1. Introduction
Diatomite, diatomaceous earth or kieselguhr is a very fine-grained sedimentary rock composed of fossilized skeletal remains (frustules) of single-celled aquatic plants (algae) called diatoms. [
1]. More than 10,000 varieties of diatoms have been classified [
2]. Various diatom shapes were well illustrated by [
3]. Porous diatomite frustules have the chemical formula of SiO
2·nH
2O in the form of amorphous silica (opal-A). They do not contain any crystalline silica, but impurities found in diatomite deposits may contain crystalline silica in the form of quartz. Depending on the deposition environment, volcanic ash, organic matter, carbonates and clay minerals may also be found as impurities along with opal-A and sometimes additionally opal-CT in the diatomite deposits [
4,
5,
6,
7].
In 2020, the USA accounted for an estimated 35% of the total world diatomite production. The USA was followed by Denmark with 17%, Turkey with 8% and China with 7%. Turkey is an important supplier with diatomite deposits that are suitable for industrial use and reserves of 44 million tons [
8]. Diatomite is an important natural resource used in a number of applications such as cement production [
9], drug delivery [
10] and insecticide [
11]. It is preferred especially in the filter aids industry for clarifying liquids due to its permeability, porosity, chemical inertness and low thermal conductivity [
4,
12,
13,
14,
15]. It reacts only with strong alkalis and hydrofluoric acid [
4]. Its high specific surface area and adsorption capacity enable it to be used in dye removal, adsorption of oils, radionuclides and microorganisms [
16,
17,
18,
19]. Diatomite is also used as a filler in paint, plastic and paper [
20]. Since mined diatomaceous earth can contain high levels of moisture up to 50% wt. [
4], a drying operation is required before or during the grinding process after crushing. Especially, the high flow rate (permeability) required in filter aid application is provided by air classification of the ground diatomite [
21,
22]. However, due to the impurities contained in the deposits, diatomite resources need to go through some further processes in order to be used commercially in industrial products and applications. Calcination or flux-calcination is one of the most frequently used methods for modifying opal-A grain size, especially to improve properties such as permeability and whiteness, which are desired in the filter aid and filler applications, respectively [
13,
14,
23,
24,
25]. By calcination, organic matter and carbonate compounds can also be removed from the diatomite and purification can be achieved [
13]. Permeability of diatomite increases with aggregation, resulting from sintering and shrinkage of the particles, and with the opening of the frustule pores after calcination [
23,
25], but the adsorption capacity of diatomite decreases [
26]. The use of flux during calcination enables the production of filter aids that offer whiter and better filtration properties [
26]. Fluxes such as Na
2CO
3 (soda ash), NaCl (halite), NaOH (caustic soda), KCl (sylvite), KOH (caustic potash), sodium silicates (water glass) and hydrated lime [
13,
25,
26] are added to the product in amounts of up to 2–12% by weight [
21] before or during calcination. The alkali or alkaline earth oxides in the chemical composition of the flux partially break up the Si–O–Si chains in the opal during calcination and occupy the spaces between the tetrahedral network structure. These broken chains recombine to the hexatomic ring network structure of cristobalite and contribute to the transformation of opal into cristobalite during cooling [
26]. In addition, the incorporation of iron ions (Fe
3+) into the Si–O mesh structure weakens the colouring effect of iron at high temperatures, resulting in whiter products. It has been reported that sodium-based fluxes are more effective than potassium-based fluxes in the transformation of opal to cristobalite. The atomic radius of K
+ is bigger than that of Na
+, making K
+ less likely to enter into the opal framework and to damage the framework [
20,
26].
In this study, the effect of thenardite (Na
2SO
4) as a flux on the properties of flux-calcined diatomite was investigated for the first time. Thenardite is a sodium sulphate mineral that occurs in arid evaporite environments, specifically lakes and playas. In Turkey, 99% of natural sodium sulphate is produced from alkaline lakes, and 1% is produced in thenardite deposits (with underground operating methods) [
27]. Thenardite, which has more demand in the industry, can also be obtained by dehydration of mirabilite [
27]. The effects of thenardite as a flux material proposed in this study were also compared with the effects of soda ash (Na
2CO
3), the most widely used flux during the calcination of diatomite. The effects of fluxes on the physico-chemical properties of two diatomite samples from the Aegean Region of Turkey (Afyon and Denizli) were revealed.
2. Materials and Methods
Diatomite occurrences in various regions in Turkey are of Neogene age and they are lacustrine formations in succession with volcanic rocks. Volcanism plays a critical role in the growth and productivity of diatoms because volcanic products such as volcanic ash supplies soluble silica into lakes and increases the silicic acid available to diatoms for frustule growth [
28,
29]. Fresh volcanic ash may also supply other chemicals, such as phosphorous and sulphur compounds, which could also increase the growth rates of diatoms [
28,
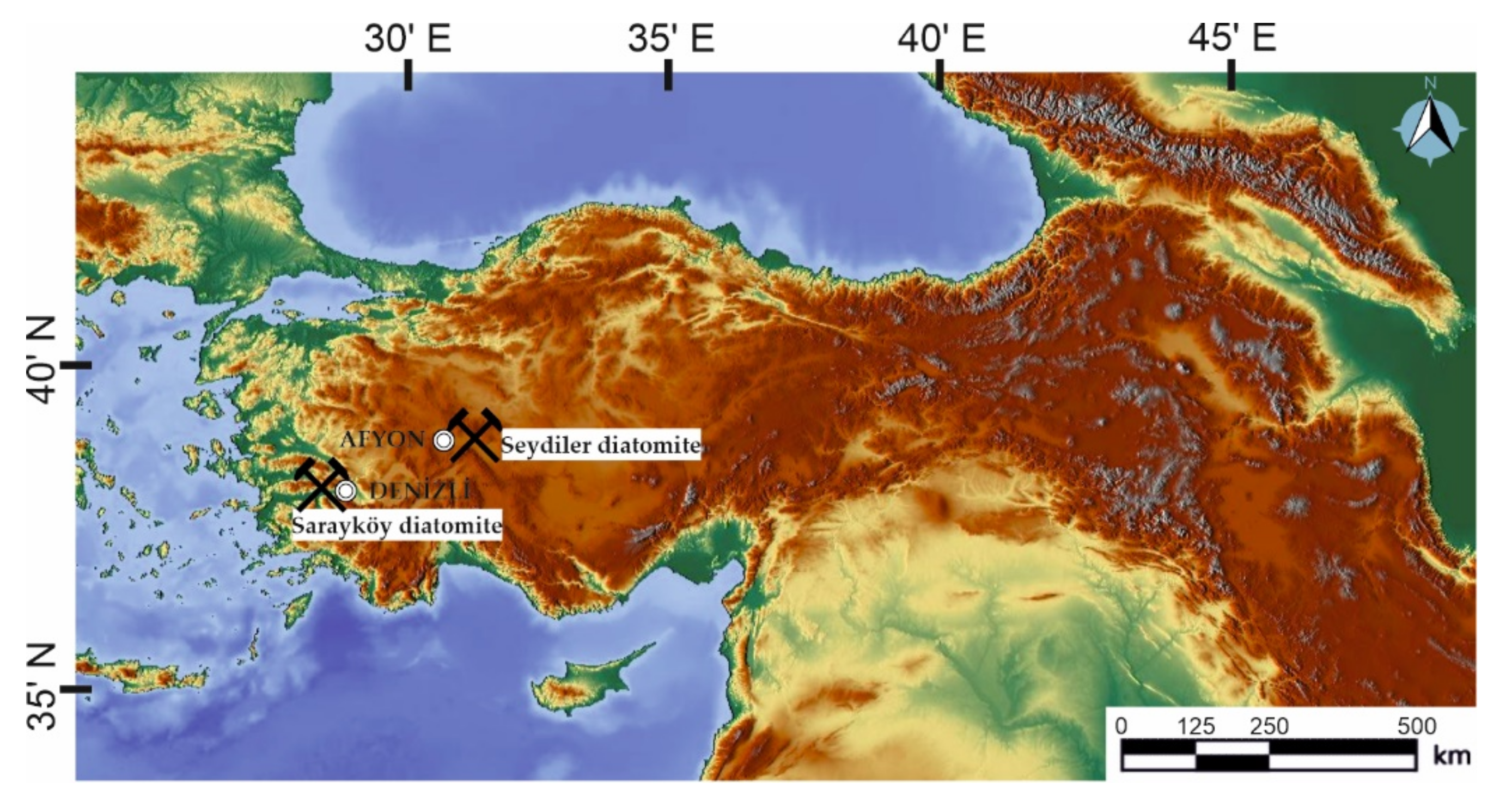
30]. The diatomite sample from Afyon (Turkey) used in this study was taken from the deposits in the town of Seydiler (
Figure 1). It was stated that this deposit has a reserve of 180,000 tons [
31]. The diatomite level was located between the rhyolitic ignimbrite deposits (Seydiler ignimbrite) formed prior to the collapse of the Köroğlu caldera, and the trachybasalt lava flows belong to the post-caldera stage [
32] (
Figure 2). At the bottom of the lava flow overlying the lacustrine sediments containing the diatomite deposits, chaotic structures indicate that the lava flow entered the lake environment [
33]. The lacustrine sediments hosting the diatomite deposits belonged to the resurgence stage of the caldera [
32]. The diatom species in the Seydiler diatomite deposits were determined as Campyloneis, Cymbella, Navicula and Trinacria [
34].
The other diatomite sample was taken from Denizli (Turkey) around the Sarayköy district (
Figure 1). It is located in Neogene series consisting of diatomite, gypsum, sulphur and marly limestones. Diatomite levels are in the form of 2–3 m thick lenses (
Figure 3). There are angular pebbles composed of opal, chalcedony and calcium carbonate in the clayey levels intercalated with diatomite lenses [
34]. It was suggested that the quality of these occurrences was not good due to the clay and sulphur they contain [
35]. Although its impurities show that it is not suitable as a filter aid, Sarayköy diatomite was used in order to see the effect of fluxes on impurities in this study. The diatom species in Sarayköy diatomite deposits were determined as Campyloneis, Synedra, Coscinodiscus and Melosira [
34].
Diatomaceous earth samples were taken from the quarries within the borders of Afyon and Denizli provinces as part of the field studies carried out by the authors in 2021. Soda ash (Na2CO3) was purchased from Merck (Darmstadt, Germany) with product number 1063921000. Thenardite (Na2SO4) was purchased from Alkim (Istanbul, Turkey).
After drying the raw diatomite samples in an oven at 100 °C for 24 h, they were ground in a ring mill and sieved with a 63 µm sieve. Samples with particle size smaller than 63 µm were used in the study. A total of 8 wt.% flux powder (Na
2CO
3 or Na
2SO
4) was blended with diatomite samples before calcination. The flux amount of 8 wt.% was used for good filtration properties considering the previous studies [
13,
26]. The samples containing flux and diatomite were calcined in a laboratory type electric muffle furnace at 1100 °C for 1 h. Control samples of the same grain size were calcined at the same temperature and time without using any flux.
One of the most important features of diatomites that determine their industrial use potential is their chemical composition. The silica (SiO
2) content of the diatomite from economic deposits is usually >86% [
4,
36]. Element abundances of raw and calcined samples were determined by XRF (X-ray fluorescence) in the Central Laboratory of Niğde Ömer Halisdemir University (Niğde, Turkey). The type of silica present and the qualitative mineral content in diatomite samples were determined by XRD (x-ray diffractometry) (Malvern Panalytical, Malvern, UK) with CuKα radiation in the Central Laboratory of Niğde Ömer Halisdemir University (Niğde, Turkey). Chemical analyses were carried out on filtrates after the permeability tests of diatomites in the laboratories of MTA General Directorate (Ankara, Turkey). Cations were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) and anions were determined by ion chromatography. Carbonate and bicarbonate determinations were made by volumetric analysis. SEM (scanning electron microscopy) analysis were carried out at Hacettepe University, Department of Geological Engineering (Ankara, Turkey). Samples were coated with a layer of gold prior to the SEM analysis to avoid charging.
The particle size distributions of the diatomite samples were analysed using a laser particle size diffraction analyser (Helos, Sympatec GmbH, Clausthal-Zellerfeld, Germany) at Niğtaş corporation (Niğde, Turkey). The colour parameters of samples were determined using a colour spectrophotometer (Elrepho, DataColor, Trenton, NJ, USA) as defined by the Commission Internationale de l’Eclairage (CIE) at Niğtaş corporation (Niğde, Turkey).
L* indicates whiteness, a* is the red/green coordinate and b* is the yellow/blue coordinate [
37]. Ry is luminous reflectance factor, expressed as a % ratio, and is primarily used for white or neutral colours as an overall indicator of reflectance. Ry is equivalent to Y Brightness [
38].
Specific surface area (SSA) is measured by the Brunauer–Emmett–Teller (BET) method on the basis of nitrogen adsorption isotherm measurements at 77 K using a surface area analyser (Quadrasorb-evo, Quantachrome, Boynton Beach, FL, USA) at Niğtaş corporation (Niğde, Turkey).
Permeability and wet cake density measurements were made according to the British Geology Survey laboratory manual [
39]. Permeability was calculated using Darcy’s law:
where
η is the viscosity of water at 20 °C,
Us is the superficial velocity,
L is the depth of the diatomite bed, Δ
P is the pressure drop across bed. For test apparatus and more detailed method, please see related appendixes in Inglethorpe (1993).
Wet cake density was calculated as follows:
where
m is dry weight of diatomite,
d is the diameter of the filter,
L is the depth of the diatomite cake.
3. Results
It is important to evaluate the diatomite samples, which will be used as filler or filter-aid, from a geochemical point of view. Geochemical analyses are given in
Table 1. Some chemical specifications state that the silica content of the diatomite used for filter aid should be >85 wt.%, and for filler >70 wt.% [
4,
39,
40]. Both diatomite samples are industrially valuable raw materials in terms of silica content. However, with a silica (SiO
2) content of more than 91 wt.%, Seydiler diatomite quarry is among the quality deposits in the world (e.g., Burney diatomite from USA) [
4]. Sarayköy diatomite has iron (III) oxide (Fe
2O
3) content (1.21 wt.%) very close to limit value (<1.5 wt.%) [
39]. Seydiler diatomite seems to be more advantageous due to its relatively low iron (III) oxide content (0.16 wt.%). Higher aluminium oxide (Al
2O
3) value (3.1 wt.%) of Sarayköy diatomite may indicate the presence of aluminosilicates, which are clay minerals. Although the loss of ignition (LOI) value may be related to the amount of moisture the sample contains, it can also indicate the presence of hydrous aluminosilicate clay minerals, which will limit the material’s industrial use. With its high LOI value (11.17 wt.%), Sarayköy diatomite is less advantageous due to the technical and financial problems it will cause in industrial processes (e.g., drying).
The crystallographic structure of diatomite samples was revealed by XRD analysis (
Figure 4). Wide bumps up to 28° (2θ) are particularly striking in the XRD patterns of raw diatomite samples. These bumps, which represent the amorphous silica phase (opal-A) in the raw diatomite samples, are accompanied by minor crystalline phases. The amorphous phase was more dominant in Seydiler raw diatomite, and there was a small amount of quartz and calcite besides the amorphous phase (
Figure 4a). The broad humps around 10° (2θ) may represent mixed clays such as illite-chlorite. In Sarayköy raw diatomite, crystalline phases consisting of quartz and clay minerals (e.g., illite-chlorite) exist besides the amorphous phase (
Figure 4b). In this raw sample, the sharper humps between 20–26° (2θ) indicate that the opal-A phase had partially transformed into the opal-CT phase. After the calcination process, the amorphous phase almost completely transformed into cristobalite in all samples, and the quartz minerals present in the raw diatomite samples took place in the crystalline phase beside cristobalite. As a result of calcination, the structure of clay minerals probably collapsed at high temperatures (e.g., 930 °C for illite) [
41]. XRD results clearly showed that flux-calcination provides more crystalline phase than straight-calcination. While bumps of amorphous phase still exist in both samples after straight-calcination, these phases were replaced by the crystalline phase (cristobalite) after flux-calcination. The effect of the flux types on the mineralogical composition of the calcined diatomite samples could not be understood from the XRD patterns. However, all calcination processes were more effective in obtaining the crystalline phase in the Seydiler diatomite sample than in the Sarayköy diatomite sample. Noise originating from the amorphous phase has left its place to clearer and narrower reflections in Seydiler diatomite by calcination.
SEM analysis of the samples showed that the diatom frustule structures were broken down as a result of grinding, but components presenting micro-nanopores were still present (
Figure 5). After calcination, all samples underwent aggregation due to sintering. There were individual agglomerations in the forms of spherical pellets, which are more common in the Sarayköy diatomite sample. It is thought that the clay minerals (e.g., illite) in the Sarayköy sample (
Figure 4) contributed to the formation of pellets by calcination. The excellent pelleting properties of clay minerals are already used in the industry [
42,
43]. It is understood that more porous aggregates, including fused grains with skeletal shapes, are formed as a result of flux-calcination compared to straight-calcination. The closed, non-porous structure resulting from the fine grains in the aggregates formed after straight-calcination probably turned into more open, porous and permeable structures as a result of these fine grains combining to form larger fused grains (
Figure 5).
The grain size of diatomite changes its porosity and filtering efficiency in applications where it is used as a filter aid. Fine-grained diatomite can cause low porosity and permeability of the filter material by blocking the pores in the filter aid. The grain size increasing with calcination in diatomite samples indicates aggregation resulting from sintering and shrinkage of the particles (
Table 2). In addition, the amount of flux used during calcination is an important parameter in the regulation of grain size distribution of diatomite. Using more or less than the required amount increases the amount of fine particles and impairs the permeability efficiency of the filter aid [
26]. In this study, particle size measurement could not be made in any of the flux-calcined diatomite samples because the size of the sample was larger than the upper limit of the particle size measuring device used (175 µm) (Sympatec Helos R3) [
44]. The fact that the grain size increased after flux-calcination showed that the amount of flux used was chosen correctly. It has been understood that the aggregation formed as a result of straight-calcination (
Figure 5) can be broken down by the vibration in the sample feeding part of the grain size measuring device. However, aggregates in pellet form consisting of fused grains after flux-calcination (
Figure 5) cannot be broken down in this mechanism and measurements cannot be taken because the dimensions of these aggregates exceed the upper limit of the device.
Whiteness is a sought-after feature for industrial minerals in many applications. In straight-calcined products, pinkish-reddish colours are obtained due to the oxidation of iron, particularly ferric iron content of the raw material [
20,
26]. In this study, the effect of flux on forming the Si–O mesh structure was observed. The iron entered this structure, and its ability to colour the diatomite was decreased. It was determined by the measurement of
L* values that the whiteness of flux-calcined diatomite samples increased (
Figure 6). Results for all colour parameters are presented in
Table 3. The whiteness of straight-calcined diatomite samples was lower than that of raw diatomite samples. The whiteness of flux-calcined diatomite samples was higher than both raw diatomite samples and straight-calcined diatomite samples, and they became more valuable industrially due to their whiteness. When diatomites are used as filtering aids in industry, whiteness is of no technical importance, but as a user-pleasing feature, it provides a preliminary idea of the purity of the product. However, especially when used as a filler, whiteness is one of the most sought-after properties in both the plastic, paint and paper industries. Here, especially in Seydiler diatomite, thenardite, which was used for the first time as a flux in this study, provided more whiteness than soda ash and provided more advantage in terms of colour for diatomite in its industrial use (
Figure 6).
In Sarayköy diatomite, soda ash was slightly more effective in whitening (
Figure 6). According to the Ry values, known as the indicator of reflectance, which is a very sought-after feature for filler minerals, fluxes increased the reflectance and caused the formation of brighter diatomite powders (
Table 3). Thenardite is more effective in increasing the brightness of the Seydiler diatomite, while soda ash is more effective in the Sarayköy diatomite. The a* value, that is, the red/green value of the samples, increased remarkably by straight-calcining, indicating the reddening of the samples. This increase was remarkable, especially in the Sarayköy sample after straight-calcination. The red colour tone was reduced in both diatomite samples with the use of fluxes. Soda ash decreased the a* value more in the Sarayköy sample, while thenardite was more effective in the Seydiler sample. Considering the b* value, which is the blue/yellow value, straight-calcination increased the b* value and thus the yellowness of both samples. Among the fluxes, thenardite was more successful than soda ash in reducing the yellowness value in both samples. It was revealed that flux-calcination with thenardite was more advantageous than with traditional flux, and soda ash if diatomite was used as a filler mineral, respectively, in plastics, paints and paper where colour and visuality were more important.
Specific surface area values of the raw diatomite samples decreased with calcination (
Figure 7). It was also demonstrated in previous studies that calcination, especially flux-calcination, reduced the specific surface area and porosity of diatomite [
20]. We can speculate that aggregation, which contributes to the increase in the permeability of diatomite, causes coarsening of the grains and, therefore, a decrease in the specific surface area. However, some researchers have suggested that the porous structure on the diatomite surface become more advanced and complete with calcination, thus increasing the permeability of the filter aid. [
13]. In this study, SEM images showed that porosity increased with flux-calcination but not with straight-calcination (
Figure 5). It is necessary to reveal whether the permeability of diatomite filter aids increases as a result of aggregation or as a result of the increase in surface porosities and thus specific surface areas. In this study, specific surface area of diatomite samples decreased significantly with calcination, especially with flux-calcination. Raw Sarayköy diatomite samples, which have a higher clay content, have a higher specific surface area value than raw Seydiler diatomite samples due to the high specific surface area of the clays without any heat treatment. As a result of calcination, the structure of clay minerals probably collapsed at high temperatures (e.g., 930 °C for illite) [
41] and the specific surface area of the sample also decreased abruptly. With the addition of aggregation to this effect in flux-calcination, the specific surface area of the coarser grains was further reduced. The lowest specific surface area values were obtained as a result of calcination with soda ash for Seydiler diatomite (1.46 m
2/g) and as a result of calcination with thenardite for Sarayköy diatomite (3.09 m
2/g).
High permeability is required for a high flow rate in filter aids. However, the porosity, which ensures the retention of the particles in the liquids filtered with filter aids, causes low wet cake density [
39,
45]. Raw diatomite samples offer very low permeability values without any heat treatment and are not capable of being used as a filter aid in the industry [
39] (
Figure 8). After straight-calcination without using flux, Sarayköy diatomite (0.31 µm
2) showed higher permeability than Seydiler diatomite (0.24 µm
2). The straight-calcination actually made the permeability value of both diatomite samples higher than that of the industrially usable products (>0.03 µm
2) [
39,
45]. However, fluxes had different effects on the permeability of the samples. Thenardite was much more effective in improving the permeability of the Sarayköy diatomite sample (0.79 µm
2). Soda ash, on the other hand, gave better results in terms of increased permeability in the Seydiler diatomite sample (0.97 µm
2). Maximum permeability values for both diatomite samples were obtained by calcination with soda ash. In this study, very coarse and very fine particles were not removed by a method such as air classification after calcination, which is an essential industrial process in the production of optimum filter aids. Nevertheless, calcined samples with sufficient permeability values (>0.80 µm
2) to be used in filter aid industrial applications were obtained.
When the specific surface area values and permeability values are compared (
Figure 9), the permeability decreases with increasing specific surface area exponentially. This shows that aggregation, which causes a decrease in specific surface area with calcination, is the most important factor in increased permeability. The fact that the flow rate (permeability) in a filter bed depends on the size of the particles comprising the bed and the void volume between the particles also supports this result [
46]. Our observation during the permeability tests is that the particle size and the void volume between them significantly affect the permeability values obtained. Our grain size and SEM analysis results also support these findings.
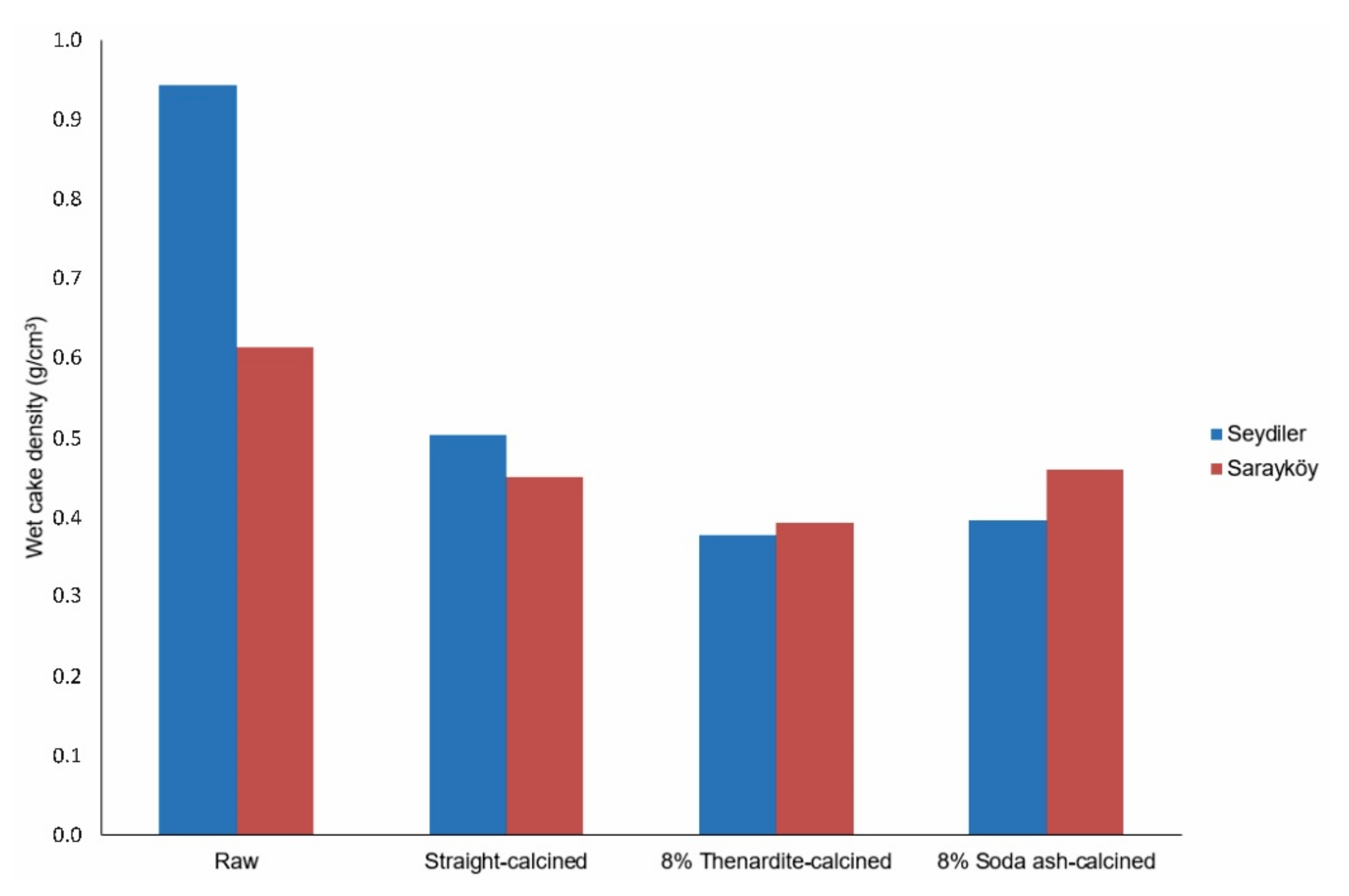
Theune and Bellet (1988) suggested wet cake density values in the range of 0.35–0.5 g/cm
3 for diatomites to be used as filter aids. Except for the raw diatomite samples, which did not undergone any heat treatment, the wet cake density values of all calcined samples remained within the specified range and they had the same values as the filter aid products currently marketed industrially [
39] (
Figure 10). If higher flow rate (permeability) is achieved with aggregation and low wet cake density is achieved with porosity, it can be interpreted that calcination with the thenardite increases the porosity of the samples more but cannot increase their permeability as much as the calcination made with soda ash. While the permeability, that is, the flow rate, determines the filtration rate of the filter aids, the wet cake density associated with porosity will determine the amount of particles in the filtrate, that is, the clarity of the filtrate [
39]. Diatomites with low wet cake density values can better retain particles removed from the liquid during filtration with their higher porosities. Thus, filter clarity can be achieved with low wet cake density. It will be beneficial for the user to evaluate these two parameters (permeability and wet cake density) according to the intended use of the filter aids. Soda-ash should be used if a high filtration rate is desired, thenardite should be used if the filtrate clarity is important.
Chemical analyses were carried out on the filtrates in order to understand whether the fluxes used were dissolved during the permeability tests and included in the filtrate. The chemistry of the filtrates was compared with the chemistry of the water used in the permeability tests (
Figure 11). It was observed that the amounts of Na
+, HCO
3−, SO
42− and the conductivity values in the filtrates filtered from the diatomites calcined with soda ash were higher than the values in the reference water (
Figure 11a). The reason for the higher amount of Na
+ and HCO
3− in the filtrate was interpreted as the dissolution of soda ash. Low CO
32− amounts (<10 ppm) in all filtrates indicate that carbonate ions were converted to bicarbonate (HCO
3−) ions. SO
42− increase may have been caused by the sulphur content in the Sarayköy sample (
Table 1). When the filtrates obtained from the samples calcined with soda ash were compared, it was observed that the chemistry of the filtered water from the Sarayköy diatomite sample deviated more from the chemistry of the reference water used. In the samples where thenardite was used as a flux, the chemicals expected to dissolve from the flux were Na
+ and SO
42−. It has been determined that these chemicals were slightly higher in the filtrate filtered from the Sarayköy diatomite sample (
Figure 11b). Regardless of the flux used, it was observed that the impurity originating from both flux and diatomite samples was higher in the waters filtered with Sarayköy diatomite. It has been understood that the filtering and grain retention properties of Seydiler diatomite are better. In terms of flux performance, soda ash passed into the filtrate at a higher rate than the thenardite. The better filtering properties of Seyitler from diatomite samples and thenardite from fluxes support the calculated wet cake density values (
Figure 10) and the comments on filtrate clarity.
4. Conclusions
In this study, the effects of the thenardite and widely used soda ash on the industrially important physico-chemical properties of diatomites after flux-calcination were investigated on the samples taken from two diatomite deposits from the Aegean region of Turkey. In raw and calcined diatomite samples, geochemical and mineralogical composition and parameters such as colour, grain size, morphology, specific surface area, permeability and wet cake density were characterized.
Seydiler (Afyon-Turkey) diatomite, with its SiO2 content of more than 98%, is an important raw material of quality that can be used both as a filter aid and filler. When examined in terms of mineralogical composition, it was understood that it was quite pure with less crystalline phase impurities and more amorphous silica phase. These properties of Seydiler diatomite made it prominent in terms of its colour parameters after calcination and made it advantageous in filler applications where whiteness is important.
Although the presence of high Fe2O3, Al2O3 content and clay minerals (e.g., illite) in Sarayköy (Denizli-Turkey) diatomite seem to be a handicap of this sample in terms of impurity and colour, clay minerals provided advantages in terms of permeability, which is an important parameter in filter aids due to their contribution to aggregation.
The specific surface area values of calcined diatomite samples decreased due to aggregation resulting from sintering and shrinkage of the fine particles. It has been understood that the reduced specific surface area is one of the most important reasons for the increased permeability of diatomites. The clay minerals in Sarayköy diatomite accelerate aggregation and even contribute to the formation of larger, porous and spherical pellets with flux calcination.
Fluxes produced different effects on diatomite samples with different geochemistry and mineralogical compositions. In terms of colour, the thenardite provided more whiteness in Seydiler diatomite, while whiter calcined products from Sarayköy diatomite were obtained after soda ash-calcination. In improving permeability, soda ash gave more effective results in both diatomite samples. This shows that higher flow and filtration rate can be obtained in diatomites calcined with soda ash. When evaluated in terms of wet cake density values, it was predicted that diatomite samples calcined with thenardite would provide a clearer filtrate as filter aids after filtration. It has been understood that flux selection should be made according to the usage areas (filler or filter aid) and desired properties of diatomite.
In this study, it was found that thenardite, which was used for the first time as a flux in the calcination of diatomite, was more effective than traditionally used soda ash in improving whiteness and filtrate clarity. However, since the diatomite deposits on earth will show differences in terms of both geochemical and mineralogical composition depending on the different formation environments and conditions of each, it will be beneficial to evaluate the results by calcining them in the laboratory with different fluxes before industrial applications.