.; Jabarvand Behrouz, M. Effect of a Natural Eye Drop, Made of Plantago Ovata Mucilage on Improvement of Dry Eye Symptoms: A Randomized, Double-blind Clinical Trial. Iran. J. Pharm. Res. 2019, 18, 1602–1611. [Google Scholar]
- Madejska, A.; Michalski, M.; Osek, J. Marine Tetrodotoxin as a Risk for Human Health. J. Vet. Res. 2019, 63, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tsutsui, H.; Yamawaki, N.; Morii, Y.; Nishihara, G.N.; Itoi, S.; Arakawa, O.; Takatani, T. Geographic Variations in the Toxin Profile of the Xanthid Crab Zosimus aeneus in a Single Reef on Ishigaki Island, Okinawa, Japan. Mar. Drugs 2021, 19, 670. [Google Scholar] [CrossRef]
- Sakkiah, S.; Guo, W.; Pan, B.; Kusko, R.; Tong, W.; Hong, H. Computational prediction models for assessing endocrine disrupting potential of chemicals. J. Environ. Sci. Health C: Toxicol. Carcinog. 2018, 36, 192–218. [Google Scholar] [CrossRef]
- Yu, E.; Xu, Y.; Shi, Y.; Yu, Q.; Liu, J.; Xu, L. Discovery of novel natural compound inhibitors targeting estrogen receptor α by an integrated virtual screening strategy. J. Mol. Model. 2019, 25, 278–288. [Google Scholar] [CrossRef]
- Sykes, D.A.; Parry, C.; Reilly, J.; Wright, P.; Fairhurst, R.A.; Charlton, S.J. Observed drug-receptor association rates are governed by membrane affinity: The importance of establishing “micro-pharmacokinetic/pharmacodynamic relationships” at the β2-adrenoceptor. Mol. Pharmacol. 2014, 85, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.; Cox, R.J.; Grime, K. Plasma Protein Binding as an Optimizable Parameter for Acidic Drugs. Drug Metab. Dispos. 2019, 47, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Y.; Yang, J.; Yin, X.X.; Yang, S.P.; Zhu, Y.G. Arsenate toxicity and stress responses in the freshwater ciliate Tetrahymena pyriformis. Eur. J. Protistol. 2012, 48, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Walum, E. Acute oral toxicity. Environ. Health Perspect. 1998, 106, 497–503. [Google Scholar] [PubMed]
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Poroikov, V.V. Online resources for the prediction of biological activity of organic compounds. Russ. Chem. Bull. 2016, 65, 384–393. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Suliman, N.A.; Mat Taib, C.N.; Mohd Moklas, M.A.; Adenan, M.I.; Hidayat Baharuldin, M.T.; Basir, R. Establishing Natural Nootropics: Recent Molecular Enhancement Influenced by Natural Nootropic. Evid.-Based Complement. Altern. Med. 2016, 2016, 4391375. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Bialecka-Florjanczyk, E.; Fabiszewska, A.U.; Krzyczkowska, J.; Kurylowicz, A. Synthetic and Natural Lipase Inhibitors. Mini-Rev. Med. Chem. 2018, 18, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R.; Abbasabadi, F.; Abdollahi, M. A Systematic Review of Plant-Derived Natural Compounds for Anxiety Disorders. Curr. Top. Med. Chem. 2016, 16, 1924–1942. [Google Scholar] [CrossRef] [PubMed]
- Sternitzke, N. The cardiovascular effect of akrinor, a circulatory analeptic with long-term effect. Z. Kreislaufforsch. 1965, 54, 10–18. [Google Scholar] [PubMed]
- Borer, J.S.; Sharma, A. Drug Therapy for Heart Valve Diseases. Circulation 2015, 132, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Bensouici, C.; Haba, H. In vitro assessment of antioxidant, anti-inflammatory, neuroprotective and antimicrobial activities of Centaurea tougourensis Boiss. & Reut. J. Pharm. Pharmacogn. Res. 2021, 9, 790–802. [Google Scholar]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Saidi, A.; Melakhsou, M.A.; Nouicer, F.; Baghiani, A.; Khennouf, S.; Kahoul, M.A.; Kadrine, N. In vivo investigation of antidiabetic, hepatoprotective, anti-inflammatory and antipyretic activities of Centaurea tougourensis Boiss. & Reut. J. Physiol. Pharmacol. 2021, 72, 439–449. [Google Scholar]
- Orlando, K.; Guo, W. Membrane organization and dynamics in cell polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a001321. [Google Scholar] [CrossRef]
- Lee, M.; Coban, C. Unforeseen pathologies caused by malaria. Int. Immunol. 2018, 30, 121–129. [Google Scholar] [CrossRef]
- Tiwary, B.K.; Bihani, S.; Kumar, A.; Chakraborty, R.; Ghosh, R. The in vitro cytotoxic activity of ethno-pharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Complement. Med. Ther. 2015, 15, 22. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Kumar, A.; Das, V.; Ghosh, S. Evaluation of chemical constituents and in vitro antimicrobial, antioxidant and cytotoxicity potential of rhizome of Astilbe rivularis (Bodho-okhati), an indigenous medicinal plant from Eastern Himalayan region of India. BMC Complement. Med. Ther. 2019, 19, 200. [Google Scholar] [CrossRef] [Green Version]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Kahoul, M.A.; Benhoula, M. Evidence of anti-inflammatory and anti-ulcer properties of aerial parts of Centaurea tougourensis Boiss and Reut. Trop. J. Pharm. Res. 2021, 20, 1647–1654. [Google Scholar]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensaad, M.S.; Dassamiour, S.; Hambaba, L.; Bensouici, C.; Ouffroukh, K.; Kahoul, M.A. HPLC-DAD phenolics screening and in vitro investigation of haemostatic, antidiabetic, antioxidant and photoprotective properties of Centaurea tougourensis Boiss. & Reut. Herba Pol. 2021, 67, 16–31. [Google Scholar]
- Raetz, E.A.; Teachey, D.T. T-cell acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Dinner, S.; Liedtke, M. Antibody-based therapies in patients with acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [Green Version]
- Melosky, B.; Blais, N.; Cheema, P.; Couture, C.; Juergens, R.; Kamel-Reid, S.; Tsao, M.S.; Wheatley-Price, P.; Xu, Z.; Ionescu, D.N. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr. Oncol. 2018, 25, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Bareschino, M.A.; Schettino, C.; Rossi, A.; Maione, P.; Sacco, P.C.; Zeppa, R.; Gridelli, C. Treatment of advanced non small cell lung cancer. J. Thorac. Dis. 2011, 3, 122–133. [Google Scholar]
- Cheng, T.Y.; Cramb, S.M.; Baade, P.D.; Youlden, D.R.; Nwogu, C.; Reid, M.E. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J. Thorac. Oncol. 2016, 11, 1653–1671. [Google Scholar] [CrossRef] [Green Version]
- Spranger, J.; Kroke, A.; Möhlig, M.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003, 361, 226–228. [Google Scholar] [CrossRef]
- Lundell, L.S.; Massart, J.; Altıntaş, A.; Krook, A.; Zierath, J.R. Regulation of glucose uptake and inflammation markers by FOXO1 and FOXO3 in skeletal muscle. Mol. Metab. 2019, 20, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone 2012, 50, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Li, W.; Hou, N.; Huang, N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, D.T.; Milovanova, T.N. Mucosal Immunity and the FOXO1 Transcription Factors. Front. Immunol. 2019, 10, 2530. [Google Scholar] [CrossRef]
- Menzaghi, C.; Trischitta, V.; Doria, A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 1198–1209. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Ovsyannikova, I.G.; Vierkant, R.A.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Extended LTA, TNF, LST1 and HLA gene haplotypes and their association with rubella vaccine-induced immunity. PLoS ONE 2010, 5, e11806. [Google Scholar] [CrossRef]
- Fabisik, M.; Tureckova, J.; Pavliuchenko, N.; Kralova, J.; Balounova, J.; Vicikova, K.; Skopcova, T.; Spoutil, F.; Pokorna, J.; Angelisova, P.; et al. Regulation of Inflammatory Response by Transmembrane Adaptor Protein LST1. Front. Immunol. 2021, 12, 618332. [Google Scholar] [CrossRef]
- Akhtar, M.; Jamal, T.; Jamal, H.; Din, J.U.; Jamal, M.; Arif, M.; Arshad, M.; Jalil, F. Identification of most damaging nsSNPs in human CCR6 gene: In silico analyses. Int. J. Immunogenet. 2019, 46, 459–471. [Google Scholar] [CrossRef]
- Hengel, H.; Hannan, S.B.; Dyack, S.; MacKay, S.B.; Schatz, U.; Fleger, M.; Kurringer, A.; Balousha, G.; Ghanim, Z.; Alkuraya, F.S.; et al. Bi-allelic loss-of-function variants in BCAS3 cause a syndromic neurodevelopmental disorder. Am. J. Hum. Genet. 2021, 108, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Fawal, M.A.; Jungas, T.; Davy, A. Inhibition of DHFR targets the self-renewing potential of brain tumor initiating cells. Cancer Lett. 2021, 503, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N.; Chen, C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann. Transl. Med. 2014, 2, 7–15. [Google Scholar] [PubMed]
- Zanger, U.M.; Klein, K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): Advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 2013, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Habtewold, A.; Amogne, W.; Makonnen, E.; Yimer, G.; Riedel, K.D.; Ueda, N.; Worku, A.; Haefeli, W.E.; Lindquist, L.; Aderaye, G.; et al. Long-term effect of efavirenz autoinduction on plasma/peripheral blood mononuclear cell drug exposure and CD4 count is influenced by UGT2B7 and CYP2B6 genotypes among HIV patients. J. Antimicrob. Chemother. 2011, 66, 2350–2361. [Google Scholar] [CrossRef] [Green Version]
- Kayed, H.; Bekasi, S.; Keleg, S.; Michalski, C.W.; Giese, T.; Friess, H.; Kleeff, J. BGLAP is expressed in pancreatic cancer cells and increases their growth and invasion. Mol. Cancer 2007, 6, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Zhao, S.; Zhao, S.; Chen, M.; Li, G.; Jiao, X.; Wang, Z.; Zhao, Y.; Qin, Y.; Gao, F.; et al. Mutations in MSH5 in primary ovarian insufficiency. Hum. Mol. Genet. 2017, 26, 1452–1457. [Google Scholar] [CrossRef] [Green Version]
- Orebaugh, C.D.; Fye, J.M.; Harvey, S.; Hollis, T.; Perrino, F.W. The TREX1 exonuclease R114H mutation in Aicardi-Goutières syndrome and lupus reveals dimeric structure requirements for DNA degradation activity. J. Biol. Chem. 2011, 286, 40246–40254. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Li, L.; Huang, Y.; Zhao, H.; Luo, Y. PBK/TOPK: A Therapeutic Target Worthy of Attention. Cells 2021, 10, 371. [Google Scholar] [CrossRef]
- Ting, S.B.; Deneault, E.; Hope, K.; Cellot, S.; Chagraoui, J.; Mayotte, N.; Dorn, J.F.; Laverdure, J.P.; Harvey, M.; Hawkins, E.D.; et al. Asymmetric segregation and self-renewal of hematopoietic stem and progenitor cells with endocytic Ap2a2. Blood 2012, 119, 2510–2522. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Welch, D.R. BRMS1: A multifunctional signaling molecule in metastasis. Cancer Metastasis Rev. 2020, 39, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Kutukculer, N.; Topyildiz, E.; Berdeli, A.; Guven Bilgin, B.; Aykut, A.; Durmaz, A.; Cogulu, O.; Aksu, G.; Edeer Karaca, N. Four diseases, PLAID, APLAID, FCAS3 and CVID and one gene (PHOSPHOLIPASE C, GAMMA-2; PLCG2): Striking clinical phenotypic overlap and difference. Clin. Case Rep. 2021, 9, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Yuan, X.; Zhuang, M.; Qiu, X.; Xu, X.; Kong, R.; Liu, Z. Histone methyltransferase SUV39H2 serves oncogenic roles in osteosarcoma. Oncol. Rep. 2019, 41, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, R.; Nozaki, H.; Kato, T.; Toyoshima, Y.; Tanaka, H.; Tsubata, Y.; Morioka, T.; Horikawa, Y.; Oyanagi, K.; Morita, T.; et al. Retinal Vasculopathy with Cerebral Leukodystrophy: Clinicopathologic Features of an Autopsied Patient with a Heterozygous TREX 1 Mutation. J. Neuropathol. Exp. Neurol. 2019, 78, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weih, F.; Carrasco, D.; Bravo, R. Constitutive and inducible Rel/NF-kappa B activities in mouse thymus and spleen. Oncogene 1994, 9, 3289–3297. [Google Scholar] [PubMed]
- Gilmore, T.D.; Gerondakis, S. The c-Rel Transcription Factor in Development and Disease. Genes Cancer 2011, 2, 695–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaharija, B.; Samardžija, B.; Bradshaw, N.J. The TRIOBP Isoforms and Their Distinct Roles in Actin Stabilization, Deafness, Mental Illness, and Cancer. Molecules 2020, 25, 4967. [Google Scholar] [CrossRef]
- Yoshina, S.; Sakaki, K.; Yonezumi-Hayashi, A.; Gengyo-Ando, K.; Inoue, H.; Iino, Y.; Mitani, S. Identification of a novel ADAMTS9/GON-1 function for protein transport from the ER to the Golgi. Mol. Biol. Cell 2012, 23, 1728–1741. [Google Scholar] [CrossRef]
- Fuseya, T.; Furuhashi, M.; Matsumoto, M.; Watanabe, Y.; Hoshina, K.; Mita, T.; Ishimura, S.; Tanaka, M.; Miura, T. Ectopic Fatty Acid-Binding Protein 4 Expression in the Vascular Endothelium is Involved in Neointima Formation After Vascular Injury. J. Am. Heart Assoc. 2017, 6, e006377. [Google Scholar] [CrossRef]
- Herroon, M.K.; Rajagurubandara, E.; Hardaway, A.L.; Powell, K.; Turchick, A.; Feldmann, D.; Podgorski, I. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 2013, 4, 2108–2123. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Wang, X.; Yue, Q. Functional loss of TAGLN inhibits tumor growth and increases chemosensitivity of non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2020, 529, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Karanian, M.; Pissaloux, D.; Gomez-Brouchet, A.; Chevenet, C.; Le Loarer, F.; Fernandez, C.; Minard, V.; Corradini, N.; Castex, M.P.; Duc-Gallet, A.; et al. SRF-FOXO1 and SRF-NCOA1 Fusion Genes Delineate a Distinctive Subset of Well-differentiated Rhabdomyosarcoma. Am. J. Surg. Pathol. 2020, 44, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Cholinesterases, A target of pharmacology and toxicology. Biomed. Pap. Med. 2011, 155, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Thompson, A.D.; Jones, C.K.; Lindsley, C.W.; Conn, P.J. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2012, 340, 595–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpa, M.; Hesse, S.; Bradley, S.J. M1 muscarinic acetylcholine receptors: A therapeutic strategy for symptomatic and disease-modifying effects in Alzheimer’s disease? Adv. Pharmacol. 2020, 88, 277–310. [Google Scholar]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Müller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Dudek, H.; Datta, S.R.; Franke, T.F.; Birnbaum, M.J.; Yao, R.; Cooper, G.M.; Segal, R.A.; Kaplan, D.R.; Greenberg, M.E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997, 275, 661–665. [Google Scholar] [CrossRef]
- Roth, L.; Kalev-Altman, R.; Monsonego-Ornan, E.; Sela-Donenfeld, D. A new role of the membrane-type matrix metalloproteinase 16 (MMP16/MT3-MMP) in neural crest cell migration. Int. J. Dev. Biol. 2017, 61, 245–256. [Google Scholar] [CrossRef]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).