Strategic Synthesis to Disperse Zeolite NaY in Lead Tree Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Lead Tree Wood-Zeolite NaY Composites
2.3. Characterization
2.4. Adsorption Experiment
3. Results and Discussion
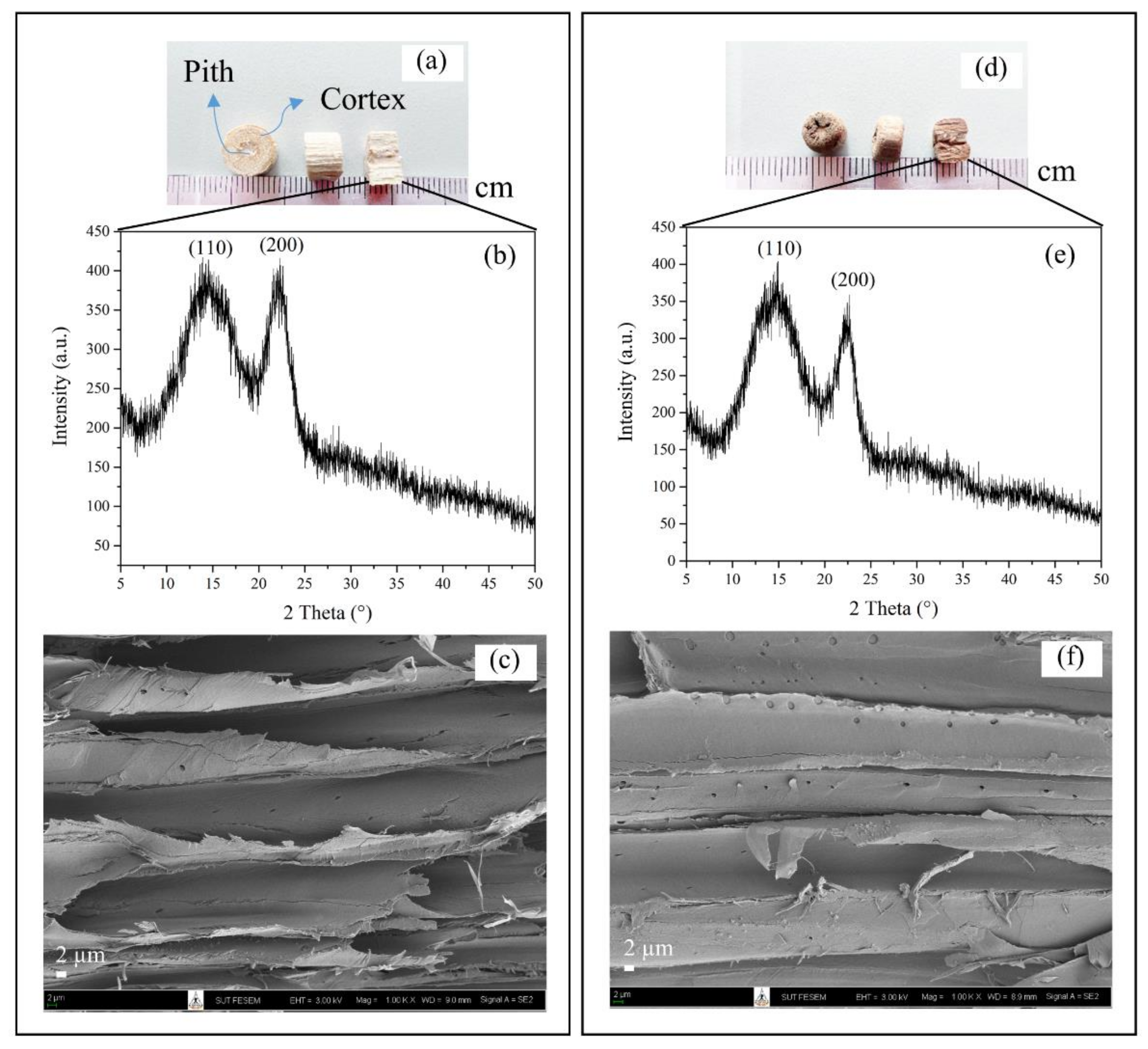
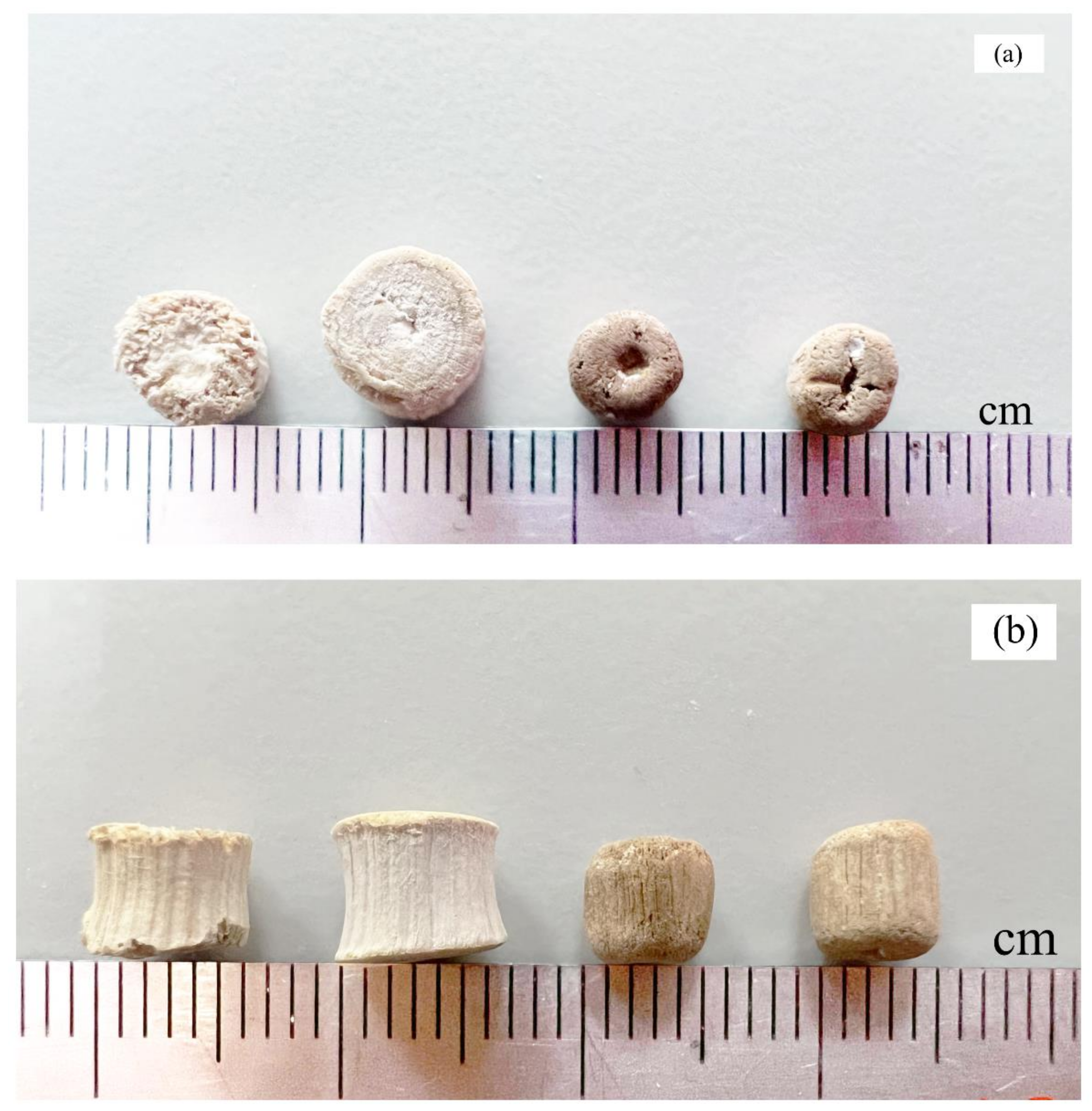
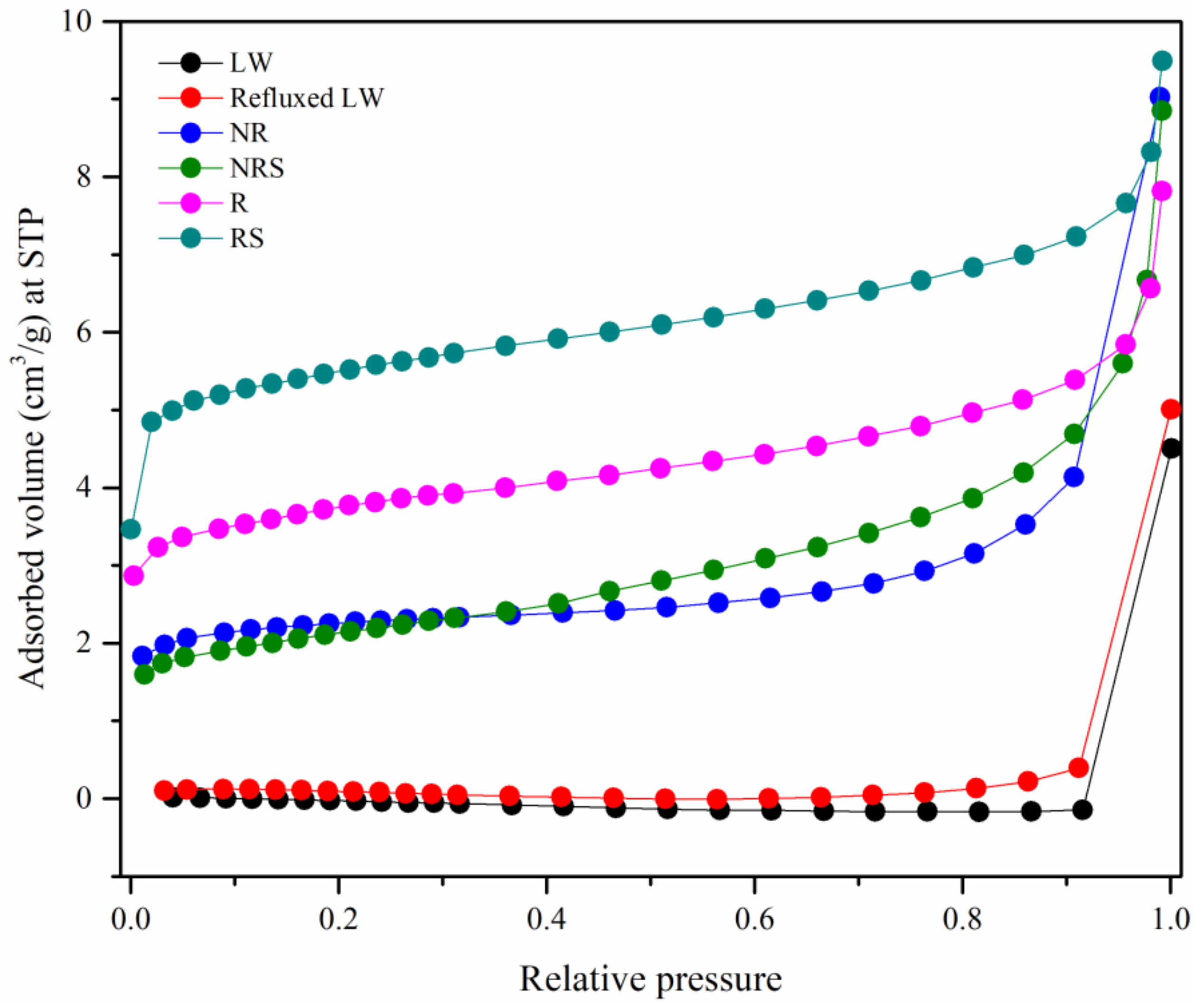
3.1. Characterization of Untreated (NR) and Refluxed (R) Lead Tree Wood
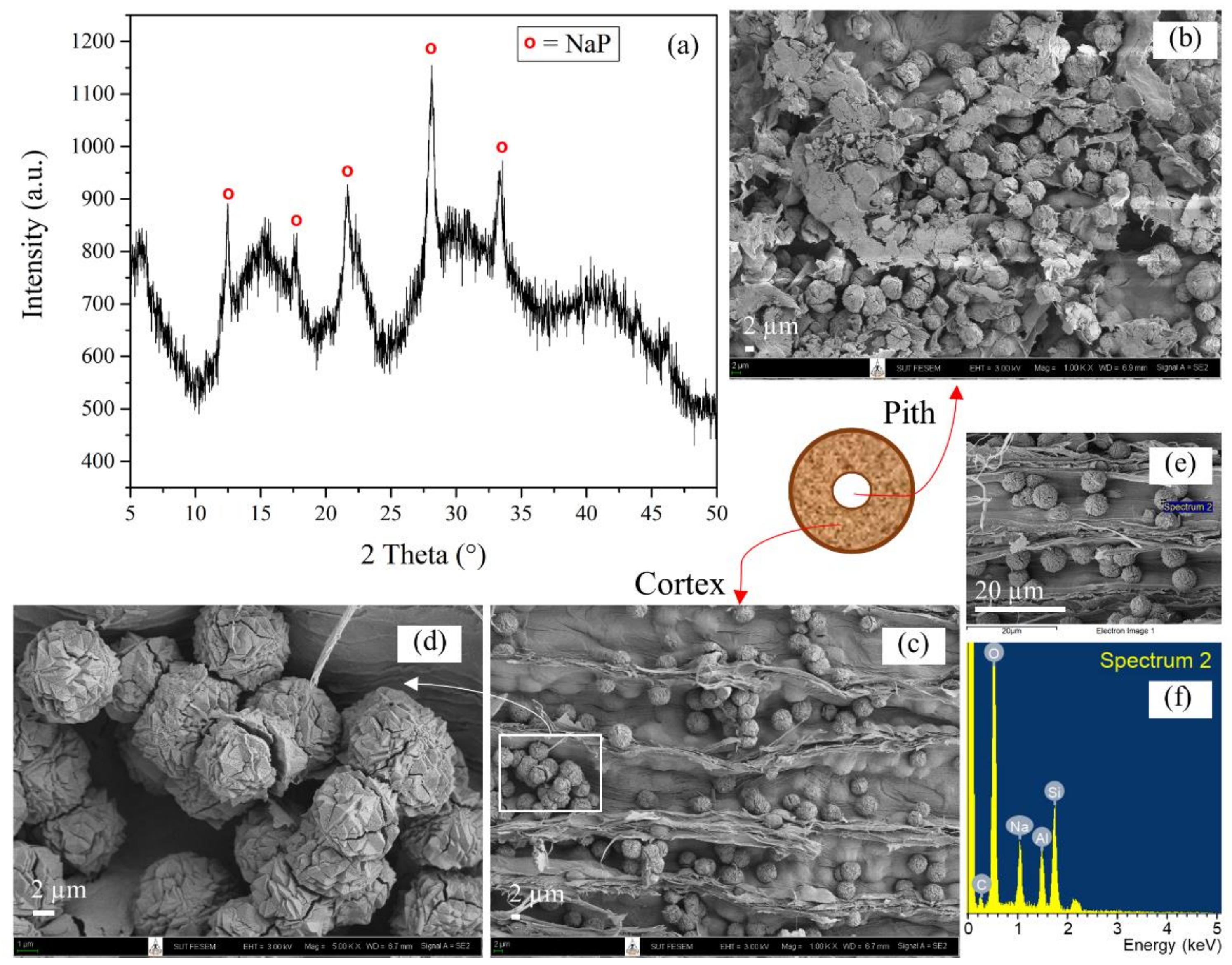
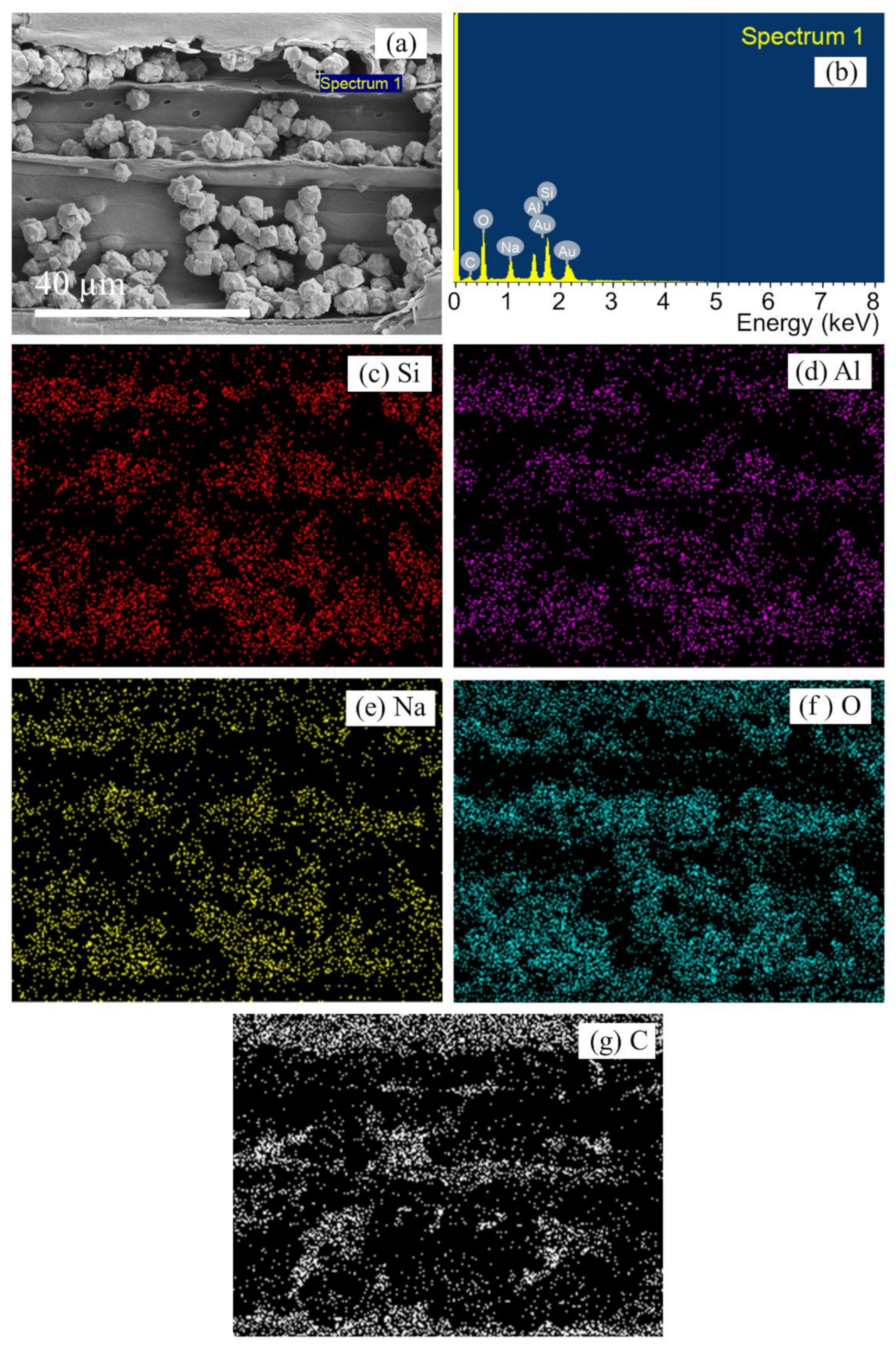
3.2. Characterization of Untreated Lead Tree Wood-Zeolite Composites
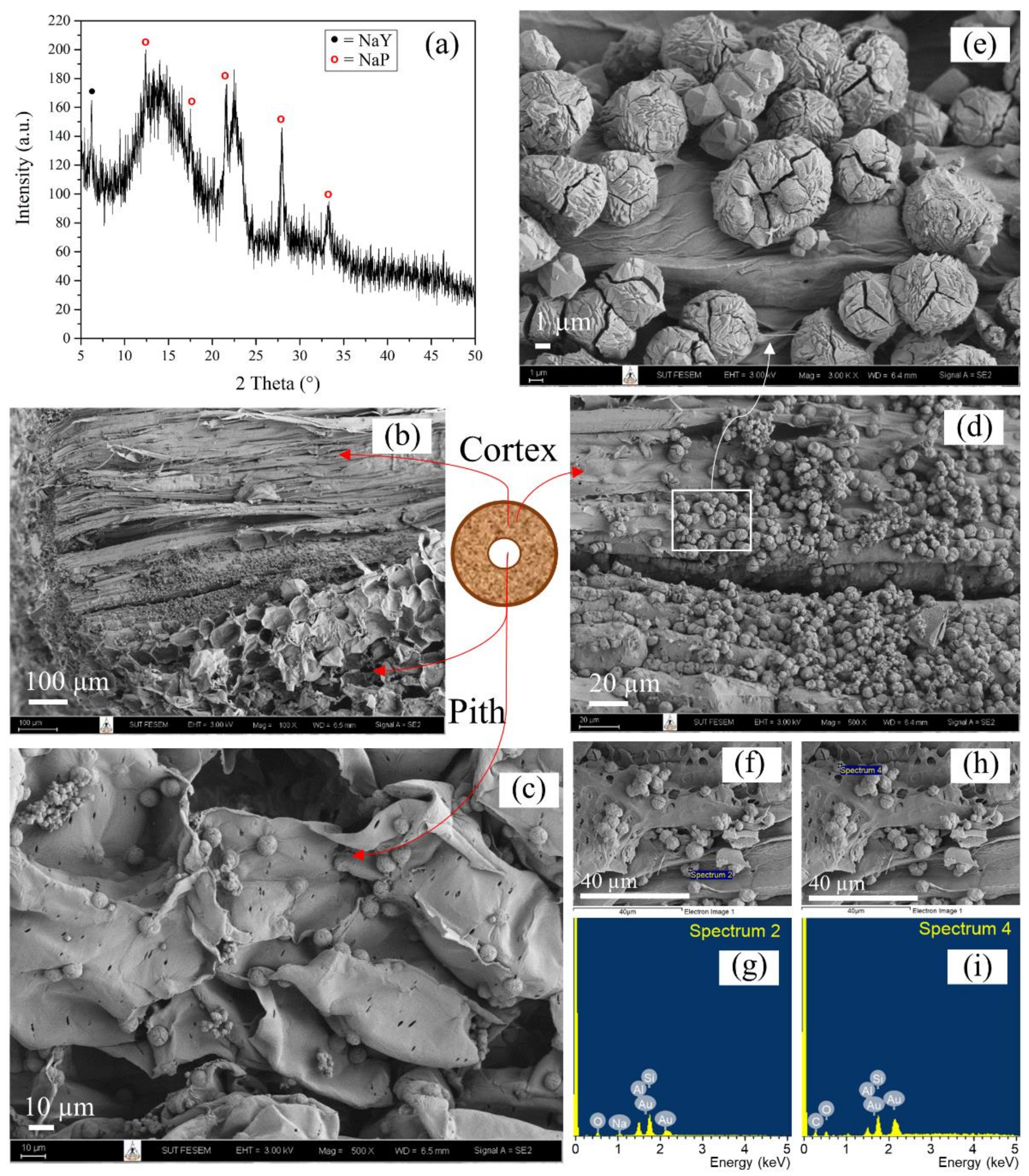
3.3. Characterization of Refluxed Lead Tree Wood-Zeolite Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rongchapo, W.; Deekamwong, K.; Loiha, S.; Prayoonpokarach, S.; Wittayakun, J. Paraquat adsorption on NaX and Al-MCM-41. Water Sci. Technol. 2015, 71, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Rongchapo, W.; Sophiphun, O.; Rintramee, K.; Prayoonpokarach, S.; Wittayakun, J. Paraquat adsorption on porous materials synthesized from rice husk silica. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2013, 68, 863–869. [Google Scholar] [CrossRef]
- Ismail, A.; Harmuni, H.; Mohd, R.R.M.A.Z. Removal of iron and manganese using granular activated carbon and zeolite in artificial barrier of riverbank filtration. AIP Conf. Proc. 2017, 1835, 020056. [Google Scholar]
- Amin, N.K. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 2008, 223, 152–161. [Google Scholar] [CrossRef]
- Chaiwon, T.; Jannoey, P.; Channei, D. Preparation of Activated Carbon from Sugarcane Bagasse Waste for the Adsorption Equilibrium and Kinetics of Basic Dye. Key Eng. Mater. 2017, 751, 671–676. [Google Scholar] [CrossRef]
- Chien, N.D.; Ivanovich, V.A.; Alexandrovna, G.N. Salinity effect on adsorption of phenol by activated carbon from sugarcane bagasse. Asian J. Chem. 2019, 32, 463–465. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Ghorbanpour, A.; Conato, M.T.; McGrail, B.P.; Grabow, L.C.; Motkuri, R.K.; Rimer, J.D. Synthesis strategies for ultrastable zeolite GIS polymorphs as sorbents for selective separations. Chemistry 2016, 22, 16078–16088. [Google Scholar] [CrossRef]
- Fujii, S.; Cha, H.; Kagi, N.; Miyamura, H.; Kim, Y.-S. Effects on air pollutant removal by plant absorption and adsorption. Build. Environ. 2005, 40, 105–112. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Bagherpour, A. Synthesis of zeolite NaY and its nanocomposites with chitosan as adsorbents for lead(II) removal from aqueous solution. Powder Technol. 2018, 338, 744–763. [Google Scholar] [CrossRef]
- Dotto, G.L.; Meili, L.; Abud, A.K.; Tanabe, E.H.; Bertuol, D.; Foletto, E.L. Comparison between Brazilian agro-wastes and activated carbon as adsorbents to remove Ni(II) from aqueous solutions. Water Sci. Technol. 2016, 73, 2713–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Beltrán-Hernández, R.I.; Coronel-Olivares, C.; Vázquez-Rodríguez, G.A. Biosorption of Water Pollutants by Fungal Pellets. Water 2020, 12, 1155. [Google Scholar] [CrossRef] [Green Version]
- Pimsuta, M.; Sosa, N.; Deekamwong, K.; Keawkumay, C.; Thathong, Y.; Rakmae, S.; Junpirom, S.; Prayoonpokarach, S.; Wittayakun, J. Charcoal and wood vinegar from pyrolysis of lead tree wood and activated carbon from physical activation. Suranaree J. Sci. Technol. 2018, 25, 177–190. [Google Scholar]
- Keawkumay, C.; Rongchapo, W.; Sosa, N.; Suthirakun, S.; Koleva, I.Z.; Aleksandrov, H.A.; Vayssilov, G.N.; Wittayakun, J. Paraquat adsorption on NaY zeolite at various Si/Al ratios: A combined experimental and computational study. Mater. Chem. Phys. 2019, 238, 121824. [Google Scholar] [CrossRef]
- Sosa, N.; Chanlek, N.; Wittayakun, J. Facile ultrasound-assisted grafting of silica gel by aminopropyltriethoxysilane for aldol condensation of furfural and acetone. Ultrason Sonochem. 2020, 62, 104857. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, D.; Sun, Q.; Liu, Y.; Zhao, H. Lignocellulose aerogel from wood-ionic liquid solution (1-allyl-3-methylimidazolium chloride) under freezing and thawing conditions. Biomacromolecules 2011, 12, 1860–1867. [Google Scholar] [CrossRef]
- Pimsuta, M. Nickel Phosphide on Activated Carbon for Hydrodeoxygenation of Palm Oil. Ph.D. Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2016. [Google Scholar]
- Joksimovic, G.; Marković, Z. Investigation of the mechanism of acidic hydrolysis of cellulose. Acta Agric. Serbia 2007, 12, 51–57. [Google Scholar]
- Huo, Z.; Xu, X.; Lü, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of zeolite NaP with controllable morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Khabuanchalad, S.; Khemthong, P.; Prayoonpokarach, S.; Wittayakun, J. Transformation of zeolite NaY synthesized from rice husk silica to NaP during hydrothermal synthesis. Suranaree J. Sci. Technol. 2008, 15, 225–231. [Google Scholar]
- Tayraukham, P.; Jantarit, N.; Osakoo, N.; Wittayakun, J. Synthesis of Pure Phase NaP2 Zeolite from the Gel of NaY by Conventional and Microwave-Assisted Hydrothermal Methods. Crystals 2020, 10, 951. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Kosri, C.; Khemthong, P.; Wittayakun, J. Fast synthesis of zeolite NaP by crystallizing the NaY gel under microwave irradiation. Mater. Lett. 2020, 272, 127845. [Google Scholar] [CrossRef]
- Shirazian, S.; Parto, S.G.; Ashrafizadeh, S.N. Effect of water content of synthetic hydrogel on dehydration performance of nanoporous LTA zeolite membranes. Int. J. Appl. Ceram. Technol. 2014, 11, 793–803. [Google Scholar] [CrossRef]
- Wittayakun, J.; Khemthong, P.; Prayoonpokarach, S. Synthesis and characterization of zeolite NaY from rice husk silica. Korean J. Chem. Eng. 2008, 25, 861–864. [Google Scholar] [CrossRef]
- Bu, N.; Liu, X.; Song, S.; Liu, J.; Yang, Q.; Li, R.; Zheng, F.; Yan, L.; Zhen, Q.; Zhang, J. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution. Adv. Powder Technol. 2020, 31, 2699–2710. [Google Scholar] [CrossRef]


| Samples | Si/Al Ratio | %wt Zeolite in Wood | Zeolite Type | |
|---|---|---|---|---|
| From XRD | From SEM | |||
| LW | - | 0 | - | - |
| Refluxed LW | - | 0 | - | - |
| NR | 2.25 | 22.4 | NaP | NaP |
| NRS | 2.74 a and 2.39 b | 17.7 | NaP + NaY | NaP + NaY |
| R | 2.16 | 20.4 | NaY | NaY |
| RS | 2.16 | 12.4 | NaY | NaY |
| Samples | SBET (m2/g) | Ni(II) Adsorption Capacity (mg/g-Adsorbent) |
|---|---|---|
| LW | 0.2 | 0.36 |
| Refluxed LW | 0.2 | 0.00 |
| NR | 6.4 | 3.75 |
| NRS | 7.0 | 4.66 |
| R | 11.2 | 5.70 |
| RS | 16.1 | 6.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krukkratoke, P.; Keawkumay, C.; Tayraukham, P.; Prompiputtanapon, K.; Khemthong, P.; Prayoonpokarach, S.; Wittayakun, J. Strategic Synthesis to Disperse Zeolite NaY in Lead Tree Wood. Crystals 2022, 12, 504. https://doi.org/10.3390/cryst12040504
Krukkratoke P, Keawkumay C, Tayraukham P, Prompiputtanapon K, Khemthong P, Prayoonpokarach S, Wittayakun J. Strategic Synthesis to Disperse Zeolite NaY in Lead Tree Wood. Crystals. 2022; 12(4):504. https://doi.org/10.3390/cryst12040504
Chicago/Turabian StyleKrukkratoke, Panot, Chalermpan Keawkumay, Pimwipa Tayraukham, Kewalee Prompiputtanapon, Pongtanawat Khemthong, Sanchai Prayoonpokarach, and Jatuporn Wittayakun. 2022. "Strategic Synthesis to Disperse Zeolite NaY in Lead Tree Wood" Crystals 12, no. 4: 504. https://doi.org/10.3390/cryst12040504
APA StyleKrukkratoke, P., Keawkumay, C., Tayraukham, P., Prompiputtanapon, K., Khemthong, P., Prayoonpokarach, S., & Wittayakun, J. (2022). Strategic Synthesis to Disperse Zeolite NaY in Lead Tree Wood. Crystals, 12(4), 504. https://doi.org/10.3390/cryst12040504









