Glycols in the Synthesis of Zinc-Anilato Coordination Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Methods
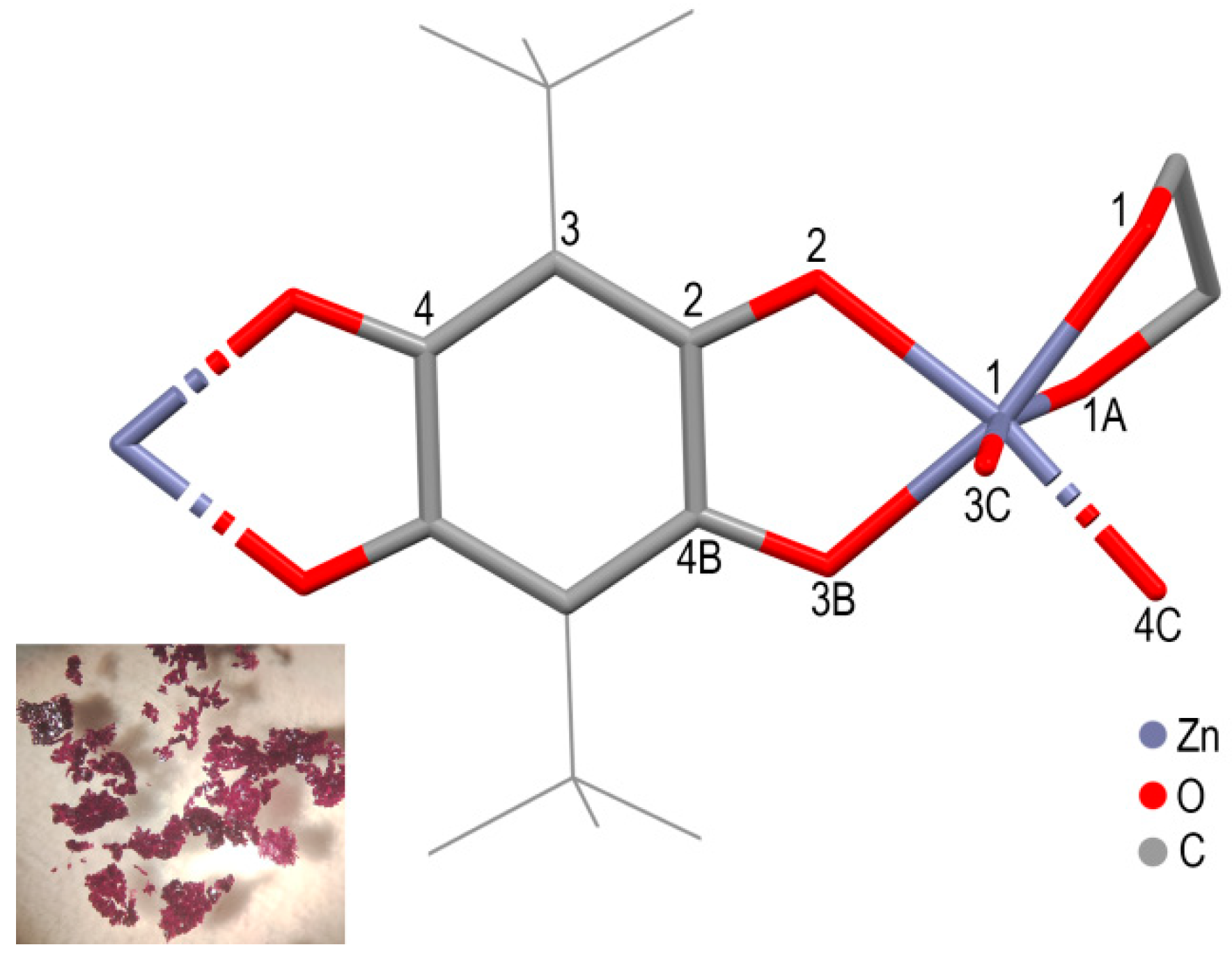
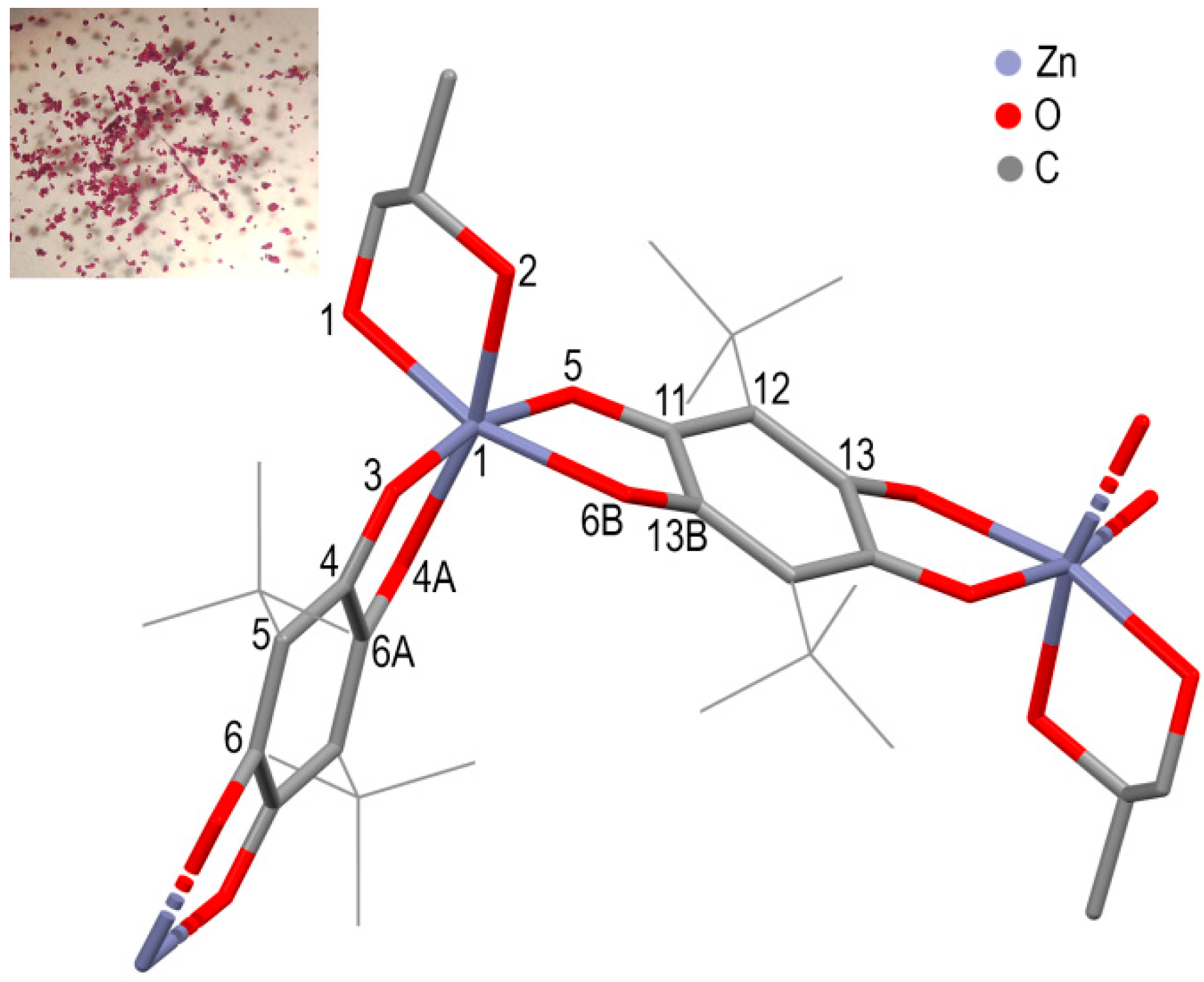
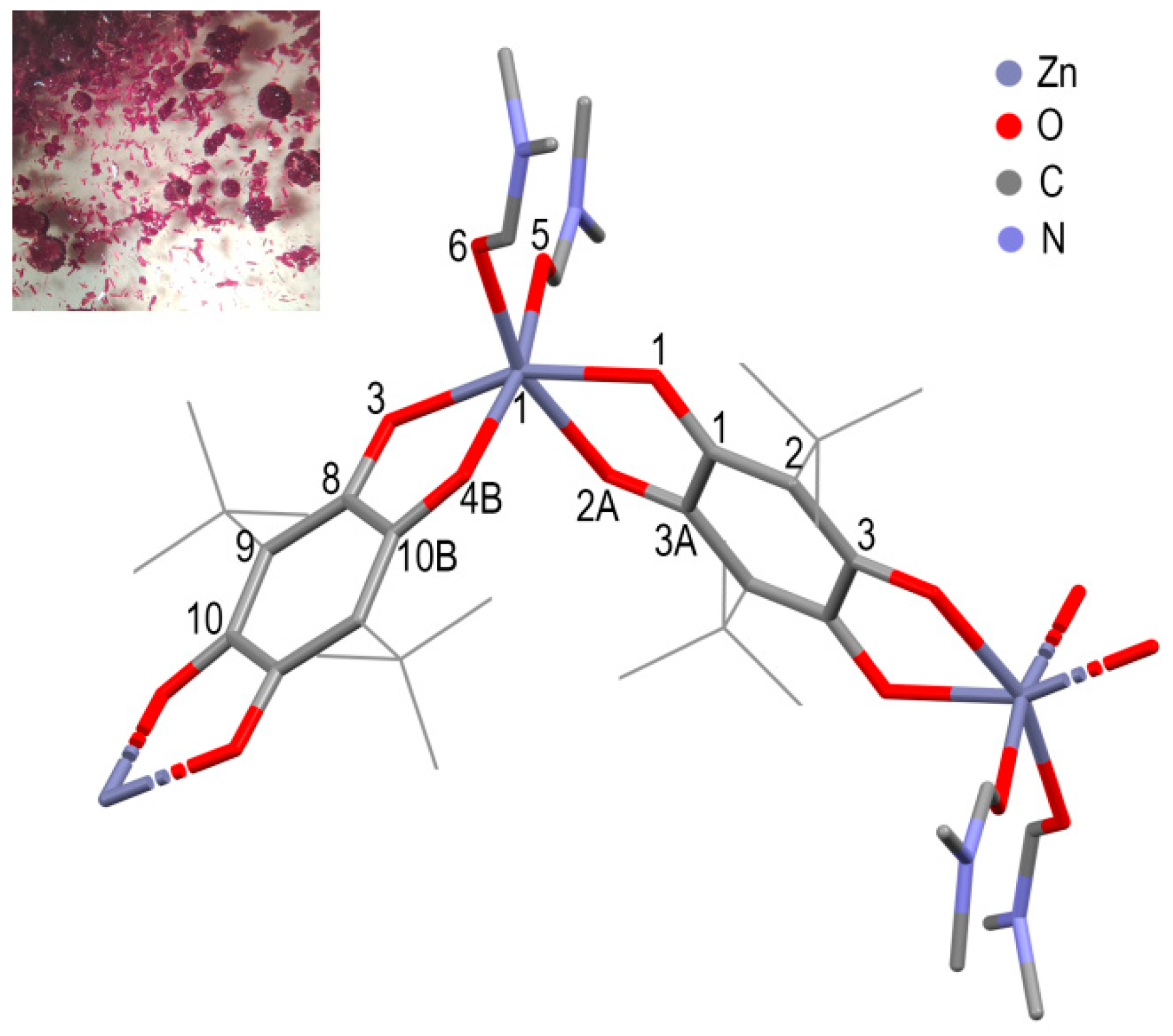
2.2. Synthesis of [Zn(pQ)EG]·2DMF (1), [Zn(pQ)1,2-PG]·2DMF (2) and [Zn(pQ)(DMF)2] (3)
2.3. Single-Crystal X-ray Diffraction Studies
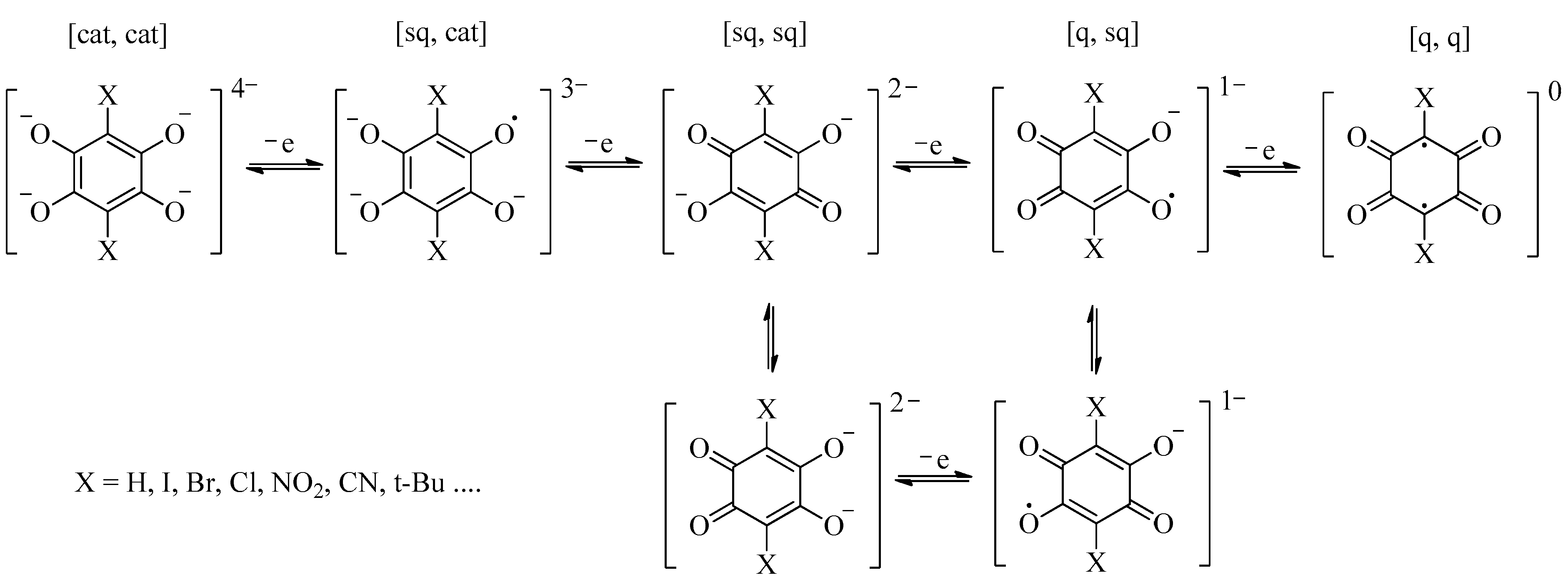
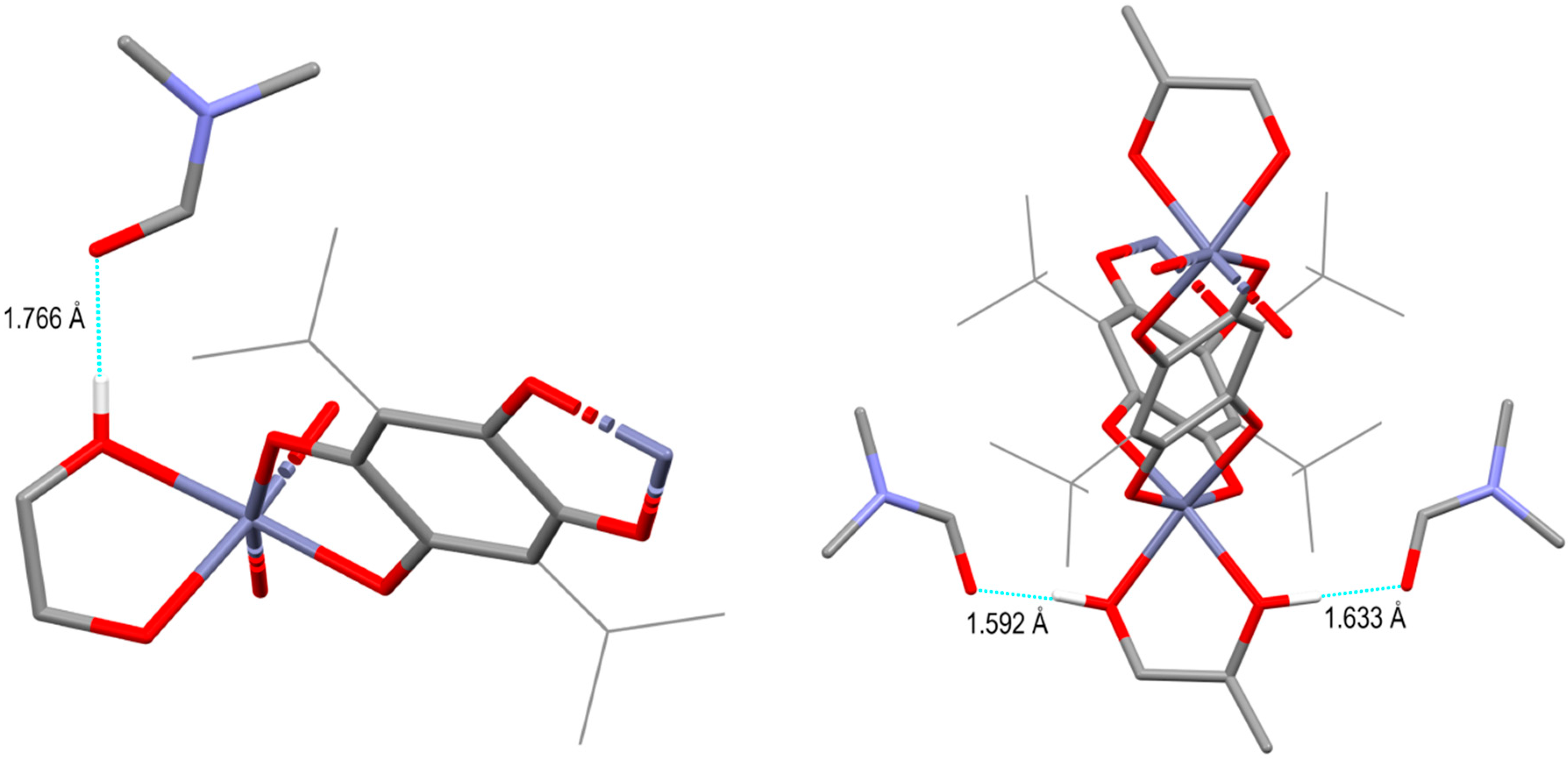
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agafonov, M.A.; Alexandrov, E.V.; Artyukhova, N.A.; Bekmukhamedov, G.E.; Blatov, V.A.; Butova, V.V.; Gayfulin, Y.M.; Gharibyan, A.A.; Gafurov, Z.N. Metal-organic coordination polymers in Russia: From synthesis and structure to functional properties and materials. J. Struct. Chem. 2022. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Abrahams, B.F.; Auckett, J.E.; Chevreau, H.; Dharma, A.D.; Duyker, S.; He, Q.; Hua, C.; Hudson, T.A.; Murray, K.S.; et al. Square Grid Metal–Chloranilate Networks as Robust HostSystems for GuestSorption. Chem. Eur. J. 2019, 25, 5222–5234. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Dharma, A.D.; Dyett, B.; Hudson, T.A.; Maynard-Casely, H.; Kingsbury, C.J.; McCormick, L.J.; Robson, R.; Sutton, A.L.; White, K.F. An indirect generation of 1D M(II)-2,5-dihydroxybenzoquinone coordination polymers, their structural rearrangements and generation of materials with a high affinity for H2, CO2 and CH4. Dalton Trans. 2016, 45, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Monni, N.; Andres-Garcia, E.; Caamaño, K.; García-López, V.; Clemente-Juan, J.M.; Giménez-Marqués, M.; Oggianu, M.; Cadoni, E.; Espallargas, G.M.; Clemente-León, M.; et al. A thermally/chemically robust and easily regenerable anilatobased ultramicroporous 3D MOF for CO2 uptake and separation. J. Mater. Chem. A 2021, 9, 25189–25195. [Google Scholar] [CrossRef]
- Fasna, P.H.F.; Sasi, S. A Comprehensive Overview on Advanced Sensing Applications of Functional Metal Organic Frameworks (MOFs). ChemistrySelect 2021, 6, 6365–6379. [Google Scholar] [CrossRef]
- Huangfu, M.; Wang, M.; Lin, C.; Wang, J.; Wu, P. Luminescent metal–organic frameworks as chemical sensors based on “mechanism–response”: A review. Dalton Trans. 2021, 50, 3429–3449. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, Z.; Zhao, Y.S.; Yan, Y. Recent advances in luminescent metal-organic frameworks and their photonic applications. Chem. Comm. 2021, 57, 13678–13691. [Google Scholar] [CrossRef] [PubMed]
- Ezugwu, C.I.; Liu, S.; Li, C.; Zhuiykov, S.; Roy, S.; Verpoort, F. Engineering metal-organic frameworks for efficient photocatalytic conversion of CO2 into solar fuels. Coord. Chem. Rev. 2021, 450, 214245. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Wang, L.Y.; Xing, Y.H.; Bai, F.Y.; Shi, Z. Construction of crystalline cadmium complex based on 1,4,5,8-naphthalene diimide derivative and photocatalytic degradation about organic dyes. Appl. Organomet. Chem. 2022, e6603, 1–13. [Google Scholar] [CrossRef]
- Wang, M.; Dong, R.; Feng, X. Two-dimensional conjugated metal–organic frameworks (2D c-MOFs): Chemistry and function for MOFtronics. Chem. Soc. Rev. 2021, 50, 2764–2793. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Feng, X. Making large single crystals of 2D MOFs. Nat. Mater. 2021, 20, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Monni, N.; Oggianu, M.; Sahadevan, S.A.; Mercuri, M.L. Redox Activity as a Powerful Strategy to Tune Magnetic and/or Conducting Properties in Benzoquinone-Based Metal-Organic Frameworks. Magnetochemistry 2021, 7, 109. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Yu, H.; Wang, L.; Liu, X.; Lin, T.; Haq, F.; Vatsadze, S.Z.; Lemenovskiy, D.A. Ferrocene-contained metal organic frameworks: From synthesis to applications. Coord. Chem. Rev. 2021, 430, 213737. [Google Scholar] [CrossRef]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- D’Alessandro, D.M. Exploiting redox activity in metal–organic frameworks: Concepts, trends and perspectives. Chem. Comm. 2016, 52, 8957–8971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Chang, C.-H.; Li, A.-C.; Popovs, I.; Kaveevivitchai, W.; Chen, J.-L.; Chou, K.-C.; Kuof, T.-S.; Chen, T.-H. Elucidating metal and ligand redox activities of a copper-benzoquinoid coordination polymer as the cathode for lithium-ion batteries. J. Mater. Chem. A 2019, 7, 23770–23774. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Bond, A.M.; Le, T.H.; McCormick, L.J.; Nafady, A.; Robson, R.; Vo, N. Voltammetric reduction and re-oxidation of solid coordination polymers of dihydroxybenzoquinone. Chem. Comm. 2012, 48, 11422–11424. [Google Scholar] [CrossRef] [PubMed]
- DeGayner, J.A.; Jeon, I.-R.; Sun, L.; Dinca, M.; Harris, T.D. 2D Conductive Iron-Quinoid Magnets Ordering up to T= 105 K via Heterogenous Redox Chemistry. J. Am. Chem. Soc. 2017, 139, 4175–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monni, N.; Angotzi, M.S.; Oggianu, A.M.; Sahadevan, S.A.; Mercuri, M.L. Redox-active benzoquinones as challenging ‘‘non-innocent’’ linkers to construct 2D frameworks and nanostructures with tunable physical properties. J. Mater. Chem. C 2022, 10, 1548–1572. [Google Scholar] [CrossRef]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Milašinović, V.; Molčanov, K. Nitranilic acid as a basis for construction of coordination polymers: From discrete monomers to 3D networks. CrystEngComm 2019, 21, 2962–2969. [Google Scholar] [CrossRef]
- Milašinović, V.; Jurić, M.; Molčanov, K. Nitrochloranilic acid: A novel asymmetrically substituted quinoid bridging ligand for design of coordination polymers. CrystEngComm 2021, 23, 2304–2315. [Google Scholar] [CrossRef]
- Khamaletdinova, N.M.; Meshcheryakova, I.N.; Piskunov, A.V.; Kuznetsova, O.V. Experimental and theoretical study of the vibrational spectra of tin(IV) complexes based on 2-hydroxy-3,6-di-tert-butyl-para-benzoquianone. J. Struct. Chem. 2015, 56, 233–242. [Google Scholar] [CrossRef]
- Kharitonov, A.D.; Trofimova, O.Y.; Meshcheryakova, I.N.; Fukin, G.K.; Khrizanforov, M.N.; Budnikova, Y.H.; Bogomyakov, A.S.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. 2D-Metal-organic coordination polymers of lanthanides (La(III), Pr(III) and Nd(III)) with redox-active dioxolene bridging ligand. CrystEngComm 2020, 22, 4675–4679. [Google Scholar] [CrossRef]
- Trofimova, O.Y.; Maleeva, A.V.; Ershova, I.V.; Cherkasov, A.V.; Fukin, G.K.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. Heteroleptic LaIII Anilate/Dicarboxylate Based Neutral 3D-Coordination Polymers. Molecules 2021, 26, 2486. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, O.Y.; Ershova, I.V.; Maleeva, A.V.; Yakushev, I.A.; Dorovatovskii, P.V.; Aisin, R.R. Metal–organic frameworks of magnesium based on 2,5-dihydroxy-3,6-di-tert-butyl-para-benzoquinone. Russ. J. Coord. Chem. 2021, 47, 610–619. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Poddel’sky, A.I.; Fukin, G.K.; Smolyaninov, I.V. Triphenylantimony(v) catecholates based on 3,6-di-tert-butyl-2,5-dihydroxy-1,4-benzoquinone. Russ. J. Coord. Chem. 2020, 46, 386–393. [Google Scholar] [CrossRef]
- Min, K.S.; DiPasquale, A.G.; Rheingold, A.L.; White, H.S.; Miller, J.S. Observation of Redox-Induced Electron Transfer and Spin Crossover for Dinuclear Cobalt and Iron Complexes with the 2,5-Di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate Bridging Ligand. J. Am. Chem. Soc. 2009, 131, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; DiPasquale, A.; Rheingold, A.L.; Miller, J.S. Room-Temperature Spin Crossover Observed for [(TPyA)FeII(DBQ2-)FeII(TPyA)]2+ [TPyA=Tris(2-pyridylmethyl)amine; DBQ2-=2,5-Di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate]. Inorg. Chem. Commun. 2007, 46, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, S.A.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting YbIII/Anilate-Based Neutral Coordination Polymer Nanosheets for Solvent Sensing. ACS Appl. Nano Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Sahadevan, S.A.; Manna, F.; Abhervé, A.; Oggianu, M.; Monni, N.; Mameli, V.; Marongiu, D.; Quochi, F.; Gendron, F.; Guennic, B.L.; et al. Combined Experimental/Theoretical Study on the Luminescent Properties of Homoleptic/Heteroleptic Erbium(III) Anilate-Based 2D Coordination Polymers. Inorg. Chem. 2021, 60, 17765–17774. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal−Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Elsevier: Oxford, UK, 1980. [Google Scholar]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA diffraction beamline for studying crystalline samples at Kurchatov synchrotron radiation source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- APEX3, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Sheldrick, G.M. TWINABS, version 2012/1; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Kabsch, W. XDS. Acta Crystallogr. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space group and crystal structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Li, L.; Ma, C.; Shen, Z.; Song, Y.; You, X. Structures and Properties of Porous Coordination Polymers Based on Lanthanide Carboxylate Building Units. Inorg. Chem. 2010, 49, 10781–10787. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; Ziebel, M.E.; Harris, T.D. Metal−Diamidobenzoquinone Frameworks via Post-Synthetic Linker Exchange. J. Am. Chem. Soc. 2020, 142, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; DeGayner, J.A.; Winegar, P.H.; Fang, Y.; Harris, T.D. Harnessing Structural Dynamics in a 2D Manganese−Benzoquinoid Framework To Dramatically Accelerate Metal Transport in Diffusion-Limited Metal Exchange Reactions. J. Am. Chem. Soc. 2018, 140, 11444–11453. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hudson, T.A.; McCormick, L.J.; Robson, R. Coordination Polymers of 2,5-Dihydroxybenzoquinone and Chloranilic Acid with the (10,3)-a Topology. Cryst. Growth Des. 2011, 11, 2717–2720. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Lu, K.D.; Moubaraki, B.; Murray, K.S.; Robson, R. X-Ray diffraction and magnetic studies on a series of isostructural divalent metal chloranilates with zigzag polymeric chain structures and on a dinuclear iron(III) chloranilate. Dalton Trans. 2000, 1793–1797. [Google Scholar] [CrossRef]
- Luo, T.-T.; Liu, Y.-H.; Tsai, H.-L.; Su, C.-C.; Ueng, C.-H.; Lu, K.-L. A Novel Hybrid Supramolecular Network Assembled from Perfect π-π Stacking of an Anionic Inorganic Layer and a Cationic Hydronium-Ion- Mediated Organic Layer. Eur. J. Inorg. Chem. 2004, 2004, 4253–4258. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Abrahams, B.F.; D’Alessandro, D.M.; Hudson, T.A.; Murase, R.; Robson, R.; White, K.F. Role of NEt4+ in Orienting and Locking Together [M2lig3]2− (6,3) Sheets (H2lig = Chloranilic or Fluoranilic Acid) to Generate Spacious Channels Perpendicular to the Sheets. Cryst. Growth Des. 2017, 17, 1465–1470. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Negru, B.; Duyne, R.P.V.; Harris, D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef] [PubMed]
- Murase, R.; Abrahams, B.F.; D’Alessandro, D.M.; Davies, C.G.; Hudson, T.A.; Jameson, G.N.L.; Moubaraki, B.; Murray, K.S.; Robson, R.; Sutton, A.L. Mixed Valency in a 3D Semiconducting Iron−Fluoranilate Coordination Polymer. Inorg. Chem. 2017, 56, 9025–9035. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S.S. The atomic radii of the elements. Russ. J. Inorg. Chem. 1991, 36, 1694–1705. [Google Scholar]
- Song, T.-Q.; Dong, J.; Gao, H.-L.; Cui, J.-Z. Three coordination polymers based on M2 (M = Co, Ni and Zn) clusters: Structures, magnetic and fluorescent properties. Inorg. Chim. Acta 2017, 466, 393–397. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Kovalenko, K.A.; Samsonenko, D.G.; Vinogradov, A.S.; Karpov, V.M.; Platonovc, V.E.; Fedin, V.P. Structural diversity of zinc(II) coordination polymers with octafluorobiphenyl-4,4′-dicarboxylate based on mononuclear, paddle wheel and cuboidal units. CrystEngComm 2019, 21, 2524–2533. [Google Scholar] [CrossRef]
- Teichert, J.; Ruck, M. Influence of Common Anions on the Coordination of Metal Cations in Polyalcohols. Eur. J. Inorg. Chem. 2019, 2019, 2267–2276. [Google Scholar] [CrossRef]



| Coordiantion Polymer | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C22H38N2O8Zn | C23H40N2O8Zn | C20H32N2O6Zn |
| Formula weight | 523.91 | 537.94 | 461.84 |
| Temperature (K) | 100(2) | 100(2) | 150(2) |
| Radiation source | microfocus sealed X-ray tube | synchrotron | microfocus sealed X-ray tube |
| Wavelength (Å) | 0.71073 | 0.74500 | 0.71073 |
| Crystal system | Monoclinic | Triclinic | Triclinic |
| Space group | C2/c | P-1 | P-1 |
| a, Å | 19.673(3) | 11.2898(11) | 10.3884(5) |
| b, Å | 11.3396(16) | 11.3959(8) | 11.0512(6) |
| c, Å | 12.9104(18) | 13.0514(7) | 12.1460(6) |
| α, ° | 90 | 71.214(3) | 69.142(2) |
| β, ° | 114.503(5) | 66.912(7) | 85.268(2) |
| γ, ° | 90 | 61.861(7) | 63.532(2) |
| V, A3 | 2620.7(7) | 1342.5(2) | 1161.60(10) |
| Z | 4 | 2 | 2 |
| ρ, g/cm3 | 1.328 | 1.331 | 1.320 |
| θ range, ° | 2.126 to 30.289 | 1.804 to 31.064 | 2.199 to 26.768 |
| Crystal size, mm | 0.360 × 0.090 × 0.030 | 0.130 × 0.090 × 0.050 | 0.095 × 0.030 × 0.013 |
| μ, mm−1 | 0.982 | 1.082 | 1.092 |
| Reflections | |||
| collected/unique | 19122/3872 | 51762/51762 | 9856/4943 |
| No. of restraints | 16 | 1 | 0 |
| No. of parameters | 178 | 325 | 272 |
| Rint | 0.0867 | - | 0.0638 |
| GOF on F2 | 1.053 | 1.027 | 1.007 |
| R1, wR2 [I > 2σ(I)] | 0.0524, 0.1098 | 0.0541, 0.1439 | 0.0505, 0.0937 |
| R1, wR2 (all data) | 0.0791, 0.1189 | 0.0729, 0.1571 | 0.0913, 0.1047 |
| Δρmax/Δρmin, e/Å3 | 0.637/−0.672 | 0.996/−1.315 | 0.376/−0.534 |
| Bond 1 | 1 | Bond 1 | 2 | Bond 1 | 3 |
|---|---|---|---|---|---|
| Zn(1)–O(1) | 2.1390(19) | Zn(1)–O(1) | 2.133(2) | Zn(1)–O(1) | 2.068(2) |
| Zn(1)–O(2) | 2.0481(17) | Zn(1)–O(2) | 2.147(2) | Zn(1)–O(2A) | 2.104(2) |
| Zn(1)–O(3B) | 2.0689(18) | Zn(1)–O(3) | 2.0411(19) | Zn(1)–O(3) | 2.045(2) |
| O(2)–C(2) | 1.263(3) | Zn(1)–O(4A) | 2.080(2) | Zn(1)–O(4B) | 2.072(2) |
| O(3B)–C(4B) | 1.265(3) | Zn(1)–O(5) | 2.0502(19) | Zn(1)–O(5) | 2.075(2) |
| C(2)–C(3) | 1.397(3) | Zn(1)–O(6B) | 2.067(2) | Zn(1)–O(6) | 2.089(2) |
| C(3)–C(4) | 1.548(3) | O(3)–C(4) | 1.267(3) | O(1)–C(1) | 1.266(3) |
| C(2)–C(4) | 1.396(3) | O(4A)–C(6A) | 1.269(3) | O(2A)–C(3A) | 1.265(3) |
| O(5)–C(11) | 1.260(3) | O(3)–C(8) | 1.276(3) | ||
| O(6B)–C(13B) | 1.265(3) | O(4B)–C(10B) | 1.262(3) | ||
| C(4)–C(5) | 1.401(3) | C(1)–C(2) | 1.402(4) | ||
| C(4)–C(6A) | 1.545(4) | C(1)–C(3A) | 1.546(4) | ||
| C(5)–C(6) | 1.405(4) | C(2)–C(3) | 1.398(4) | ||
| C(11)–C(12) | 1.401(3) | C(8)–C(9) | 1.399(4) | ||
| C(11)–C(13B) | 1.550(4) | C(8)–C(10B) | 1.547(4) | ||
| C(12)–C(13) | 1.394(4) | C(9)–C(10) | 1.403(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimova, O.Y.; Maleeva, A.V.; Arsenyeva, K.V.; Klimashevskaya, A.V.; Yakushev, I.A.; Piskunov, A.V. Glycols in the Synthesis of Zinc-Anilato Coordination Polymers. Crystals 2022, 12, 370. https://doi.org/10.3390/cryst12030370
Trofimova OY, Maleeva AV, Arsenyeva KV, Klimashevskaya AV, Yakushev IA, Piskunov AV. Glycols in the Synthesis of Zinc-Anilato Coordination Polymers. Crystals. 2022; 12(3):370. https://doi.org/10.3390/cryst12030370
Chicago/Turabian StyleTrofimova, Olesya Y., Arina V. Maleeva, Kseniya V. Arsenyeva, Anastasiya V. Klimashevskaya, Il’ya A. Yakushev, and Alexandr V. Piskunov. 2022. "Glycols in the Synthesis of Zinc-Anilato Coordination Polymers" Crystals 12, no. 3: 370. https://doi.org/10.3390/cryst12030370






