Properties of Sm-Doped SrCl2 Crystalline Scintillators
Abstract
:1. Introduction
2. Materials and Methods
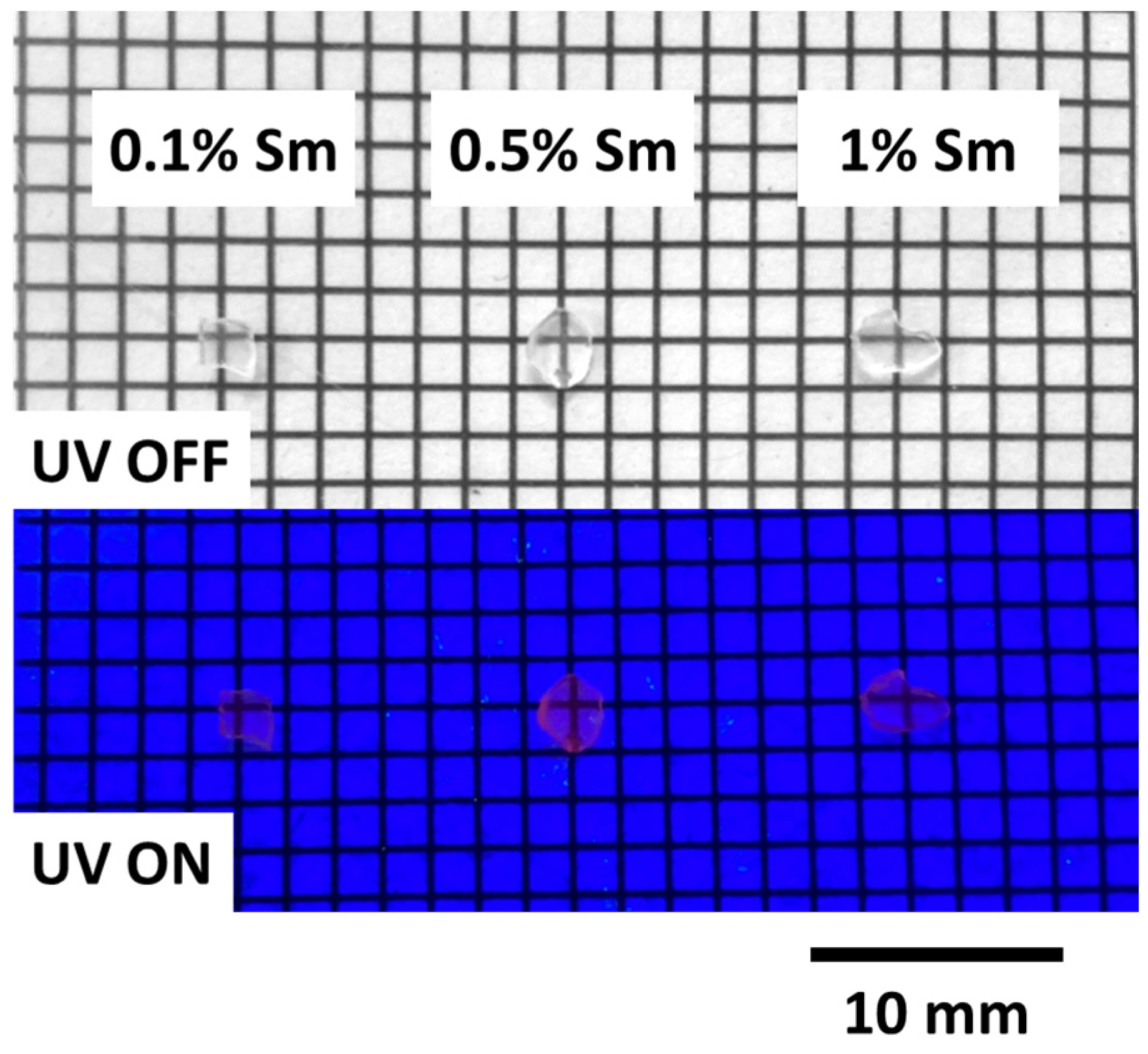
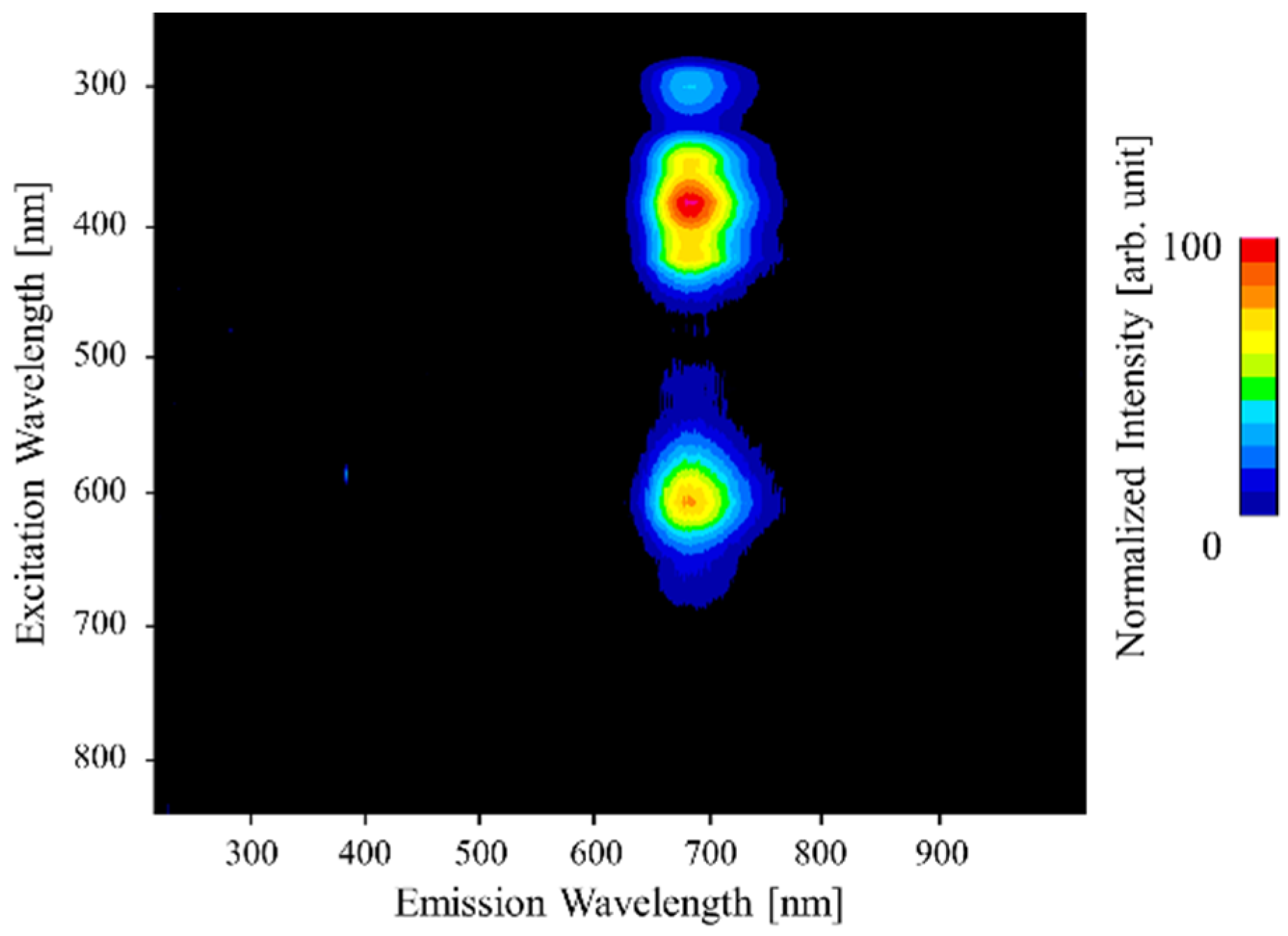
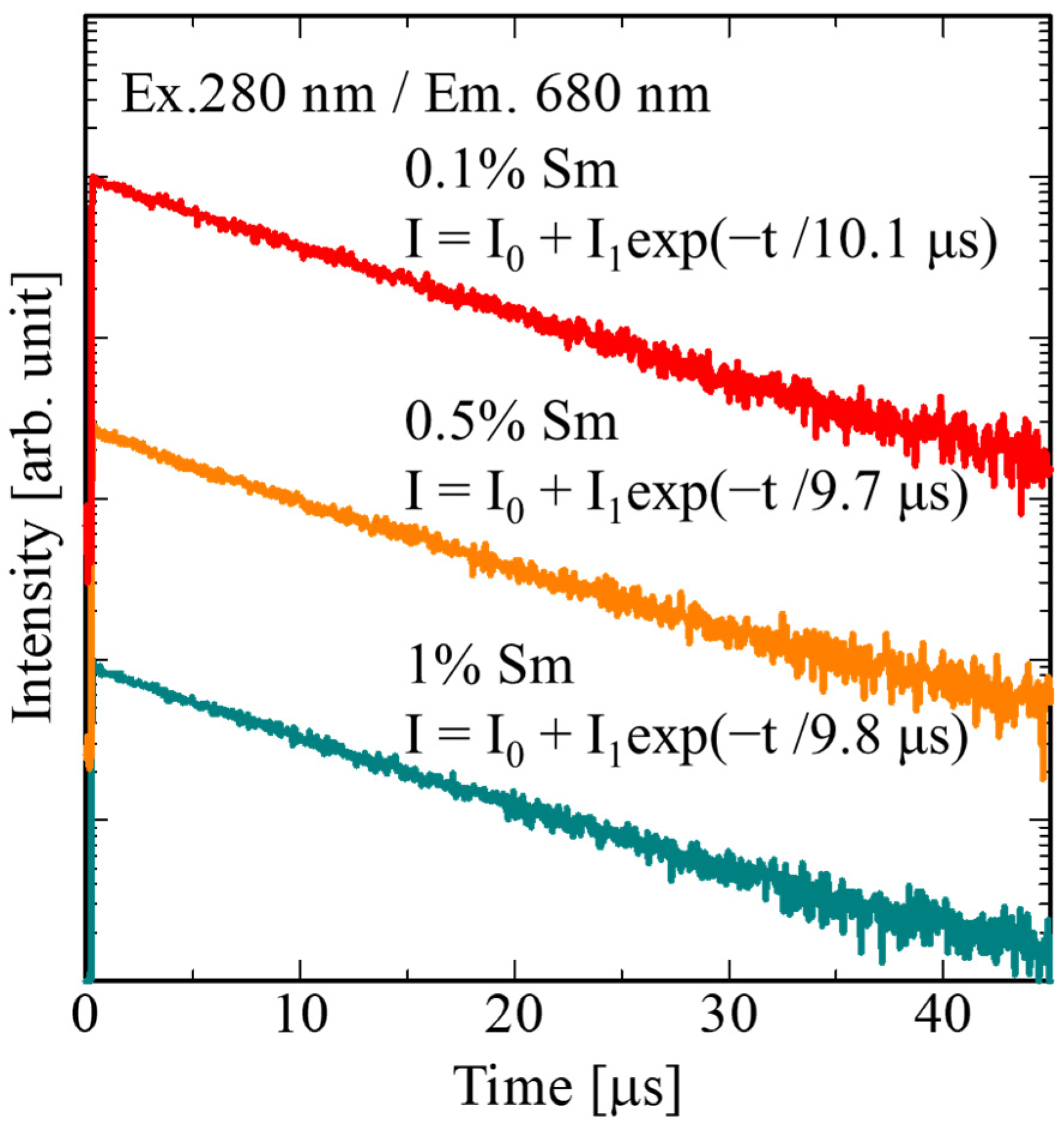
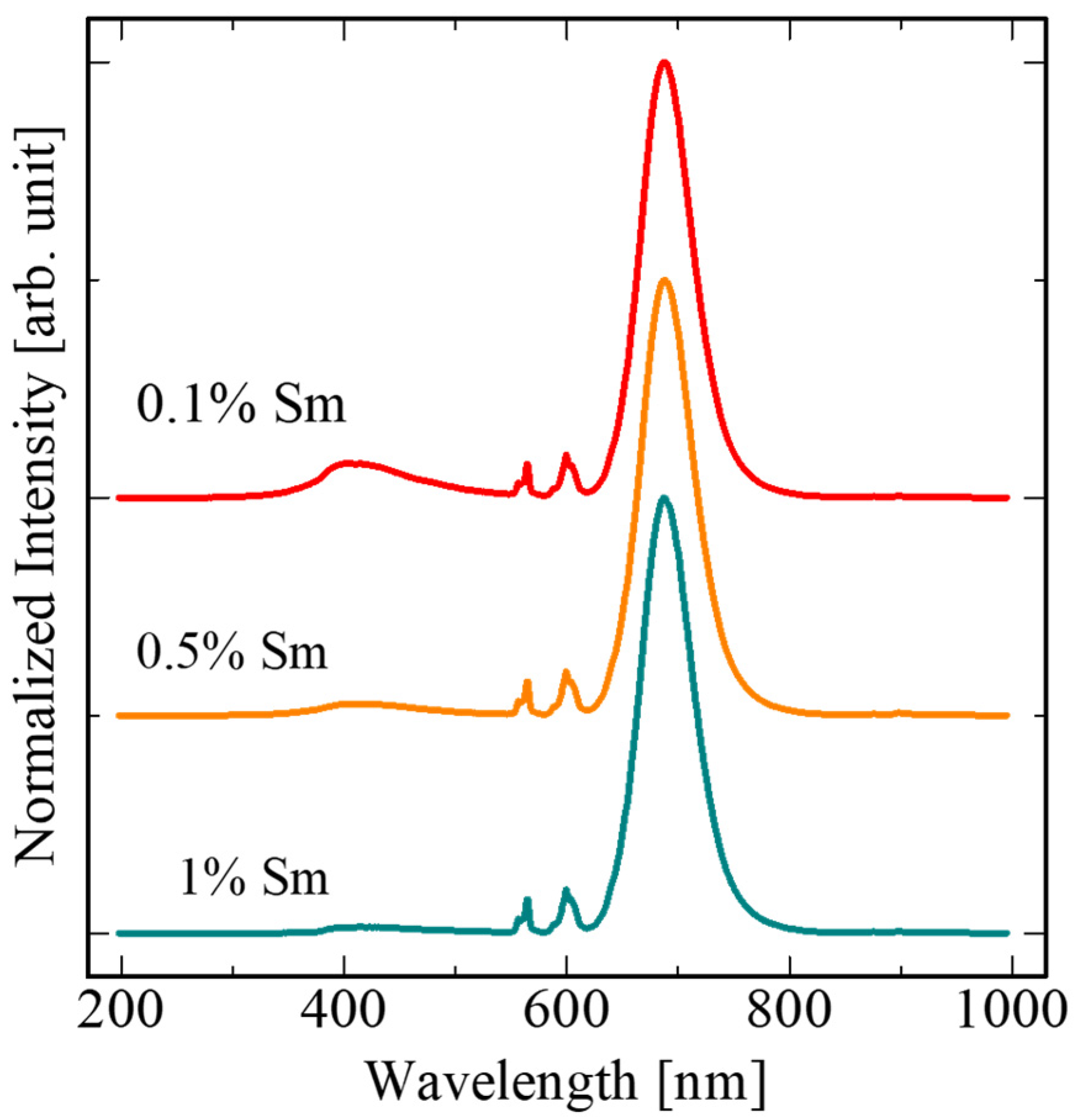
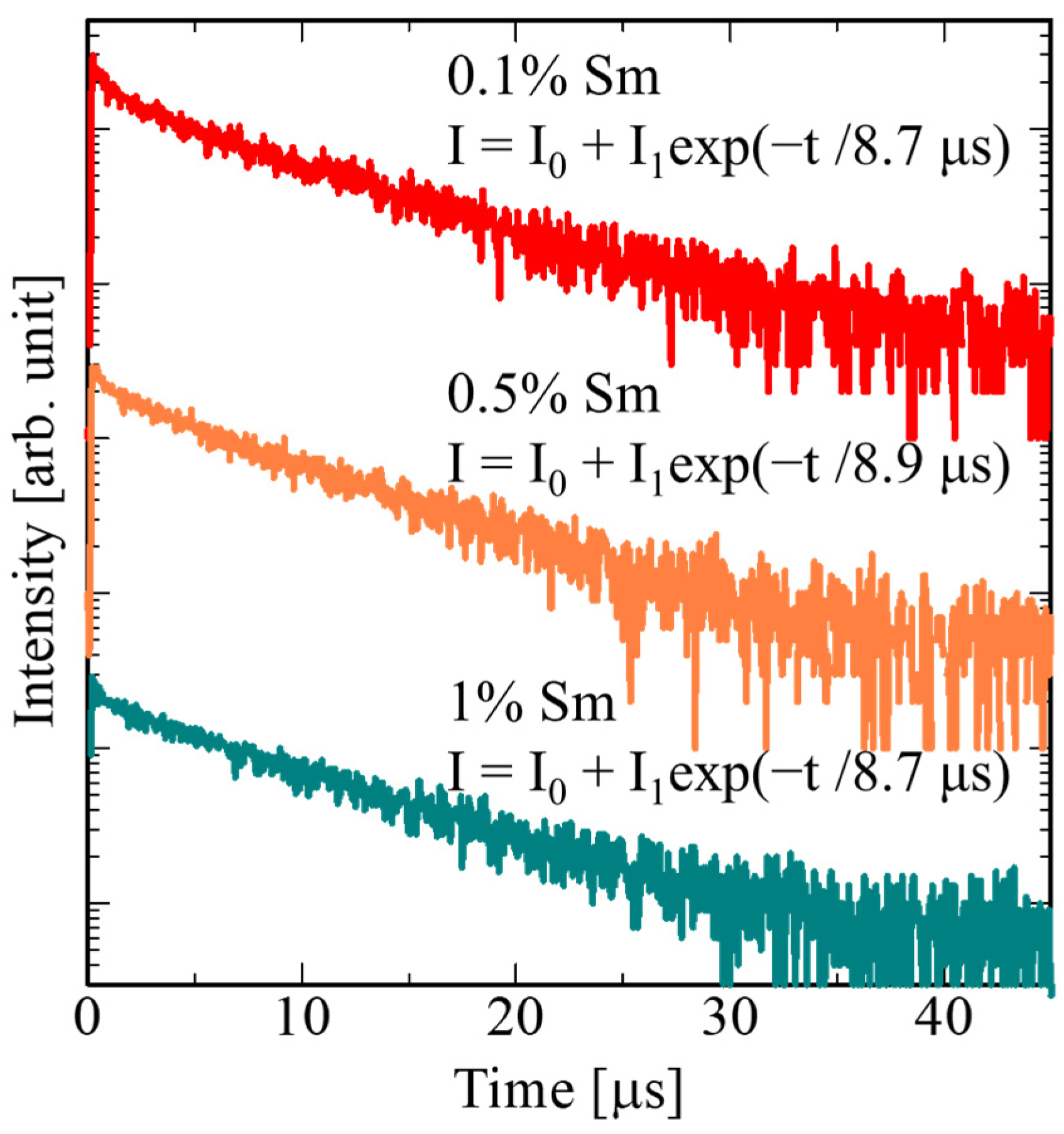
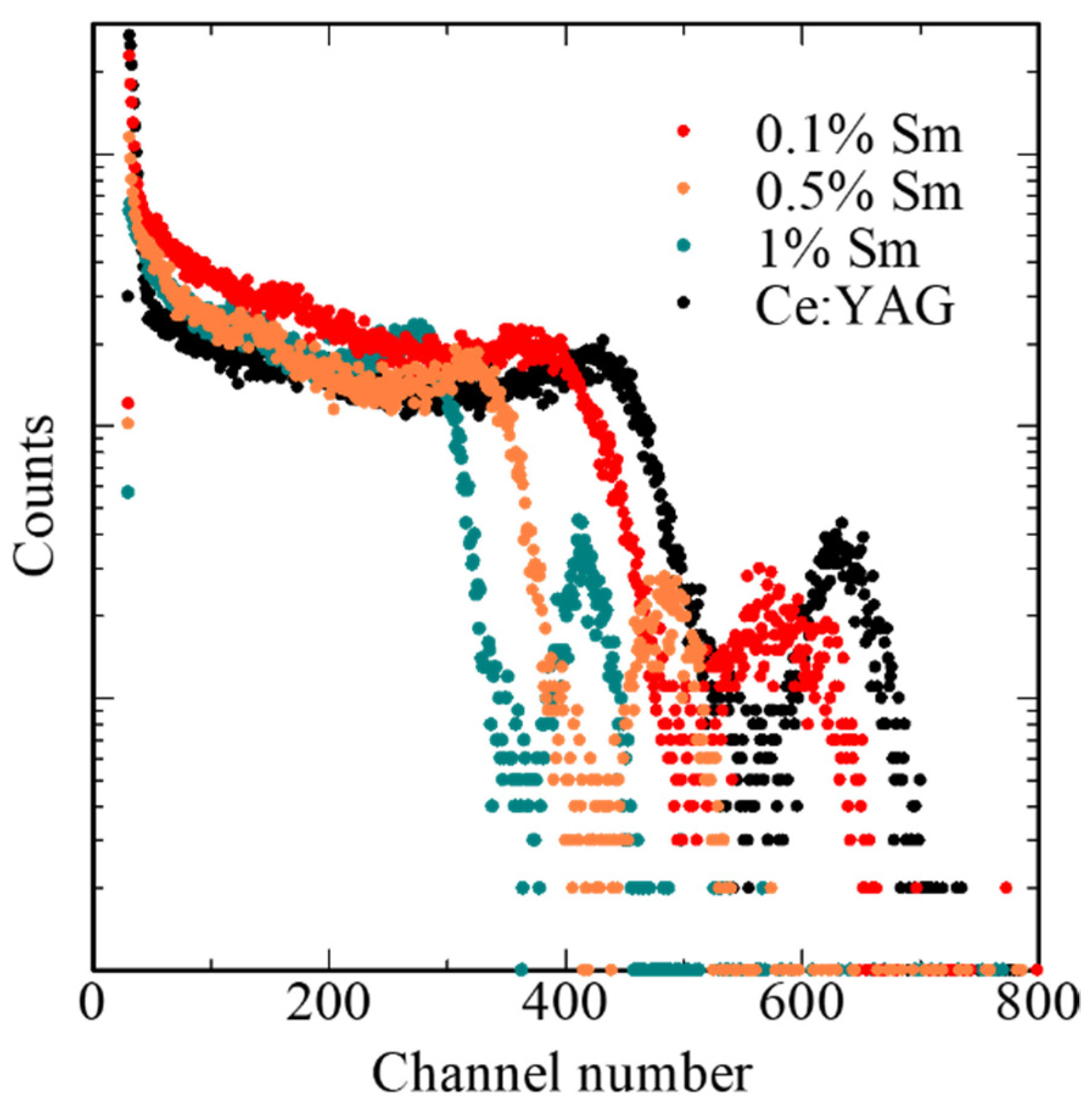
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, S.; Okumura, S.; Kato, N.; Yeom, J.Y. Timing measurements of lutetium based scintillators combined with silicon photomultipliers for TOF-PET system. J. Instrum. 2015, 10, T09002. [Google Scholar] [CrossRef]
- Ishikawa, A.; Yamazaki, A.; Watanabe, K.; Yoshihashi, S.; Uritani, A.; Sakurai, Y.; Tanaka, H.; Ogawara, R.; Suda, M.; Hamano, T. Development of optical-fiber-based neutron detector using Li glass scintillator for an intense neutron field. Sens. Mater. 2020, 32, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Melcher, C.L. Scintillators for well logging applications. Nucl. Instrum. Methods Phys. Res. B 1989, 40, 1214–1218. [Google Scholar] [CrossRef]
- Glodo, J.; Wang, Y.; Shawgo, R.; Brecher, C.; Hawrami, R.H.; Tower, J.; Shah, K.S. New Developments in Scintillators for Security Applications. Phys. Procedia 2017, 90, 285–290. [Google Scholar] [CrossRef]
- Itoh, T.; Yanagida, T.; Kokubun, M.; Sato, M.; Miyawaki, R.; Makishima, K.; Takashima, T.; Tanaka, T.; Nakazawa, K.; Takahashi, T.; et al. A 1-dimensional γ-ray position sensor based on GSO:Ce scintillators coupled to a Si strip detector. Nucl. Instrum. Methods Phys. Res. A 2007, 579, 239–242. [Google Scholar] [CrossRef]
- Salonen, L. A rapid method for monitoring of uranium and radium in drinking water. Sci. Total Environ. 1993, 130, 23–35. [Google Scholar] [CrossRef]
- Shirakawa, Y. Development of a direction finding gamma-ray detector. Nucl. Instrum. Methods Phys. Res. B 2007, 263, 58–62. [Google Scholar] [CrossRef]
- Dorenbos, P. The quest for high resolution γ-ray scintillators. Opt. Mater. X 2019, 1, 100021. [Google Scholar] [CrossRef]
- Kim, C.; Lee, W.; Melis, A.; Elmughrabi, A.; Lee, K.; Park, C.; Yeom, J.-Y. A Review of Inorganic Scintillation Crystals for Extreme Environments. Crystals 2021, 11, 669. [Google Scholar] [CrossRef]
- Kumar, V.; Luo, Z. A Review on X-ray Excited Emission Decay Dynamics in Inorganic Scintillator Materials. Photonics 2021, 8, 71. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Quek, C.-H.; Leong, K.W. Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging. Nanomaterials 2012, 2, 92–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijnheer, B.; Beddar, S.; Izewska, J.; Reft, C. In vivo dosimetry in external beam radiotherapy. Med. Phys. 2013, 40, 070903. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Sapre, A.A.; Novitskaya, E.; Vakharia, V.; Cota, A.; Wrasidlo, W.; Hanrahan, S.M.; Derenzo, S.; Makale, M.T.; Graeve, O.A. Optimized scintillator YAG:Pr nanoparticles for X-ray inducible photodynamic therapy. Mater. Lett. 2018, 228, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lv, B.; Tang, Z.; Zhang, M.; Ge, W.; Liu, Y.; He, X.; Zhao, K.; Zheng, X.; He, M.; et al. Scintillator-Based Nanohybrids with Sacrificial Electron Prodrug for Enhanced X-ray-Induced Photodynamic Therapy. Nano Lett. 2018, 18, 5768–5774. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kondo, K.; Hirabayashi, H.; Kurokawa, S.; Taino, M.; Yamamoto, A.; Sugimoto, S.; Yoshida, H.; Wada, T.; Nakagawa, Y.; et al. Radiation damage of BGO crystals due to low energy γ rays, high energy protons and fast neutrons. Nucl. Instrum. Methods Phys. Res. 1983, 206, 107–117. [Google Scholar] [CrossRef]
- Gurzhiev, A.N.; Turchanovich, L.K.; Vasil’chenko, V.G.; Bogatyrjov, V.A.; Mashinsky, V.M. Radiation damage in optical fibers. Nucl. Instrum. Methods Phys. Res. A 1997, 391, 417–422. [Google Scholar] [CrossRef]
- Dai, X.; Rollin, E.; Bellerive, A.; Hargrove, C.; Sinclair, D.; Mifflin, C.; Zhang, F. Wavelength shifters for water Cherenkov detectors. Nucl. Instrum. Methods Phys. Res. A 2008, 589, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Jiang, S.; Jia, M.; Gunn, J.; Miao, T.; Davis, S.C.; Bruza, P.; Pogue, B.W. Observation of short wavelength infrared (SWIR) Cherenkov emission. Opt. Lett. 2018, 43, 3854–3857. [Google Scholar] [CrossRef]
- Kimura, H.; Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Radiation-induced Luminescence Properties of SrBr2 Transparent Ceramics Doped with Different Eu Concentrations. Sens. Mater. 2020, 32, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Sekine, D.; Fujimoto, Y.; Koshimizu, M.; Nakauchi, D.; Yanagida, T.; Asai, K. Photoluminescence and scintillation properties of Yb2+-doped SrCl2 crystals. Jpn. J. Appl. Phys. 2020, 59, 012005. [Google Scholar] [CrossRef]
- Mizoi, K.; Arai, M.; Fujimoto, Y.; Nakauchi, D.; Koshimizu, M.; Yanagida, T.; Asai, K. Photoluminescence and scintillation properties of Yb2+-doped SrCl2-xBrx (x = 0, 1.6, 2.0) crystals. Appl. Phys. Express 2020, 13, 112008. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Hull, G.; Drobshoff, A.D.; Payne, S.A.; Van Loef, E.; Wilson, C.M.; Shah, K.S.; Roy, U.N.; Burger, A.; Boatner, L.A.; et al. Strontium and barium iodide high light yield scintillators. Appl. Phys. Lett. 2008, 92, 083508. [Google Scholar] [CrossRef] [Green Version]
- Mizoi, K.; Arai, M.; Fujimoto, Y.; Nakauchi, D.; Koshimizu, M.; Yanagida, T.; Asai, K. Evaluation of photoluminescence and scintillation properties of Yb2+-doped SrCl2-xBrx crystals. J. Ceram. Soc. Jpn. 2021, 129, 406–414. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Li, S.; Xu, X. Dynamic studies on the time-resolved fluorescence of Sm2+ in BaCl2. J. Lumin. 2002, 97, 102–106. [Google Scholar] [CrossRef]
- Dixie, L.C.; Edgar, A.; Reid, M.F. Sm2+ fluorescence and absorption in cubic BaCl2: Strong thermal crossover of fluorescence between 4f6 and 4f55d1 configurations. J. Lumin. 2012, 132, 2775–2782. [Google Scholar] [CrossRef]
- Karbowiak, M.; Solarz, P.; Lisiecki, R.; Ryba-Romanowski, W. Optical spectra and excited state relaxation dynamics of Sm2+ ions in SrCl2, SrBr2 and SrI2 crystals. J. Lumin. 2018, 195, 159–165. [Google Scholar] [CrossRef]
- Dixie, L.C.; Edgar, A.; Bartle, M.C. Spectroscopic and radioluminescence properties of two bright X-ray phosphors: Strontium barium chloride doped with Eu2+ or Sm2+ ions. J. Lumin. 2014, 149, 91–98. [Google Scholar] [CrossRef]
- Dixie, L.C.; Edgar, A.; Bartle, C.M. Samarium doped calcium fluoride: A red scintillator and X-ray phosphor. Nucl. Instrum. Methods Phys. Res. A 2014, 753, 131–137. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Alekhin, M.S.; Biner, D.A.; Krämer, K.W.; Dorenbos, P. Converting SrI2:Eu2+ into a near infrared scintillator by Sm2+ co-doping. J. Lumin. 2019, 212, 1–4. [Google Scholar] [CrossRef]
- Wolszczak, W.; Krämer, K.W.; Dorenbos, P. CsBa2I5:Eu2+,Sm2+—The First High-Energy Resolution Black Scintillator for γ-Ray Spectroscopy. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 201900158. [Google Scholar] [CrossRef]
- Alekhin, M.S.; Awater, R.H.P.; Biner, D.A.; Krämer, K.W.; De Haas, J.T.M.; Dorenbos, P. Luminescence and spectroscopic properties of Sm2+ and Er3+ doped SrI2. J. Lumin. 2015, 167, 347–351. [Google Scholar] [CrossRef]
- Nakauchi, D.; Fujimoto, Y.; Kato, T.; Kawaguchi, N.; Yanagida, T. X- And γ-ray response of Sm-doped SrBr2 crystalline scintillators emitting red-NIR photons. Jpn. J. Appl. Phys. 2021, 60, 092002. [Google Scholar] [CrossRef]
- Kanchana, V.; Vaitheeswaran, G.; Svane, A. Calculated structural, elastic and electronic properties of SrCl2. J. Alloys Compd. 2008, 455, 480–484. [Google Scholar] [CrossRef]
- Chaminade, J.P.; Viraphong, O.; Guillen, F.; Fouassier, C.; Czirr, B. Crystal growth and optical properties of new neutron detectors Ce3+:Li6R(BO3)3 (R=Gd,Y). IEEE Trans. Nucl. Sci. 2001, 48, 1158–1161. [Google Scholar] [CrossRef]
- Yanagida, T.; Kamada, K.; Fujimoto, Y.; Yagi, H.; Yanagitani, T. Comparative study of ceramic and single crystal Ce:GAGG scintillator. Opt. Mater. 2013, 35, 2480–2485. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Ito, T.; Uchiyama, K.; Mori, K. Development of X-ray-induced afterglow characterization system. Appl. Phys. Express 2014, 7, 062401. [Google Scholar] [CrossRef]
- Antonyak, O.T.; Chornodolskyy, Y.M.; Syrotyuk, S.V.; Gloskovska, N.V.; Gamernyk, R. V High-energy electronic excitations and radiation defects in SrCl2 crystals. Mater. Res. Express 2017, 4, 116306. [Google Scholar] [CrossRef]
- Yanagida, T.; Takahashi, H.; Ito, T.; Kasama, D.; Enoto, T.; Sato, M.; Hirakuri, S.; Kokubun, M.; Makishima, K.; Yanagitani, T.; et al. Evaluation of properties of YAG (Ce) ceramic scintillators. IEEE Trans. Nucl. Sci. 2005, 52, 1836–1841. [Google Scholar] [CrossRef]
- Bourret-Courchesne, E.D.; Bizarri, G.A.; Borade, R.; Gundiah, G.; Samulon, E.C.; Yan, Z.; Derenzo, S.E. Crystal growth and characterization of alkali-earth halide scintillators. J. Cryst. Growth 2012, 352, 78–83. [Google Scholar] [CrossRef]
- Robbins, D.J. On Predicting the Maximum Efficiency of Phosphor Systems Excited by Ionizing Radiation. J. Electrochem. Soc. 1980, 127, 2694–2702. [Google Scholar] [CrossRef]
- Lempicki, A.; Wojtowicz, A.J.; Berman, E. Fundamental limits of scintillator performance. Nucl. Instrum. Methods Phys. Res. A 1993, 333, 304–311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakauchi, D.; Fujimoto, Y.; Kato, T.; Kawaguchi, N.; Yanagida, T. Properties of Sm-Doped SrCl2 Crystalline Scintillators. Crystals 2022, 12, 517. https://doi.org/10.3390/cryst12040517
Nakauchi D, Fujimoto Y, Kato T, Kawaguchi N, Yanagida T. Properties of Sm-Doped SrCl2 Crystalline Scintillators. Crystals. 2022; 12(4):517. https://doi.org/10.3390/cryst12040517
Chicago/Turabian StyleNakauchi, Daisuke, Yutaka Fujimoto, Takumi Kato, Noriaki Kawaguchi, and Takayuki Yanagida. 2022. "Properties of Sm-Doped SrCl2 Crystalline Scintillators" Crystals 12, no. 4: 517. https://doi.org/10.3390/cryst12040517
APA StyleNakauchi, D., Fujimoto, Y., Kato, T., Kawaguchi, N., & Yanagida, T. (2022). Properties of Sm-Doped SrCl2 Crystalline Scintillators. Crystals, 12(4), 517. https://doi.org/10.3390/cryst12040517




