Preparation of Niobium Aluminium Alloy Based on Shock Compression Method
Abstract
:1. Introduction
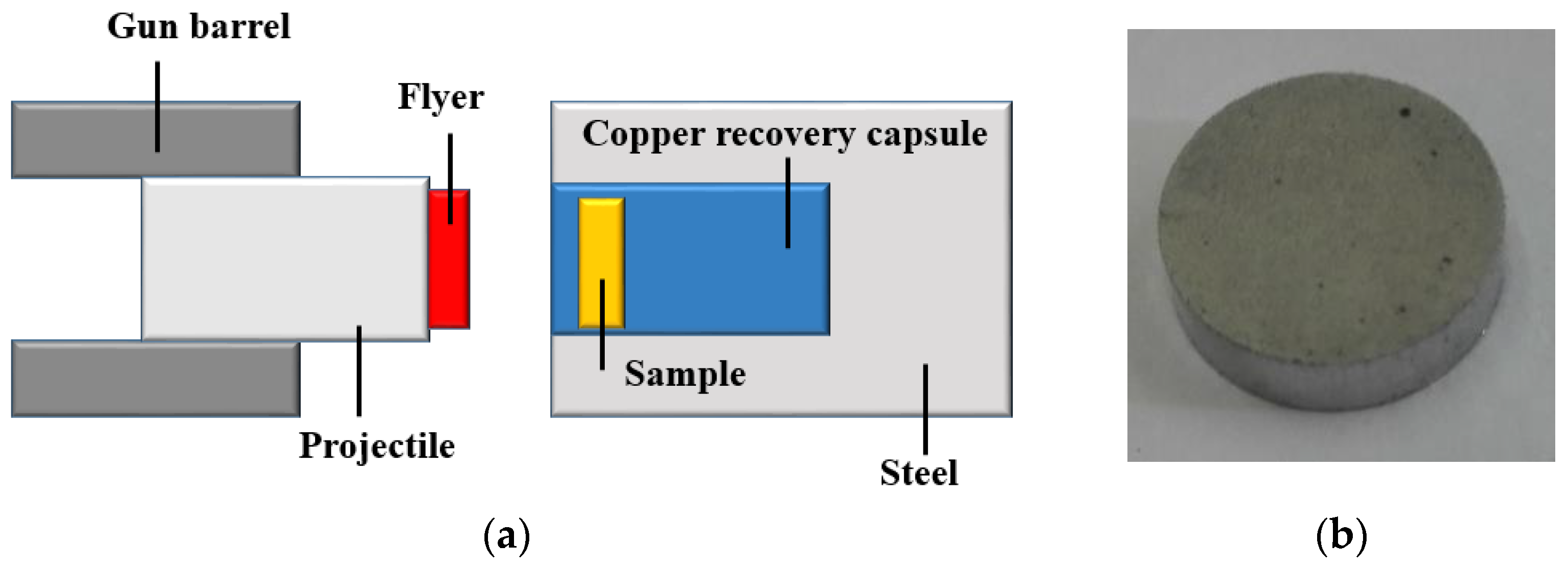
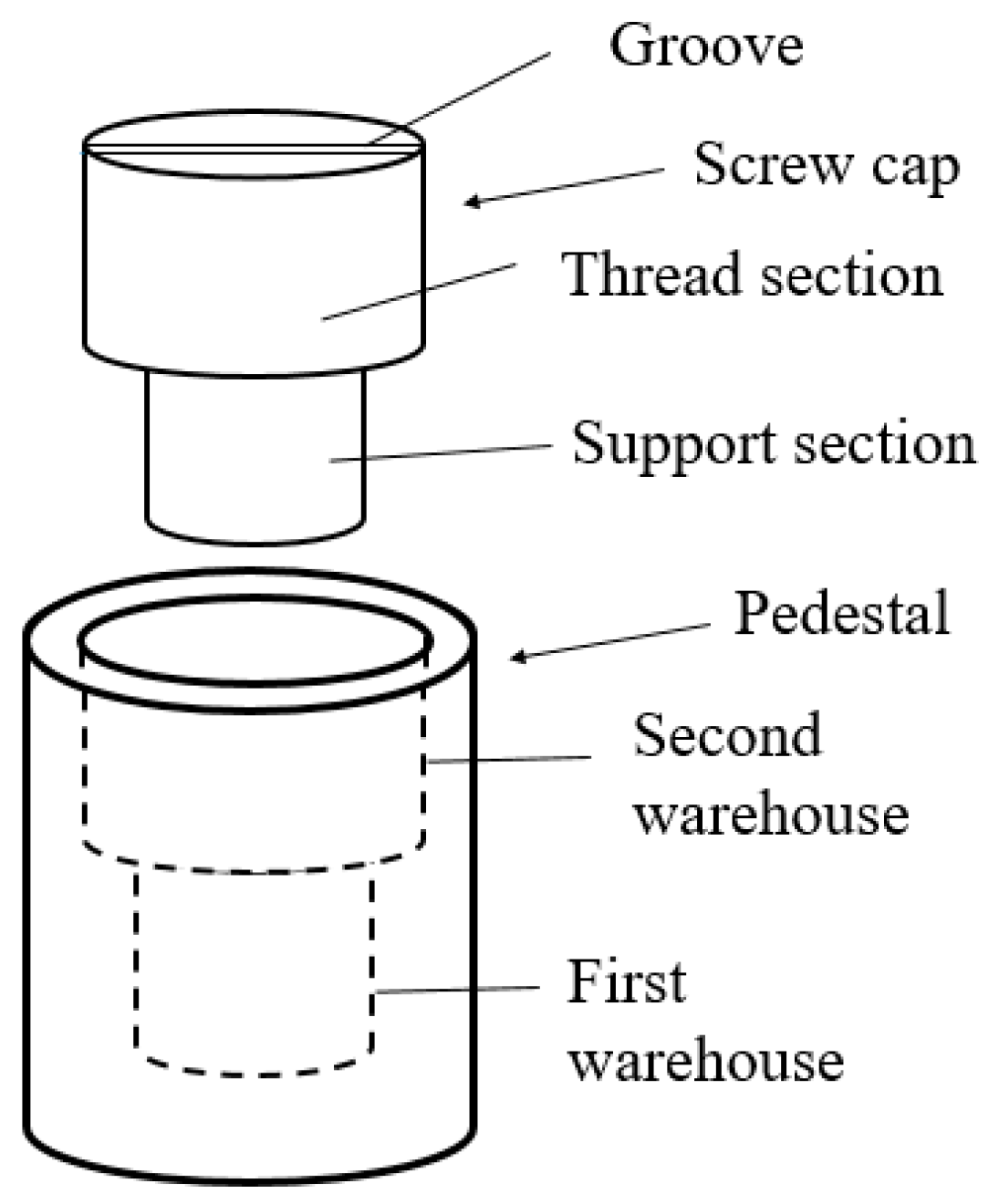
2. Experimental Methods
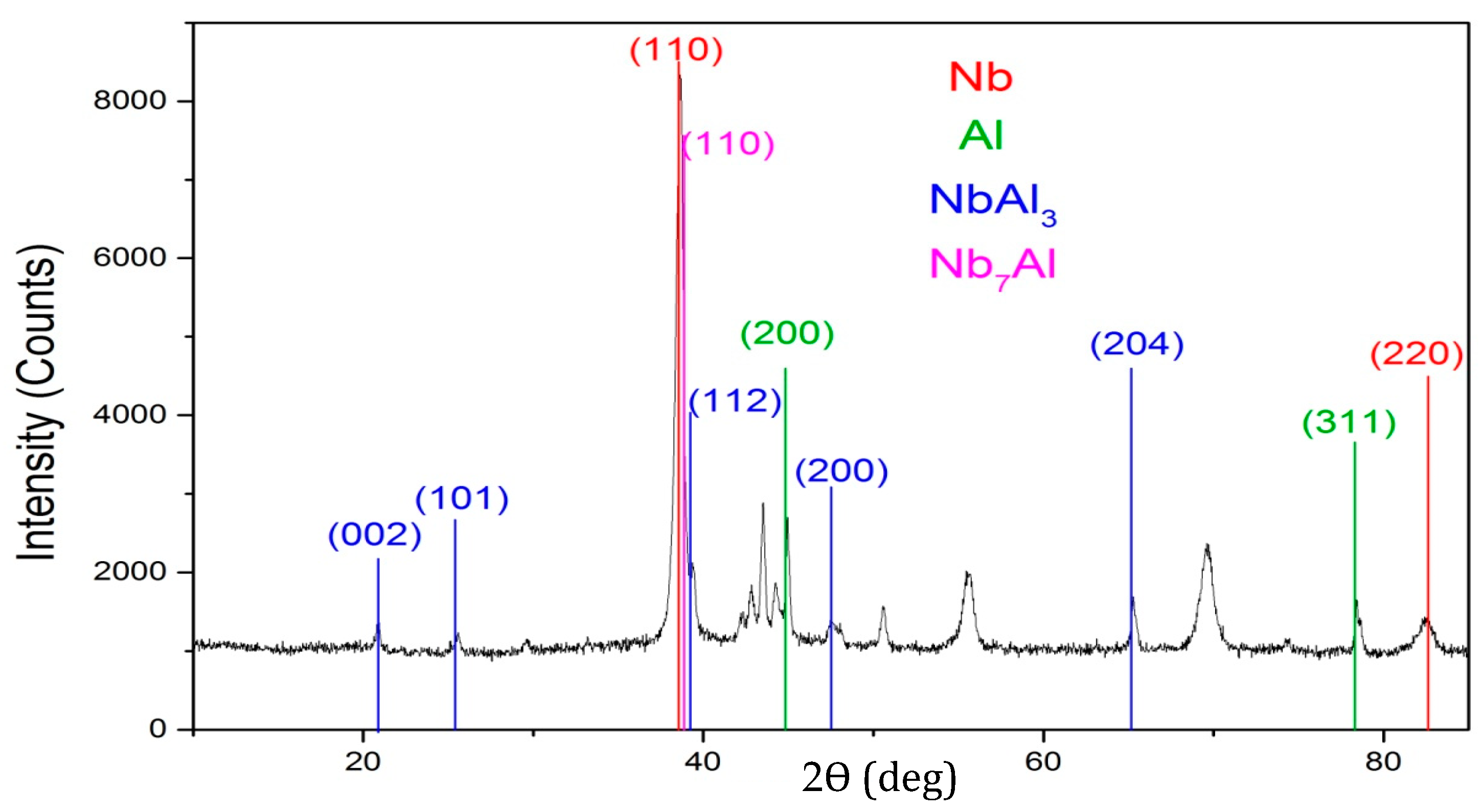
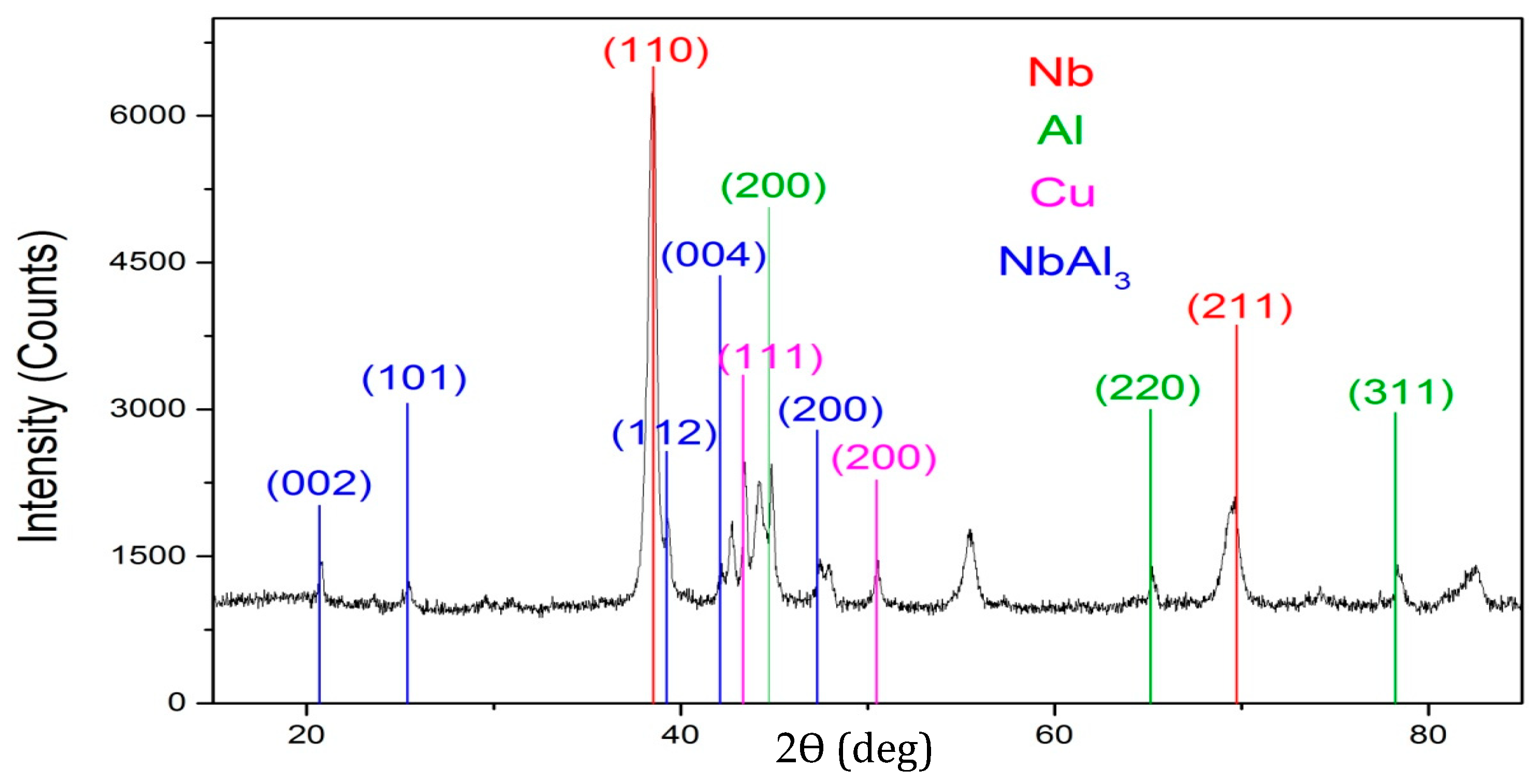
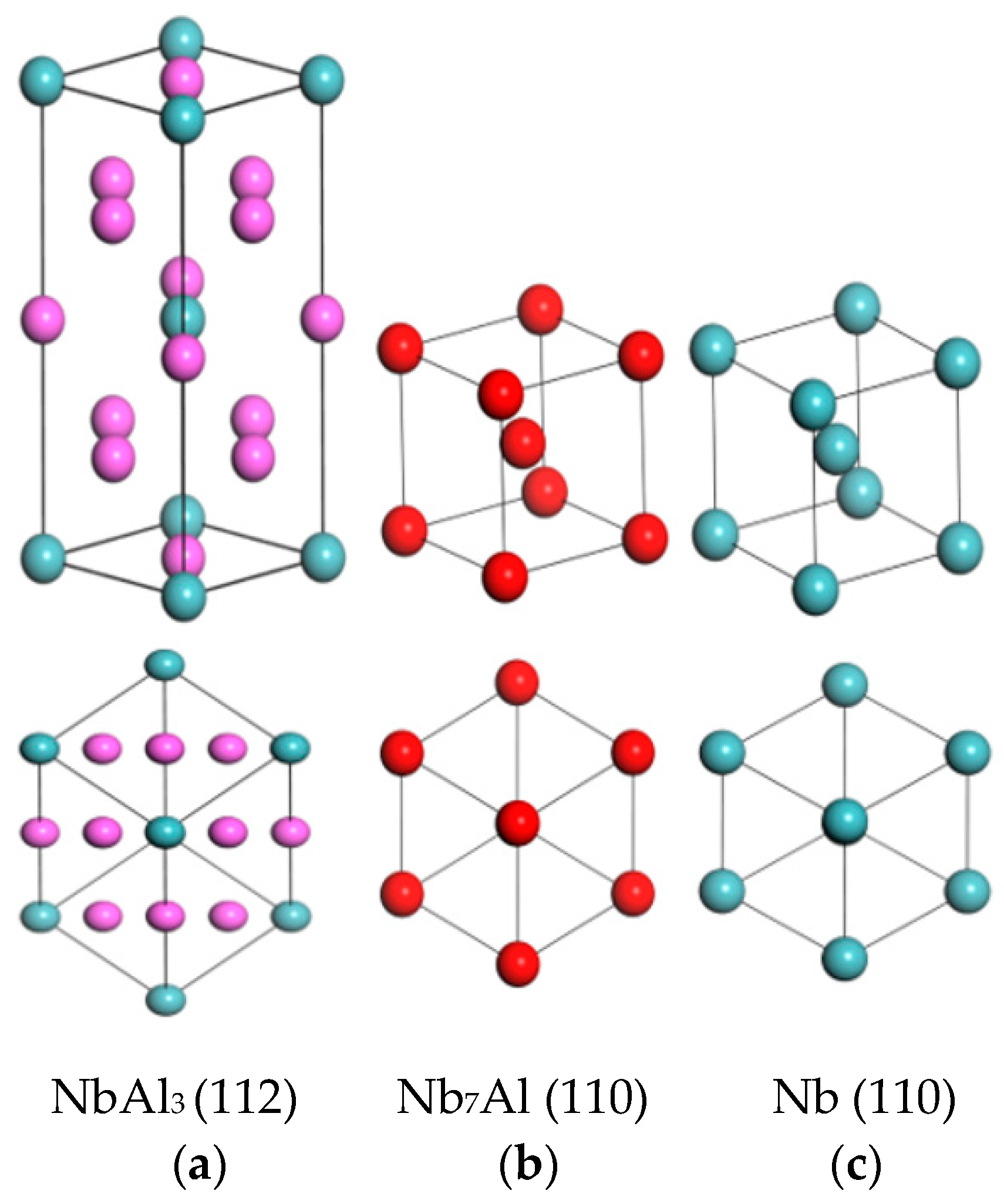
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seiler, F.; Igra, O. Hypervelocity Launchers; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–19. [Google Scholar] [CrossRef]
- Crozier, W.D.; Hume, W. High-Velocity, Light-Gas Gun. J. Appl. Phys. 1957, 28, 892–894. [Google Scholar] [CrossRef]
- Dlott, D.D. New Developments in the Physical Chemistry of Shock Compression. Annu. Rev. Phys. Chem. 2011, 62, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Lexow, B.; Wickert, M.; Thoma, K.; Schäfer, F.; Poelchau, M.H.; Kenkmann, T. The extra-large light-gas gun of the Fraunhofer EMI: Applications for impact cratering research. Meteorit. Planet. Sci. 2013, 48, 3–7. [Google Scholar] [CrossRef]
- Erskine, D.; Nellis, W.J. Shock-induced martensitic phase transformation of oriented graphite to diamond. Nature 1991, 349, 317–319. [Google Scholar] [CrossRef]
- Sato, T.; Ishii, T.; Setaka, N. Formation of Cubic Boron Nitride from Rhombohedral Boron Nitride by Explosive Shock Compression. J. Am. Ceram. Soc. 1982, 65, c162. [Google Scholar] [CrossRef]
- Sōma, T.; Sawaoka, A.; Saito, S. Characterization of wurtzite type boron nitride synthesized by shock compression. Mater. Res. Bull. 1974, 9, 755–762. [Google Scholar] [CrossRef]
- Sekine, T.; He, H.; Kobayashi, T.; Zhang, M.; Xu, F. Shock-induced transformation of β-Si3N4 to a high-pressure cubic-spinel phase. Appl. Phys. Lett. 2000, 76, 3706–3708. [Google Scholar] [CrossRef]
- Sekine, T.; He, H.; Kobayashi, T.; Shibata, K. Sinoite (Si2N2O) shocked at pressures of 28 to 64 GPa. Am. Miner. 2006, 91, 463–466. [Google Scholar] [CrossRef]
- Sekine, T.; Kobayashi, T.; Li, X. Hugoniot of beta-SiAlON and high-pressure phase transitions. J. Appl. Phys. 2006, 99, 53501. [Google Scholar] [CrossRef]
- Hanada, S. Niobium aluminides. Curr. Opin. Solid State Mater. Sci. 1997, 2, 279–283. [Google Scholar] [CrossRef]
- Wang, N.; Du, C.; Hou, J.; Zhang, Y.; Huang, K.; Jiao, S.; Zhu, H. Direct synthesis of Nb–Al intermetallic nanoparticles by sodiothermic homogeneous reduction in molten salts. Intermetallics 2013, 43, 45–52. [Google Scholar] [CrossRef]
- Leyarovski, E.; Leyarovska, L.; Krasnopyorov, E.; Kokot, L.; Horyń, R.; Mydlarz, T. Superconductivity and magnetic properties of Nb—Al phases: Nb2Al and NbAl3. Eur. Phys. J. B 1977, 27, 57–60. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. Ab initio investigation of the Nb–Al system. Comput. Mater. Sci. 2015, 107, 116–121. [Google Scholar] [CrossRef]
- Neumeier, J.J.; Nellis, W.J.; Maple, M.B.; Torikachvili, M.S.; Yang, K.N.; Ferreira, J.M.; Summers, L.T.; Miller, J.I.; Sales, B.C. Metastable A15 phase Nb3Si synthesized by high dynamic pressure. High Press. Res. 1989, 1, 267–289. [Google Scholar] [CrossRef]
- Yu, L.H.; Meyers, M.; Thadhani, N.N. Reaction-assisted shock consolidation of RSR Ti–Al alloys. J. Mater. Res. 1990, 5, 302–312. [Google Scholar] [CrossRef]
- Jiao, Z.; Liu, Q.J.; Liu, F.S. Study on preparation and properties of Nb-Al superalloy under shock compression. In Proceedings of the 18th China High Pressure Science Conference, Chengdu, China, July 2016; Available online: https://cpfd.cnki.com.cn/Article/CPFDTOTAL-SCYZ201607001242.htm (accessed on 20 February 2022).
- Jiao, Z.; Liu, Q.J.; Wang, Y.G.; Zhang, M.J.; Liu, F.S.; Chang, X.H.; Fan, D.H. Shock Compression Loading Recycling Box and Method for Preparing Material by Shock Compression, Public No. CN107008207A. Available online: https://pss-system.cnipa.gov.cn/sipopublicsearch/portal/uiIndex.shtml (accessed on 20 February 2022).
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.; Liu, Q.-J.; Liu, F.-S.; Tang, B. Structural and electronic properties of low-index surfaces of NbAl3 intermetallic with first-principles calculations. Appl. Surf. Sci. 2017, 419, 811–816. [Google Scholar] [CrossRef]
- Lundin, C.E.; Yamamoto, A.S. Equilibrium Phase Diagram, Niobium (Columbium)-Aluminum. Trans. Met. Soc. AIME 1966, 236, 863. [Google Scholar] [CrossRef]
- Pialoux, A.; Joyeux, M.; Cizeron, G. Étude du comportement du niobium sous vide par diffraction des rayons X à haute température. J. Less Common Met. 1982, 87, 1–19. [Google Scholar] [CrossRef]
- Tougait, O.; Noël, H. Stoichiometry of UAl4. Intermetallics 2004, 12, 219–223. [Google Scholar] [CrossRef]
- Suh, I.-K.; Ohta, H.; Waseda, Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray diffraction. J. Mater. Sci. 1988, 23, 757–760. [Google Scholar] [CrossRef]
- Huang, H.-J.; Jing, F.-Q.; Cai, L.-C.; Bi, Y. Grüneisen Parameter along Hugoniot and Melting Temperature of ε-Iron: A Result from Thermodynamic Calculations. Chin. Phys. Lett. 2005, 22, 836–838. [Google Scholar] [CrossRef]
- Chau, R.; Stölken, J.; Asoka-Kumar, P.; Kumar, M.; Holmes, N.C. Shock Hugoniot of single crystal copper. J. Appl. Phys. 2010, 107, 023506. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.Q.; Cheng, J.X. Principle and Technology of Dynamic High Pressure; National Defense Industry Press: Beijing, China, 2006. [Google Scholar]

| Number | Diameter (mm) | Thickness (mm) | Mass (g) | Density | Porosity |
|---|---|---|---|---|---|
| No. 1 | 12 | 3.16 | 1.361 | 3.81 | 38.8% |
| No. 2 | 12 | 3.21 | 1.382 | 3.81 | 38.8% |
| No. 3 | 12 | 3.56 | 1.537 | 3.82 | 38.6% |
| No. 4 | 12 | 3.36 | 1.455 | 3.82 | 38.6% |
| No. 5 | 12 | 3.54 | 1.526 | 3.81 | 38.8% |
| No. 6 | 12 | 3.26 | 1.408 | 3.81 | 38.8% |
| Number. | Mass of Sheet (g) | Impact Velocity (km/s) | Impact Pressure (GPa) |
|---|---|---|---|
| No. 1 | 1.361 | 2.14 | 44.31 |
| No. 2 | 1.382 | 1.63 | 31.79 |
| No. 3 | 1.537 | 2.52 | 54.44 |
| No. 4 | 1.45 | 1.28 | 23.91 |
| No. 5 | 1.526 | 2.60 | 56.65 |
| No. 6 | 1.408 | 3.28 | 86.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.-L.; Jiao, Z.; Liu, Z.-T.; Jiang, C.-L.; Liu, Q.-J. Preparation of Niobium Aluminium Alloy Based on Shock Compression Method. Crystals 2022, 12, 381. https://doi.org/10.3390/cryst12030381
Tao Y-L, Jiao Z, Liu Z-T, Jiang C-L, Liu Q-J. Preparation of Niobium Aluminium Alloy Based on Shock Compression Method. Crystals. 2022; 12(3):381. https://doi.org/10.3390/cryst12030381
Chicago/Turabian StyleTao, Ya-Le, Zhen Jiao, Zheng-Tang Liu, Cheng-Lu Jiang, and Qi-Jun Liu. 2022. "Preparation of Niobium Aluminium Alloy Based on Shock Compression Method" Crystals 12, no. 3: 381. https://doi.org/10.3390/cryst12030381






