The Use of Fe-26Si-9B Alloy as Phase Change Material in Si3N4 Container
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Wettability Experimental Procedure
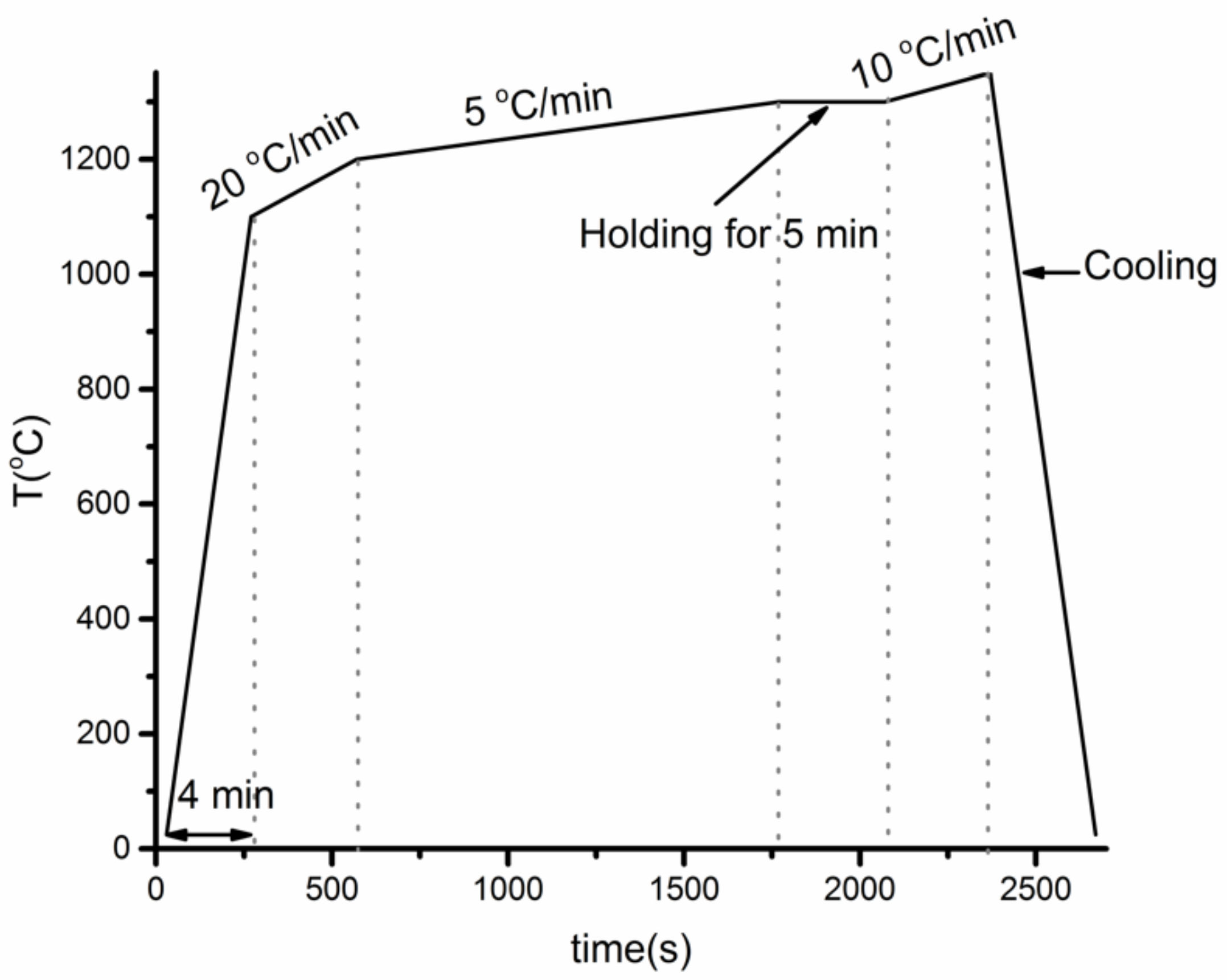
2.3. Thermal Cycle Experiments Procedure
2.4. Characterization Method
3. Results and Discussion
3.1. Wettability Property of Fe-26Si-9B Alloy on Si3N4 Substrate
3.2. Phase Stability
3.3. Interface of Fe-26Si-9B Alloy and Si3N4
4. Conclusions
- FeSi, FeB, SiB6, and FeSiB3 formed in Fe-26Si-9B alloy after thermal cycle experiments. FeB was in the form of large crystals. FeSi and FeSiB3 were in the form of eutectic structures.
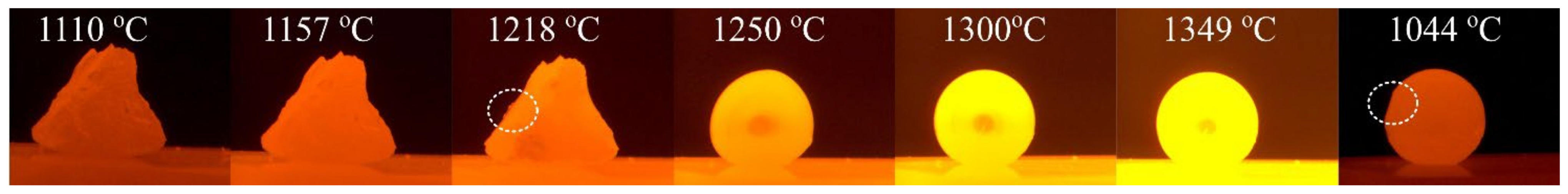
- Fe-26Si-9B alloy started to melt at ~1218 °C and became completely molten at ~1250 °C, indicating that Fe-26Si-9B alloy is not a eutectic alloy. The eutectic point was calculated to be about 61 mass % Fe, 29 mass % Si, and 10 mass % B (Fe-29Si-10B) in the Fe-Si-B system.
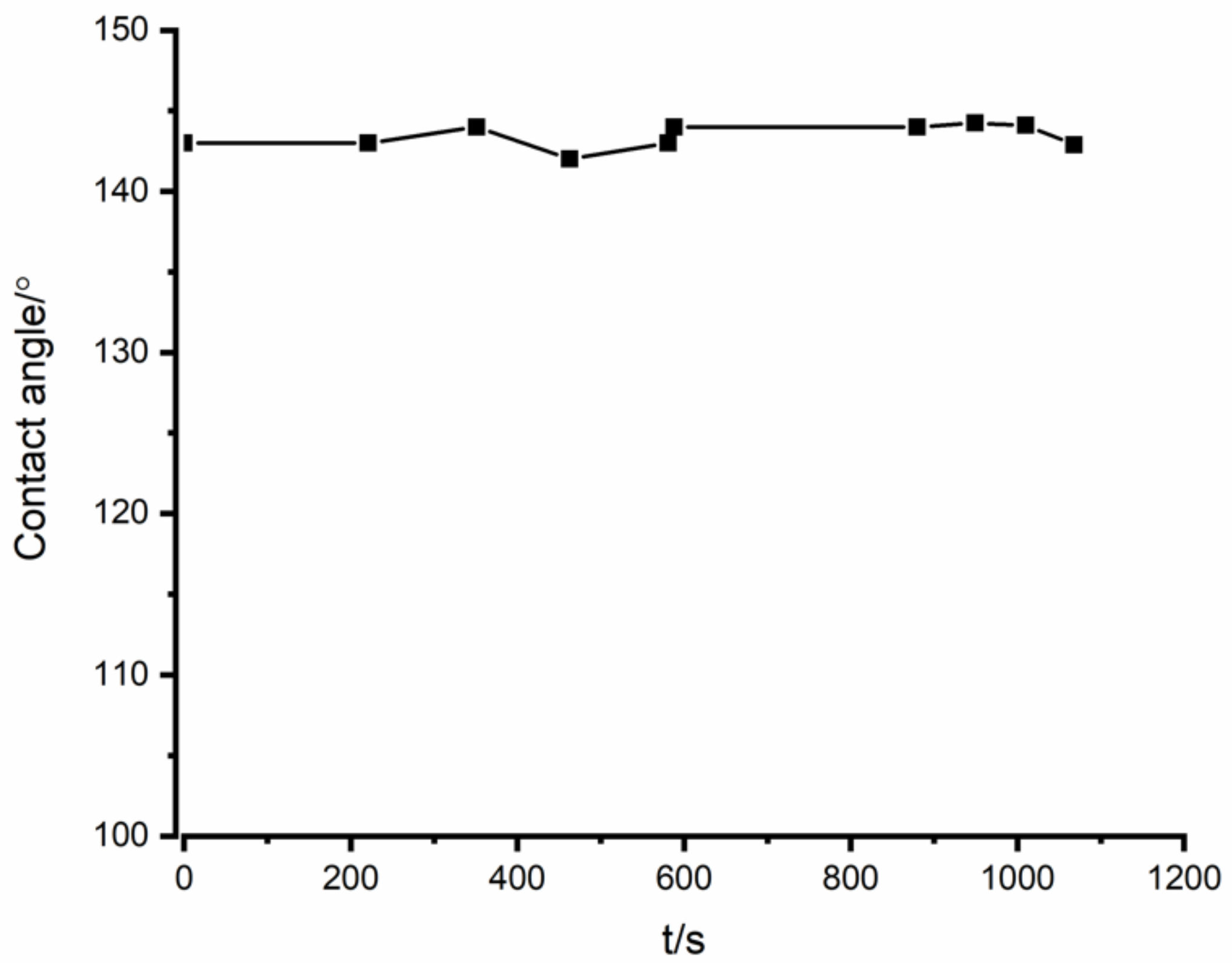
- In the interaction between Si3N4 and Fe-26Si-9B alloy, the equilibrium contact angle was measured to be ~143°.
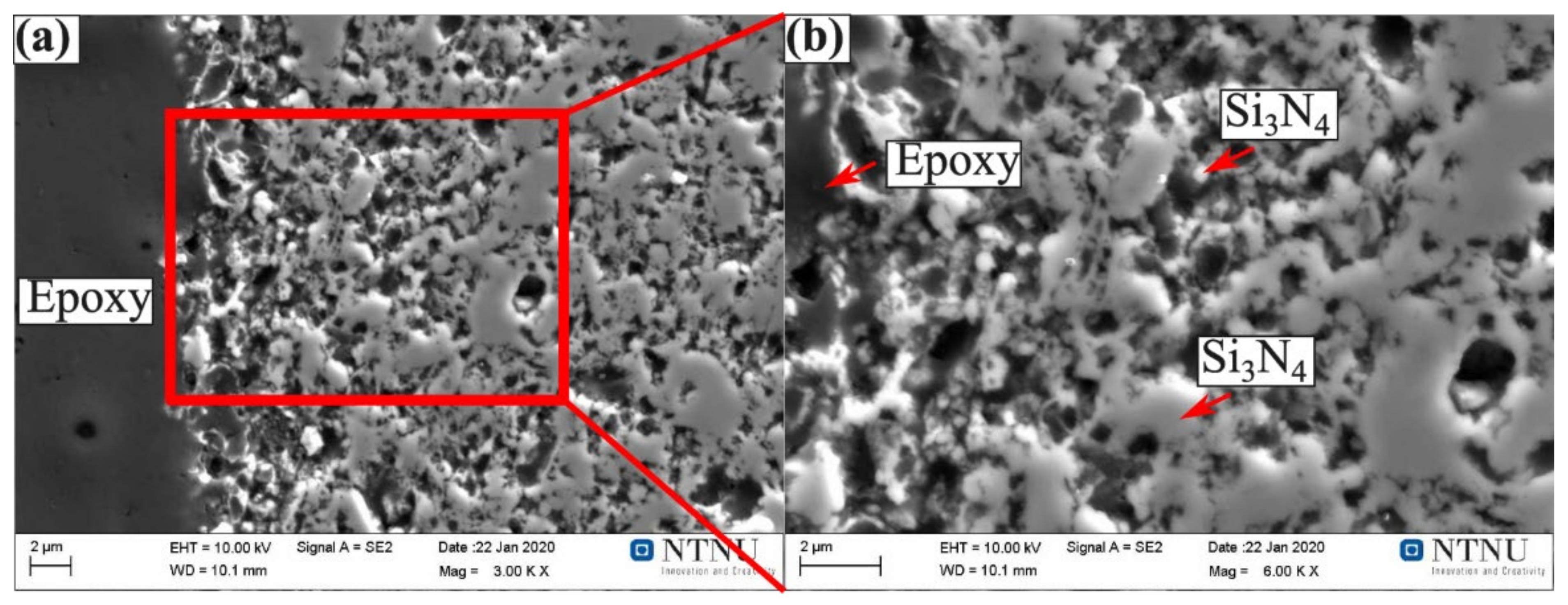
- No nitride phases were present in Fe-26Si-9B alloy or at their interface. This shows the possibility of using Si3N4 refractory material as an Fe-26Si-9B alloy container at high temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA Net Zero by 2050. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 10 February 2022).
- Kumar, N.; Banerjee, D. Phase change materials. In Handbook of Thermal Science and Engineering; Springer International Publishing: Cham, Switzerland, 2018; pp. 2213–2275. ISBN 9783319266954. [Google Scholar]
- Magomedov, A.M. Netraditsionnye Istochniki Energii (Non-Traditional Sources of Energy); Yupiter: Makhachkala, India, 1996. [Google Scholar]
- Jiao, J.; Grorud, B.; Sindland, C.; Safarian, J.; Tang, K.; Sellevoll, K.; Tangstad, M. The use of eutectic Fe-Si-B alloy as a phase change material in thermal energy storage systems. Materials 2019, 12, 2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellevoll, K. Interactions of Eutectic Fe-Si-B Alloy with Graphite Crucibles; Norwegian University of Science and Technology: Trondheim, Norway, 2018. [Google Scholar]
- Jayakumari, S. Making of Prototypes: Fe-Si, Fe-Si-B Alloys and SiC-Crucibles for Phase Change Materials (PCMs); Norwegian Univerisity of Science and Technology: Trondheim, Norway, 2019. [Google Scholar]
- Grorud, B. Interaction of Eutectic Fe-Si-B Alloy with Graphite Crucibles. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2018. [Google Scholar]
- Jiao, J.M. Si-Based Phase Change Materials in Thermal Energy Storage Systems; Norwegian University of Science and Technology (NTNU): Trondheim, Norway, 2020. [Google Scholar]
- Grorud, B. Interaction of Liquid Si-B Alloys with Graphite Crucibles; Norwegian University of Science and Technology (NTNU): Trondheim, Norway, 2017. [Google Scholar]
- Sellevoll, K. Interactions of FeSi Alloys with Graphite Crucibles. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2019. [Google Scholar]
- Datas, A.; Ramos, A.; Martí, A.; del Cañizo, C.; Luque, A. Ultra high temperature latent heat energy storage and thermophotovoltaic energy conversion. Energy 2016, 107, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Datas, A.; Cristobal, A.B.; Del Cañizo, C.; Antolín, E.; Beaughon, M.; Nikolopoulos, N.; Nikolopoulos, A.; Zeneli, M.; Sobczak, N.; Polkowski, W.; et al. AMADEUS: Next generation materials and solid state devices for ultra high temperature energy storage and conversion. AIP Conf. Proc. 2018, 2033, 170004. [Google Scholar]
- Gilpin, M.R. High Temperature Latent Heat Thermal Energy Storage to Augment Solar Thermal Propulsion for Microsatellites; Defense Technical Information Center: Fort Belvoir, VA, USA, 2015. [Google Scholar]
- Rhim, W.K.; Ohsaka, K. Thermophysical properties measurement of molten silicon by high-temperature electrostatic levitator: Density, volume expansion, specific heat capacity, emissivity, surface tension and viscosity. J. Cryst. Growth 2000, 208, 313–321. [Google Scholar] [CrossRef]
- Jiao, J.; Safarian, J.; Grorud, B.; Tangstad, M. High temperature interaction of Si-B alloys with graphite crucible in thermal energy storage systems. Materials 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Søiland, A.K.; Øvrelid, E.J.; Lohne, O.; Tuset, J.K.; Engh, T.A.; Gjerstad, Ø. Carbon and nitrogen contents and inclusion formation during crystallization of multi-crystalline silicon. In Proceedings of the 19th EUPVSEC, Paris, France, 7–11 June 2004; pp. 7–11. [Google Scholar]
- Mitsuru, T.; Toshiharu, F.; Yamauchi, C. Activity of Boron in Molten Silicon. Min. Mater. Process. Inst. Japan 1998, 114, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi1, M.; Iguchi, Y.; Narushima, T. Nitrogen Solubility in Liquid Silicon. Mater. Trans. JIM 1994, 35, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Morita, K. Thermodynamic Property of B in Molten Si and Phase Relations in the Si-Al-B System. Mater. Trans. 2005, 46, 1335–1340. [Google Scholar] [CrossRef] [Green Version]
- Yatsurugi, Y.; Akiyama, N.; Endo, Y.; Nozaki, T. Concentration, Solubility, and Equilibrium Distribution Coefficients of Nitrogen and Oxygen in Semiconductor Silicon. J. Electrochem. Soc. 1973, 120, 975–978. [Google Scholar] [CrossRef]
- Kaiser, W.; Thurmond, C.D. Nitrogen in silicon. J. Appl. Phys. 1959, 30, 427–431. [Google Scholar] [CrossRef]
- Ciftja, A.; Engh, T.A.; Tangstad, M. Wetting properties of molten silicon with graphite materials. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 3183–3195. [Google Scholar] [CrossRef]
- FactSage. Available online: http://www.factsage.com/fs_general.php (accessed on 1 March 2022).
- Gao, H.; Dong, B.S.; Zhong, J.; Li, Z.Z.; Xu, M.; Zhou, S.X. The influence of substrate and atmosphere on the properties of FeSiB(Cu,Nb) alloy melts. Sci. China Technol. Sci. 2016, 59, 1892–1898. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Aronsson, B.; Engström, I. X-Ray Investigations on M-Si-B Systems (M = Mn, Fe, Co). II. Some Features of the Fe-Si-B and Mn-Si-B Systems. Acta Chem. Scand. 1960, 14, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.M.; Tang, K.; Safarian, J.; Grorud, B.; Sellevoll, K.; Tangstad, M. High temperature interaction between Si–B alloys and Si3N4. Ceram. Int. 2021, 47, 13837–13844. [Google Scholar] [CrossRef]






| Sample | Si | Fe | B | Al | Mn |
|---|---|---|---|---|---|
| Fe-Si-B | 25.65 | 63.33 | 8.47 | 0.18 | 0.23 |
| Phase | Fe | Std. Dev. | Si | Std. Dev. | B | Std. Dev. |
|---|---|---|---|---|---|---|
| FeSi | 45.5 | ±1.2 | 43.2 | ±1.1 | 11.3 | ±1.7 |
| FeSiB3 | 22.0 | ±1.0 | 20.6 | ±1.4 | 57.3 | ±0.4 |
| FeB | 47.7 | ±0.9 | 0.23 | ±0.9 | 52.0 | ±0.1 |
| SiB6 | 0.31 | ±0.1 | 10.6 | ±0.2 | 89.3 | ±0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Safarian, J.; Tangstad, M. The Use of Fe-26Si-9B Alloy as Phase Change Material in Si3N4 Container. Crystals 2022, 12, 376. https://doi.org/10.3390/cryst12030376
Jiao J, Safarian J, Tangstad M. The Use of Fe-26Si-9B Alloy as Phase Change Material in Si3N4 Container. Crystals. 2022; 12(3):376. https://doi.org/10.3390/cryst12030376
Chicago/Turabian StyleJiao, Jianmeng, Jafar Safarian, and Merete Tangstad. 2022. "The Use of Fe-26Si-9B Alloy as Phase Change Material in Si3N4 Container" Crystals 12, no. 3: 376. https://doi.org/10.3390/cryst12030376
APA StyleJiao, J., Safarian, J., & Tangstad, M. (2022). The Use of Fe-26Si-9B Alloy as Phase Change Material in Si3N4 Container. Crystals, 12(3), 376. https://doi.org/10.3390/cryst12030376







