Sol-Gel Synthesis and Characterization of Highly Selective Poly(N-methyl pyrrole) Stannous(II)Tungstate Nano Composite for Mercury (Hg(II)) Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Preparation of Poly(N-methyl pyrrole)/Nano-Stannous(II)WO3 Composite
2.3. Modification of GCE with PNMPy/Nano-Stannous(II)WO3 Composite
3. Results and Discussion
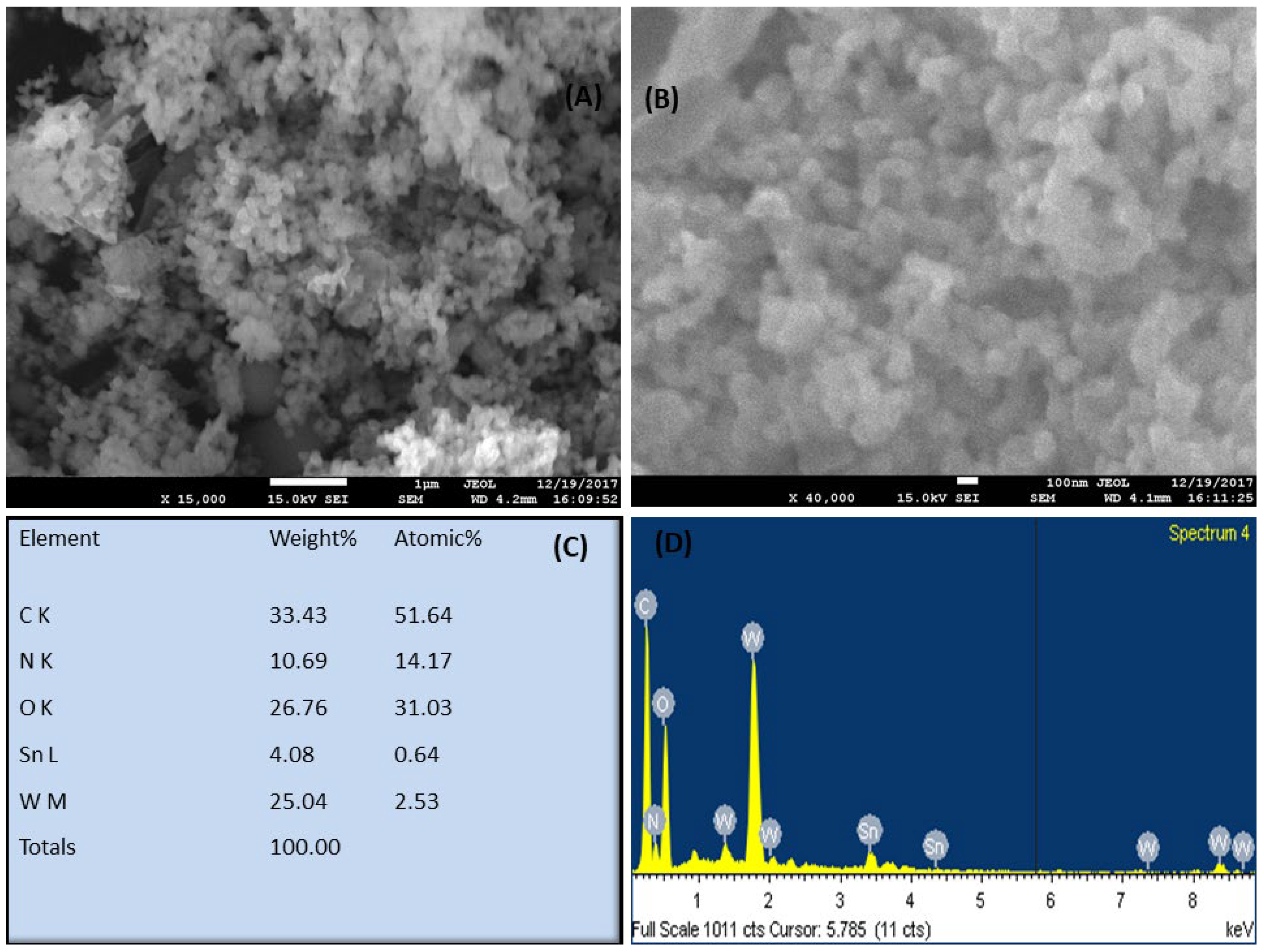
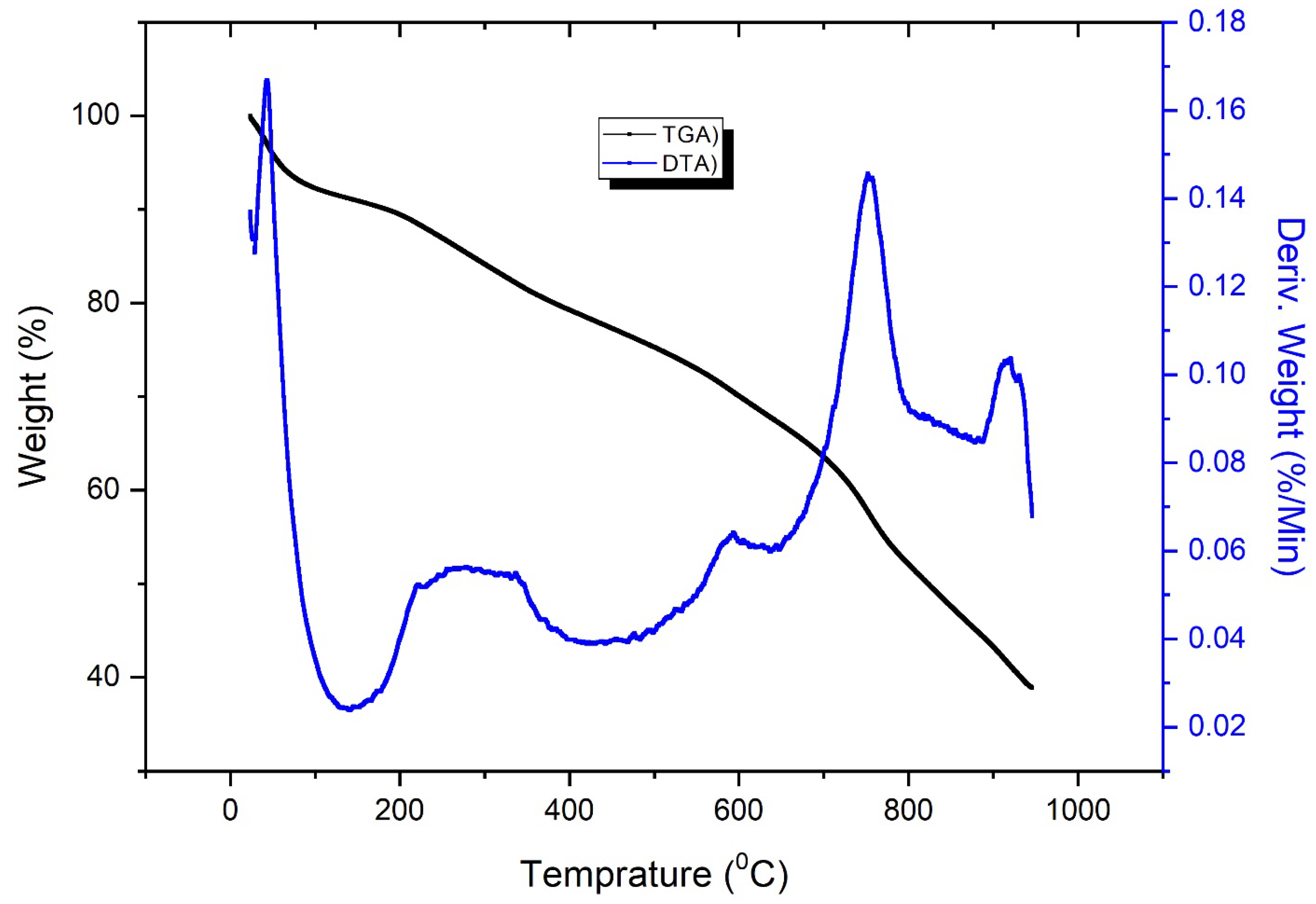
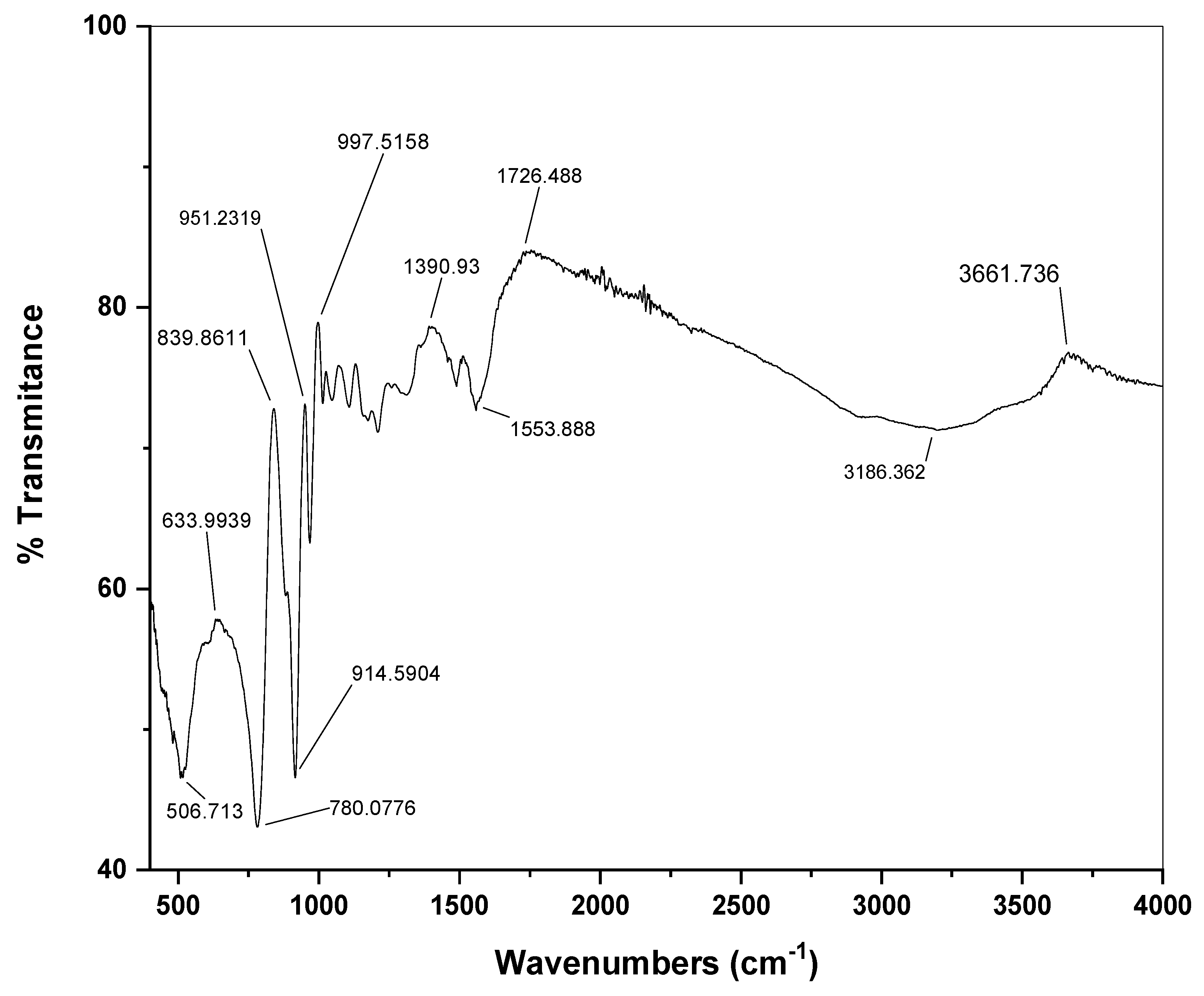
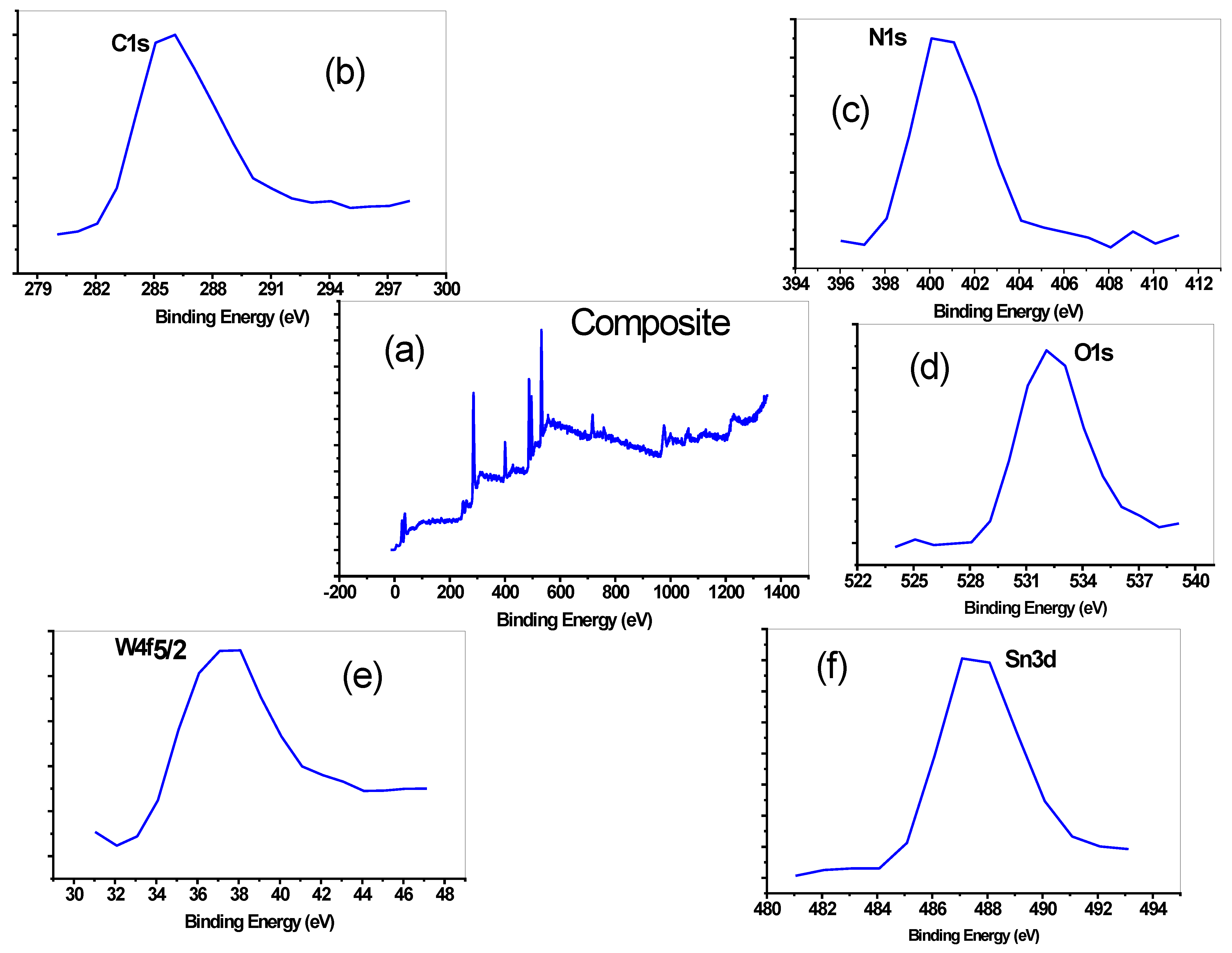
3.1. Characterization
3.2. Applications: Chemical Sensor Development
3.2.1. Detection of Hg2+ Ion Using PNMPY/NANO-Stannous(II)WO3/GCE by the I-V Method
3.2.2. Real Sample Analysis by PNMPY/NANO-Stannous(II)WO3/GCE Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M.; Inamuddin; Alamry, K.A.; Hameed, S.A. Preparation and characterization of PANI@G/CWO nanocomposite for enhanced 2-nitrophenol sensing. Appl. Surf. Sci. 2018, 433, 696–704. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Khan, A.A.P.; Rub, M.A.; Azum, N.; Rahman, M.M.; Al-Youbi, A.O.; Qusti, A.H. Dual nature, self oxidized poly(o-anisidine) functionalized multiwall carbon nanotubes composite: Preparation, thermal and electrical studies. Compos. Part B Eng. 2014, 58, 451–456. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.P.; Asiri, A.M.; Rahman, M.M.; Alhogbi, B.G. Preparation and properties of novel sol-gel-derived quaternized poly(n-methyl pyrrole)/Sn(II)SiO3/CNT composites. J. Solid State Electrochem. 2015, 19, 1479–1489. [Google Scholar] [CrossRef]
- Khan, A. Electrical conductivity and cation exchange kinetic studies on poly-o-toluidine Th(IV) phosphate nano-composite cation exchange material. Talanta 2007, 73, 850–856. [Google Scholar] [CrossRef]
- Khan, A.A.; Habiba, U.; Khan, A. Synthesis and Characterization of Organic-Inorganic Nanocomposite Poly-o-anisidine Sn(IV) Arsenophosphate: Its Analytical Applications as Pb(II) Ion-Selective Membrane Electrode. Int. J. Anal. Chem. 2009, 2009, 659215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Liu, J.; Tanaka, T.; Sivula, K.; Alivisatos, A.P.; Fréchet, J.M.J. Employing End-Functional Polythiophene to Control the Morphology of Nanocrystal−Polymer Composites in Hybrid Solar Cells. J. Am. Chem. Soc. 2004, 126, 6550–6551. [Google Scholar] [CrossRef]
- Zhang, M.; Nautiyal, A.; Du, H.; Li, J.; Liu, Z.; Zhang, X.; Wang, R. Polypyrrole film based flexible supercapacitor: Mechanistic insight into influence of acid dopants on electrochemical performance. Electrochim. Acta 2020, 357, 136877. [Google Scholar] [CrossRef]
- Shimoga, G.; Palem, R.; Choi, D.-S.; Shin, E.-J.; Ganesh, P.-S.; Saratale, G.; Saratale, R.; Lee, S.-H.; Kim, S.-Y. Polypyrrole-Based Metal Nanocomposite Electrode Materials for High-Performance Supercapacitors. Metals 2021, 11, 905. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Hasik, M.; Kloc, M. Pd/polyaniline as the catalysts for 2-ethylanthraquinone hydrogenation. The effect of palladium dispersion. Catal. Lett. 2000, 64, 41–47. [Google Scholar] [CrossRef]
- Radford, P.; Creager, S. Dual-stream flow injection method for amplified electrochemical detection of ferrocene derivatives. Anal. Chim. Acta 2001, 449, 199–209. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Khan, A.A.P.; Rub, M.A.; Azum, N.; Rahman, M.M.; Khan, S.B.; Alamry, K.A.; Ab Ghani, S. Sol–gel synthesis and characterization of conducting polythiophene/tin phosphate nano tetrapod composite cation-exchanger and its application as Hg(II) selective membrane electrode. J. Sol-Gel Sci. Technol. 2013, 65, 160–169. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Xu, Y.; Liu, Y.; Li, D.; Fan, C. A highly sensitive chemiluminescence sensor for detecting mercury (II) ions: A combination of Exonuclease III-aided signal amplification and graphene oxide-assisted background reduction. Sci. China Ser. B Chem. 2015, 58, 514–518. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Hou, Y.; Chen, J. Ultrasensitive Quantum Dot Fluorescence quenching Assay for Selective Detection of Mercury Ions in Drinking Water. Sci. Rep. 2015, 4, 5624. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; She, S.; Zhang, J.; Bayaguud, A.; Wei, Y. Label-free colorimetric detection of mercury via Hg2+ ions-accelerated structural transformation of nanoscale metal-oxo clusters. Sci. Rep. 2015, 5, 16316. [Google Scholar] [CrossRef] [Green Version]
- Moreton, J.A.; Delves, H.T. Simple direct method for the determination of total mercury levels in blood and urine and nitric acid digests of fish by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1998, 13, 659–665. [Google Scholar] [CrossRef]
- Xiao, X.; Ulstrup, J.; Li, H.; Wang, M.; Zhang, J.; Si, P. Nanoporous gold assembly of glucose oxidase for electrochemical biosensing. Electrochim. Acta 2014, 130, 559–567. [Google Scholar] [CrossRef]
- Lang, X.-Y.; Fu, H.-Y.; Hou, C.; Han, G.-F.; Yang, P.; Liu, Y.-B.; Jiang, Q. Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nat. Commun. 2013, 4, 2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Kim, Y. Gold nanoparticle-based fluorescent “turn-on” sensing system for the selective detection of mercury ions in aqueous solution. RSC Adv. 2015, 5, 95268–95272. [Google Scholar] [CrossRef]
- Wegner, S.V.; Okesli, A.; Chen, A.P.; He, C. Design of an Emission Ratiometric Biosensor from MerR Family Proteins: A Sensitive and Selective Sensor for Hg2+. J. Am. Chem. Soc. 2007, 129, 3474–3475. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Togashi, H. Highly Selective Oligonucleotide-Based Sensor for Mercury(II) in Aqueous Solutions. Angew. Chem. 2004, 116, 4400–4402. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ting, R.; Perrin, D.M. High affinity DNAzyme-based ligands for transition metal cations? A prototype sensor for Hg2+. Org. Biomol. Chem. 2004, 2, 307–312. [Google Scholar] [CrossRef]
- Duval, C. Inorganic Thermogravimetric Analysis; Elsevier: Amsterdam, The Netherlands, 1963. [Google Scholar]
- Wang, Y.; Rubner, M. Stability studies of the electrical conductivity of various poly(3-alkylthiophenes). Synth. Met. 1990, 39, 153–175. [Google Scholar] [CrossRef]
- Wang, J.; Keene, F. Mechanism of mediation of the electrochemical oxidation of K4Fe(CN)6 at poly-[tris(3-{ω-[4-(2,2′-bipyridyl)] alkyl}-thiophene)iron(II)]-film modified electrodes in aqueous solutions. Electrochim. Acta 1996, 41, 2563–2569. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly(thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Toshima, N.; Hara, S. Direct synthesis of conducting polymers from simple monomers. Prog. Polym. Sci. 1995, 20, 155–183. [Google Scholar] [CrossRef]
- Solonin, Y.M.; Khyzhun, O.Y.; Graivoronskaya, E.A. Nonstoichiometric Tungsten Oxide Based on Hexagonal WO3. Cryst. Growth Des. 2001, 1, 473–477. [Google Scholar] [CrossRef]
- Utsumi, S.; Honda, H.; Hattori, Y.; Kanoh, H.; Takahashi, K.; Sakai, H.; Abe, M.; Yudasaka, M.; Iijima, S.; Kaneko, K. Direct Evidence on C−C Single Bonding in Single-Wall Carbon Nanohorn Aggregates. J. Phys. Chem. C 2007, 111, 5572–5575. [Google Scholar] [CrossRef]
- Siaj, M.; Maltais, C.; Zahidi, E.M.; Oudghiri-Hassani, H.; Wang, J.; Rosei, A.F.; McBreen, P.H. Carbon−Nitrogen Place Exchange on NO Exposed β-Mo2C. J. Phys. Chem. B 2005, 109, 15376–15382. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, S.B.; Faisal, M.; Rub, M.A.; Al-Youbi, A.O.; Asiri, A.M. Electrochemical determination of olmesartan medoxomil using hydrothermally prepared nanoparticles composed SnO2–Co3O4 nanocubes in tablet dosage forms. Talanta 2012, 99, 924–931. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alenazi, N.A.; Hussein, M.A.; Alam, M.M.; Alamry, K.A.; Asiri, A.M. Hybride ZnCdCrO embedded aminated polyethersulfone nanocomposites for the development of Hg2+ ionic sensor. Mater. Res. Express 2018, 5, 065019. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alamry, K.A.; Awual, R.; Mekky, A.E. Efficient Hg(II) ionic probe development based on one-step synthesized diethyl thieno[2,3-b]thiophene-2,5-dicarboxylate (DETTDC2) onto glassy carbon electrode. Microchem. J. 2020, 152, 104291. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Arshad, M.N.; Asiri, A.M. Hg2+ Sensor Development Based on (E)-N′-Nitrobenzylidene-Benzenesulfonohydrazide (NBBSH) Derivatives Fabricated on a Glassy Carbon Electrode with a Nafion Matrix. ACS Omega 2017, 2, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.N.; Rahman, M.M.; Asiri, A.M.; Sobahi, T.R.; Yu, S.-H. Development of Hg2+ sensor based on N′-[1-(pyridin-2-yl)ethylidene]benzenesulfono-hydrazide (PEBSH) fabricated silver electrode for environmental remediation. RSC Adv. 2015, 5, 81275–81281. [Google Scholar] [CrossRef]
- Awual, R.; Hasan, M.; Eldesoky, G.E.; Khaleque, A.; Rahman, M.M.; Naushad, M. Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem. Eng. J. 2016, 290, 243–251. [Google Scholar] [CrossRef]
- Katowah, D.F.; Alqarni, S.; Mohammed, G.I.; Al Sheheri, S.Z.; Alam, M.M.; Ismail, S.H.; Asiri, A.M.; Hussein, M.A.; Rahman, M.M. Selective Hg2+ sensor performance based various carbon-nanofillers into CuO-PMMA nanocomposites. Polym. Adv. Technol. 2020, 31, 1946–1962. [Google Scholar] [CrossRef]
- Soman, S.; PV, A. Covalently modified graphene quantum dot using a thiourea based imprinted polymer for the selective electrochemical sensing of Hg(II) ions. J. Polym. Res. 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Koshki, M.-S.; Baghayeri, M.; Fayazi, M. Application of sepiolite/FeS2 nanocomposite for highly selective detection of mercury(II) based on stripping voltammetric analysis. J. Food Meas. Charact. 2021, 15, 5318–5325. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Fox, D.; Stanberry, J.; Anagnostopoulos, V.; Zhai, L.; Lee, W. Direct Mercury Detection in Landfill Leachate Using a Novel AuNP-Biopolymer Carbon Screen-Printed Electrode Sensor. Micromachines 2021, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.-C.; Ke, X.-X.; Chen, X.; Weerasooriya, R.; Hong, Z.-Y.; Wang, L.-C.; Wu, Y.-C. Assembling reduced graphene oxide with sulfur/nitrogen-“hooks” for electrochemical determination of Hg(II). Anal. Chim. Acta 2020, 1100, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Raicopol, M.D.; Chira, N.A.; Pandele, A.M.; Hanganu, A.; Ivanov, A.A.; Tecuceanu, V.; Bugean, I.G.; Buica, G.-O. Electrodes modified with clickable thiosemicarbazone ligands for sensitive voltammetric detection of Hg(II) ions. Sens. Actuators B Chem. 2020, 313, 128030. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Li, J.; Chen, L. Highly sensitive and selective voltammetric detection of mercury(II) using an ITO electrode modified with 5-methyl-2-thiouracil, graphene oxide and gold nanoparticles. Mikrochim. Acta 2013, 180, 493–499. [Google Scholar] [CrossRef]
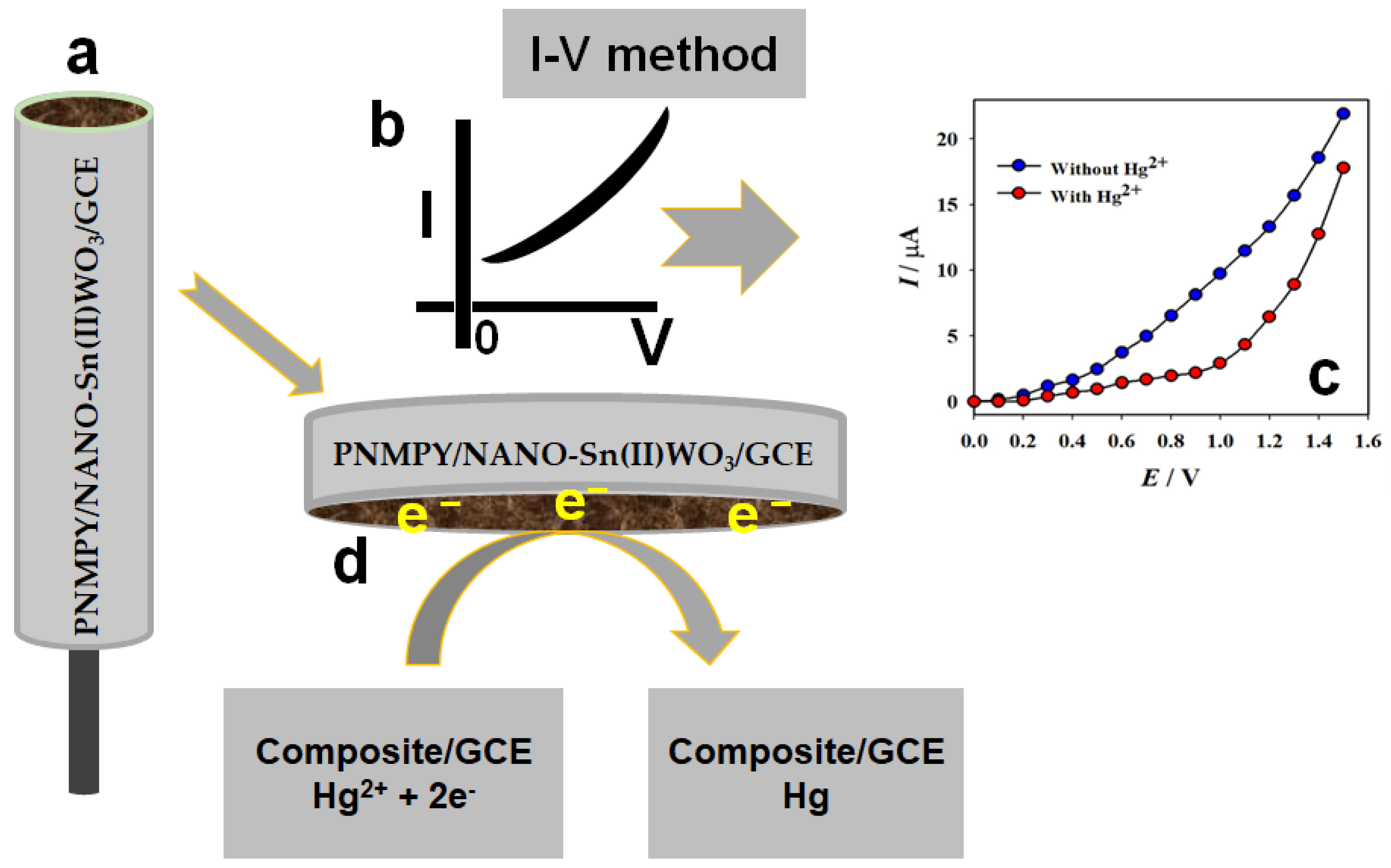


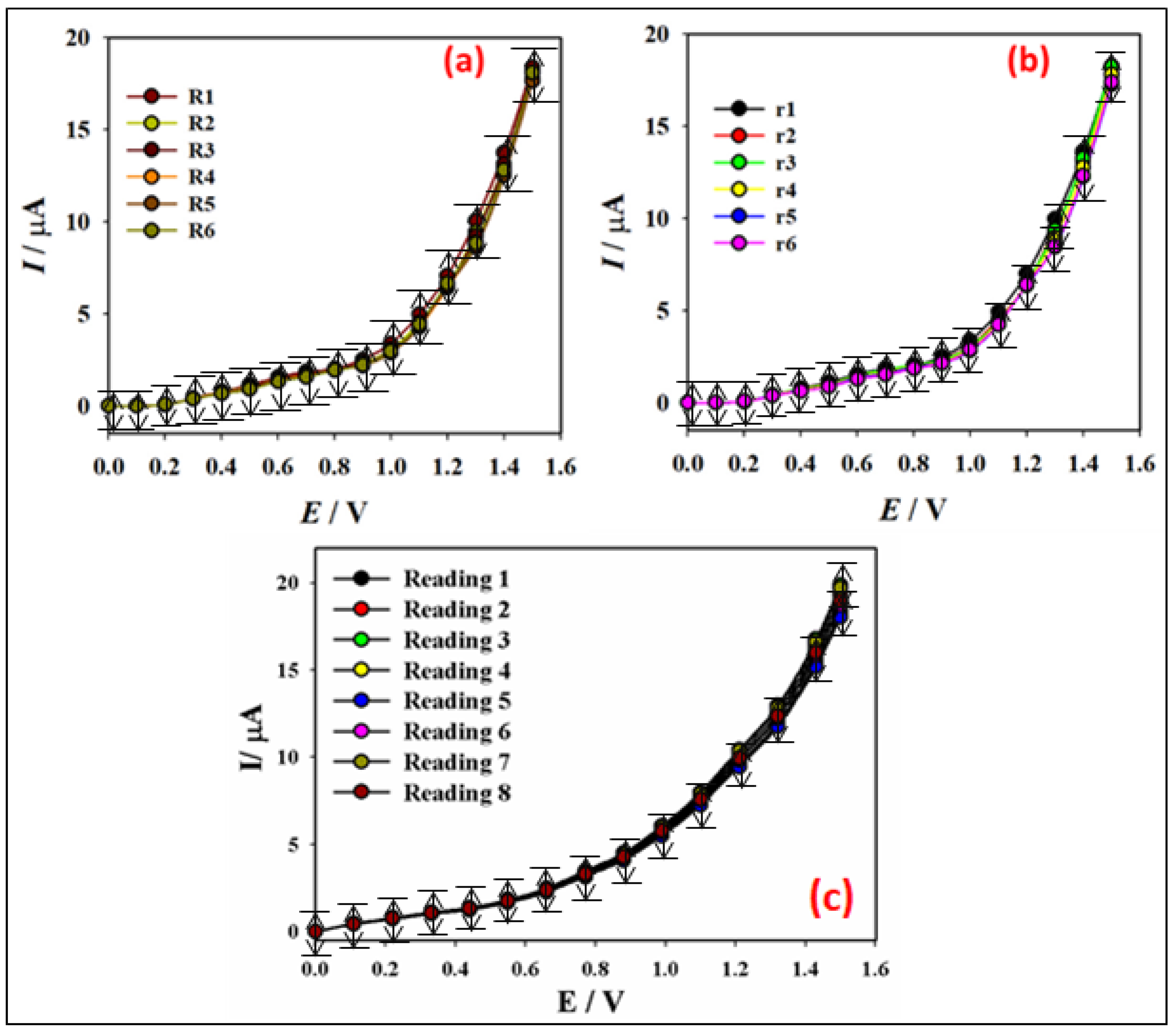
| Real Sample | Hg2+ Ion Conc. a Added | Hg2+ Ion Conc. a Deter b by the PNMPy/Nano-Stannous(II)WO3/GCE | Recovery b (%) | RSD c (%) (n = 3) |
|---|---|---|---|---|
| S1 | 1.000 nM | 1.015 nM | 101.5 | 3.4 |
| S1 | 1.000 µM | 1.056 µM | 105.6 | 3.1 |
| S1 | 1.000 mM | 1.021 mM | 102.1 | 2.8 |
| S2 | 1.000 nM | 0.983 nM | 98.3 | 3.3 |
| S2 | 1.000 µM | 1.001 µM | 100.1 | 3.8 |
| S2 | 1.000 mM | 0.998 mM | 99.8 | 2.9 |
| Electrode | Methods | Detection Limit | Linear Range | References |
|---|---|---|---|---|
| Graphene quantum dot using a thiourea-based imprinted polymer | CV | 30.2 nM | 5 × 10(−8) M to 2.3 × 10(−5) M | [41] |
| Sepiolite/FeS2 nanocomposite | SWASV | 4.12 nM | 10–120 nM | [42] |
| AuNP-biopolymer carbon screen-printed | SWASV | 1.69 ppb | 50–500 nM | [43] |
| SN-rGO modified GCE | SWASV | 8.93 nM | 0.6–1.7 μM | [44] |
| Thiosemicarbazone ligands bearing azido groups | ASV | 0.5 nM | 1 nM–0.8 μM | [45] |
| GO-chitosan-AuNPs/MTU modified ITO | DPV | 0.78 nM | 5.0–110.0 nM | [46] |
| PNMPy/nano-Stannous(II)WO3/GCE | I-V | 3.401 pM | 1.0 nM to 0.1 mM | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Khan, A.A.P.; Khan, I.; Asiri, A.M.; Rahman, M.M. Sol-Gel Synthesis and Characterization of Highly Selective Poly(N-methyl pyrrole) Stannous(II)Tungstate Nano Composite for Mercury (Hg(II)) Detection. Crystals 2022, 12, 371. https://doi.org/10.3390/cryst12030371
Khan A, Khan AAP, Khan I, Asiri AM, Rahman MM. Sol-Gel Synthesis and Characterization of Highly Selective Poly(N-methyl pyrrole) Stannous(II)Tungstate Nano Composite for Mercury (Hg(II)) Detection. Crystals. 2022; 12(3):371. https://doi.org/10.3390/cryst12030371
Chicago/Turabian StyleKhan, Anish, Aftab Aslam Parwaz Khan, Imran Khan, Abdullah M. Asiri, and Mohammed M. Rahman. 2022. "Sol-Gel Synthesis and Characterization of Highly Selective Poly(N-methyl pyrrole) Stannous(II)Tungstate Nano Composite for Mercury (Hg(II)) Detection" Crystals 12, no. 3: 371. https://doi.org/10.3390/cryst12030371
APA StyleKhan, A., Khan, A. A. P., Khan, I., Asiri, A. M., & Rahman, M. M. (2022). Sol-Gel Synthesis and Characterization of Highly Selective Poly(N-methyl pyrrole) Stannous(II)Tungstate Nano Composite for Mercury (Hg(II)) Detection. Crystals, 12(3), 371. https://doi.org/10.3390/cryst12030371









