Abstract
The objective of this work was to obtain glass-ceramics from stable glasses, with a composition of barium, lead, and potassium titanate phases, for use as semiconductors. For this purpose, the glass-ceramic technique was used to control crystal growth and obtain a fine-grained microstructure. Various glasses containing K2O, PbO, BaO, Al2O3, B2O3, and TiO2 were prepared using a melt-quenching method. X-ray diffraction (XRD) and scanning electron microscopy (SEM) showed a single amorphous phase of all samples. Infrared spectra confirmed the presence of B-O bonds stretching vibrations of (B3O6)3− boroxol rings and BO3 triangles, as well as Ti-O stretching vibrations of (TiO6/2) and (AlO6/2) octahedral units. Thermal analyses confirmed the presence of one or more crystallization peaks in the range of 700 to 744 °C. On this base, they were heat-treated to promote crystal growth. XRD and SEM detected Ba4Ti12O27, Ti7O13, and BaTiO3 phases, homogeneously distributed throughout the material with fine crystallite size. In addition, crystallized glasses’ (glass-ceramics) properties were determined; the density values were 2.8–3.55 g/cm3; the chemical resistance to acidic and basic media was low; and the band-gap values were in the range of 2.88 to 3.05 eV. These results suggest that crystallized glasses may have application in photocatalysis.
1. Introduction
The crystallization of glasses is a process by which polycrystalline solids (glass-ceramic, GC) can be obtained, and they have advantages on precursor glasses such as higher mechanical and chemical resistance. Moreover, during processing, it can control the growth of crystalline phases, resulting in fine-sized microstructure materials with specific properties for varied applications. In this sense, for many years, TiO2 glasses and glass-ceramics have had a great interest in fundamental and technologic research due to their optical and chemical properties [1]. However, the main problem reported is obtaining stable glasses (without superficial or volume crystallization) since it is well-known that TiO2 increases spontaneous crystallization tendency during cooling. Despite this, several researchers have obtained stable glasses in oxide systems such as BaO-TiO2-B2O3, K2O-Al2O3-TiO2, BaO-PbO-B2O3-Al2O3-TiO2, K2O-BaO-B2O3-Al2O3-TiO2, PbO-TiO2-Al2O3-SiO2, BaO-PbO-TiO2-B2O3-SiO2, 55[(PbxBi1-x)TiO3]-44[2SiO2·B2O3]-1CeO2, and others [2,3,4,5,6,7,8,9]. In these systems, the introduction of fluxes and modifier oxides was necessary to obtain a stable glassy structure. Furthermore, the controlled crystallization of glasses was reported to obtain glass-ceramics with titanate phases, such as BaTiO3, PbTiO3 (K0.58Ba0.36)2Ti6O13, BaTi2O5, and Bi2Ti2O7, among others.
There is extensive research on titanate-type materials, with sufficient information concerning barium, bismuth, strontium, lead, copper, lanthanum, and lithium titanates, among others. For example, BaTiO3 is the most widely investigated ferroelectric material, and it possesses very stable chemical and mechanical behavior. Additionally, it presents dielectric, ferroelectric, and piezoelectric properties [3,4,10]. It can also act as a semiconductor by adding specific doping [10] and together with PbTiO3 are two of the most relevant ferroelectric ceramics useful in many technologic applications [11,12]. Another titanate reported in the literature is CaCu3Ti4O12, which exhibits the highest dielectric constant (>105) but a considerably high dielectric loss [13]. In addition, SrTiO3 is used to fabricate capacitive sensors at cryogenic temperatures. On the other hand, Li2O·La2O3·4TiO2 is useful in sensors and solid-state batteries because of Li+’s high ionic conductivity [14,15,16,17,18,19]. Likewise, β-Al2TiO5 or tialite present high mechanical and thermal shock resistance, which allows its use in applications where these properties are required [20]. One more of the titanates is K2Ti6O13, fibrous material, considered a lower bandgap semiconductor, photo-catalytic, and reinforcing agent used to produce hydrogen from water-splitting reaction [21,22,23].
In previous work, we reported several stable one-phase glasses in the K2O-BaO-B2O3-Al2O3-TiO2 system; however, a high quantity of K2O (20–25 mol%) and a relatively low amount of BaO (3–12 mol%) were used [9]. On the other hand, Ruiz-Valdez et al. [3] reported stable titanate glasses with a composition containing BaO (18–22 mol%) and PbO (4–9 mol%), successfully crystallized. The results showed a mixture of barium and lead titanate polymorphs with crystal sizes between 90 and 200 nm. Considering the mentioned works, in this study, we proposed the following mixture (mol%): (9) K2O, (23.5) BaO, (0.5) PbO, (13) B2O3, (16) Al2O3, and (38) TiO2 as base composition, varying only in the BaO and PbO content. The objective was to obtain stable one-phase glasses and glass-ceramics, including crystalline phases such as barium, lead, and potassium titanates, with homogeneous fine grain structure, as promising materials for optical and electronic applications. In addition, taking into account that most of the research is focused on the study of ferroelectric properties (dielectric constant and losses) [2,3,24,25,26,27,28], another aim of this work was to obtain glass-ceramics materials with a mixture of crystalline titanate phases to understand their structural, chemical, and optical properties in detail. The above is to discover the great variety of technological applications, principally, semiconductors.
2. Materials and Methods
2.1. Glass Synthesis
The reagents (>99% purity) used for the synthesis of the glasses were: TiO2, K2CO3, Ba(NO3)2, PbO, Al2O3, and H3BO3; stoichiometric amounts of each reagent, according to Table 1, were weighed, homogenized in an agate mortar, and deposited in a platinum crucible and introduced in a furnace (Lindberg blue M, BF51433PC-1). The furnace program included heating up to 400 °C and maintained for 1 h (to decompose boric acid and avoid losses due to volatilization [29]), followed by heating up to 1350 °C and maintained for 1 h; the heating rate used in both stages was 10 °C/min. The molten glass was poured onto a stainless-steel plate at room temperature.

Table 1.
Samples identification, composition, Tg, and TC.
2.2. Glass Characterization
A piece of each glass was ground in an agate mortar, so the powder was characterized by differential thermal analysis (DTA, Netzsch STA 449 F1 Jupiter). The studies were performed from room temperature up to 1000 °C in the N2 atmosphere and using a heating rate of 10 °C/min. The glass transition temperature (Tg) was determined by the inflection point method, and the crystallization temperature (Tc) was taken as the higher point of each peak. In addition, the structure was studied by X-ray diffraction (XRD, Philips X’pert, Cu Kα radiation) using pulverized samples with particle size less than 212 μm (mesh 70). The infrared analyses (FTIR, ABB MB3000) were performed using a mixture of KBr and glass powder, pressed into a disk form, and carried out from 500 to 3500 cm−1. The microstructure was investigated by scanning electron microscopy (SEM, Jeol microscope JSM-6610, equipped with an EDS detector), a piece of glass was mounted into quick-setting resin, then sanded with silicon carbide paper of different grain, subsequently were diamond-paste polished, and, finally, were carbon-coated to make them conductive.
2.3. Glass Crystallization
Based on the DTA results, the glasses were heat-treated to crystallize at the temperature of the first crystallization peak. The resulting materials were designated as GC1 to GC5, Table 1. The heat treatment was carried out in an electric furnace (Kodiak) from room temperature to the desired temperature using a heating rate of 10 °C/min for 2 h; a new piece of parent glass was used for each treatment.
2.4. Glass-Ceramic Characterization
The glass-ceramics obtained were characterized by XRD and SEM. The preparation of the samples was performed in the same way described for glasses characterization. The glassy and crystalline phases were calculated from the XRD patterns; first, a baseline was drawn where the diffractogram begin and end, then the area of each peak was delimited and calculated using the number of counts multiplied by the angle two theta (°). The % area gives us an estimated content of each phase. In addition, samples were analyzed using the Scherrer equation to estimate the average size of the crystals. For the calculation the first four peaks of each diffractogram, Equation (1) was used:
where: L = Crystallite size (nm); K = Scherrer constant; l = wavelength; B = FWHM (full width at half maximum); and θ = diffraction angle.
The chemical resistance in acid and basic media was measured by the Russian standard GOST 10134-82, using HCl and NaOH 1N solutions, respectively [30]. For the analyses, powder samples were deposited in PE containers with the corresponding solution with a ratio of 1:10 (glass:water) and then introduced in a stove (Lab-Line, Imperial V) at 96 °C for 3 h. Then, the solutions were filtered and dried, and the losses (in weight %) were calculated using Equation (2):
where: mi = initial mass, and mf = final mass.
Density was measured by the pycnometer method using a calibrated 25 mL glass pycnometer, 5 g of sample powder, and toluene as the immersion liquid. Density was calculated using Equation (3) [31]:
where: d = density; d1 = immersion liquid density (toluene = 0.8623 g/cm3 at 25 °C); m = pycnometer mass; m1 = pycnometer mass plus sample mass; m2 = pycnometer plus sample plus liquid mass; and m3 = pycnometer plus liquid mass.
For both chemical resistance and density, each sample was measured in triplicate, and the values obtained were averaged.
On the other hand, band-gap energy was measured using a UV-Vis spectrophotometer (Perkin Elmer LAMBDA-10) equipped with an integration sphere for diffuse reflectance studies. The results were converted to Kubelka–Munk units [22] through Equation (4):
where: R is the diffuse reflectance, and F(R) is the Kubelka–Munk function.
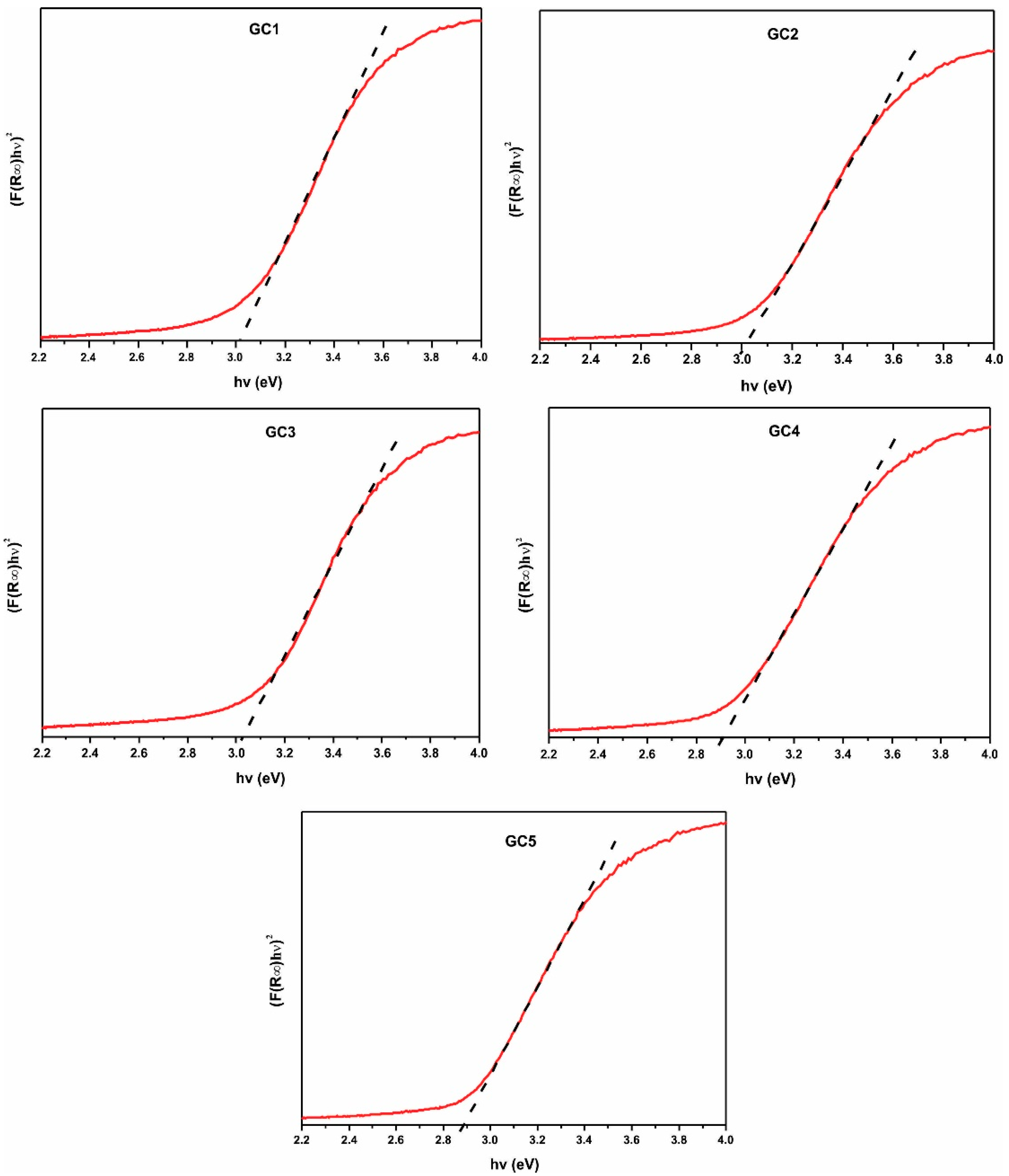
To determine the direct bandgap energy of the samples, it was extrapolated the linear region from the inflection point of the diffuse reflectance spectrum to the intersection of the photon energy axis (X-axis). This point gives the value of the material bandgap.
3. Results and Discussion
3.1. Glass Characterization
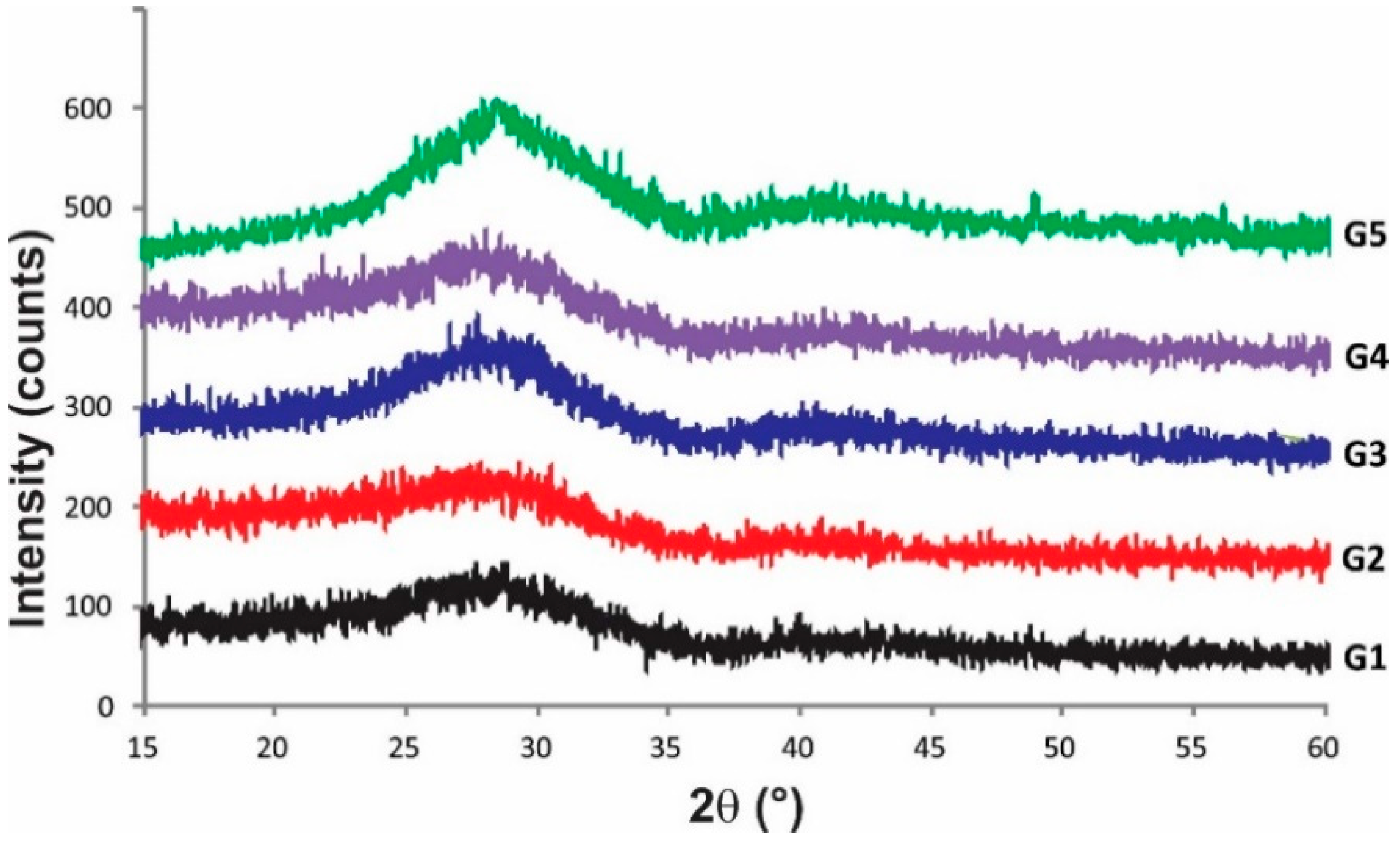
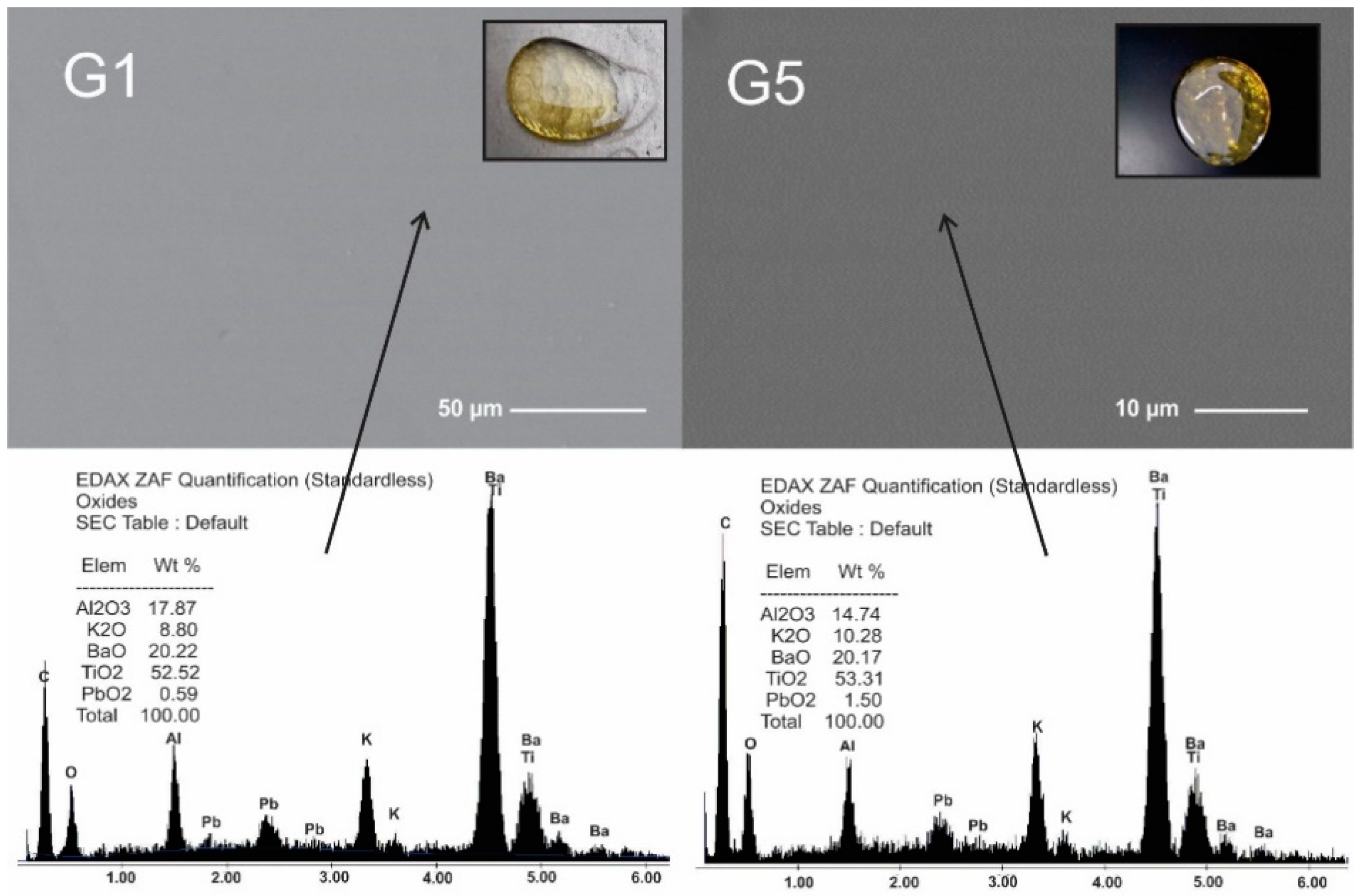
All selected compositions fused and formed yellowish stable glass. Figure 1 presents XRD patterns and can be appreciated the absence of diffraction peaks which indicated that materials were fully amorphous. The microstructure of glasses showed unique homogeneous phase free pores and cracks, Figure 2. These glasses had similar microstructure to those reported in previous work [9], but different from the microstructure reported by Ruiz-Valdes et al. [5]. Although the EDS results are semi-quantitative, since no boron can be detected, the increase in PbO in the compositions can be seen.
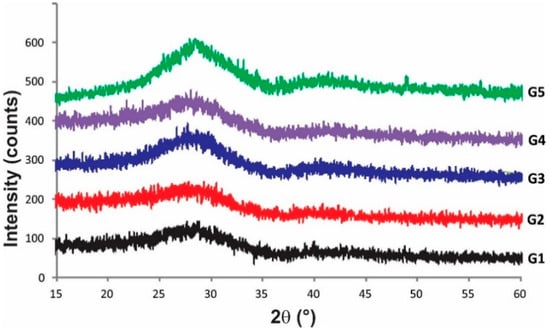
Figure 1.
The XRD patterns of synthesized glasses G1–G5 show their amorphous nature.
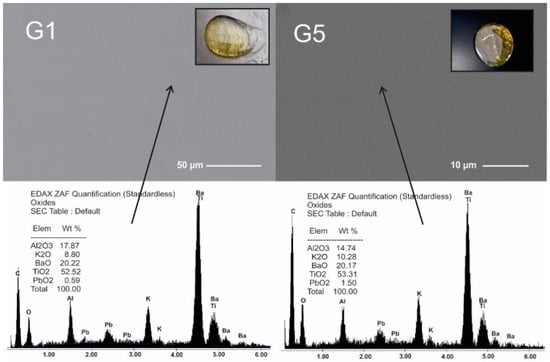
Figure 2.
SEM Micrographs and EDS quantification of G1 and G5 glasses. The arrows indicate the point where EDS was performed. The image of each micrograph shows the glass obtained.
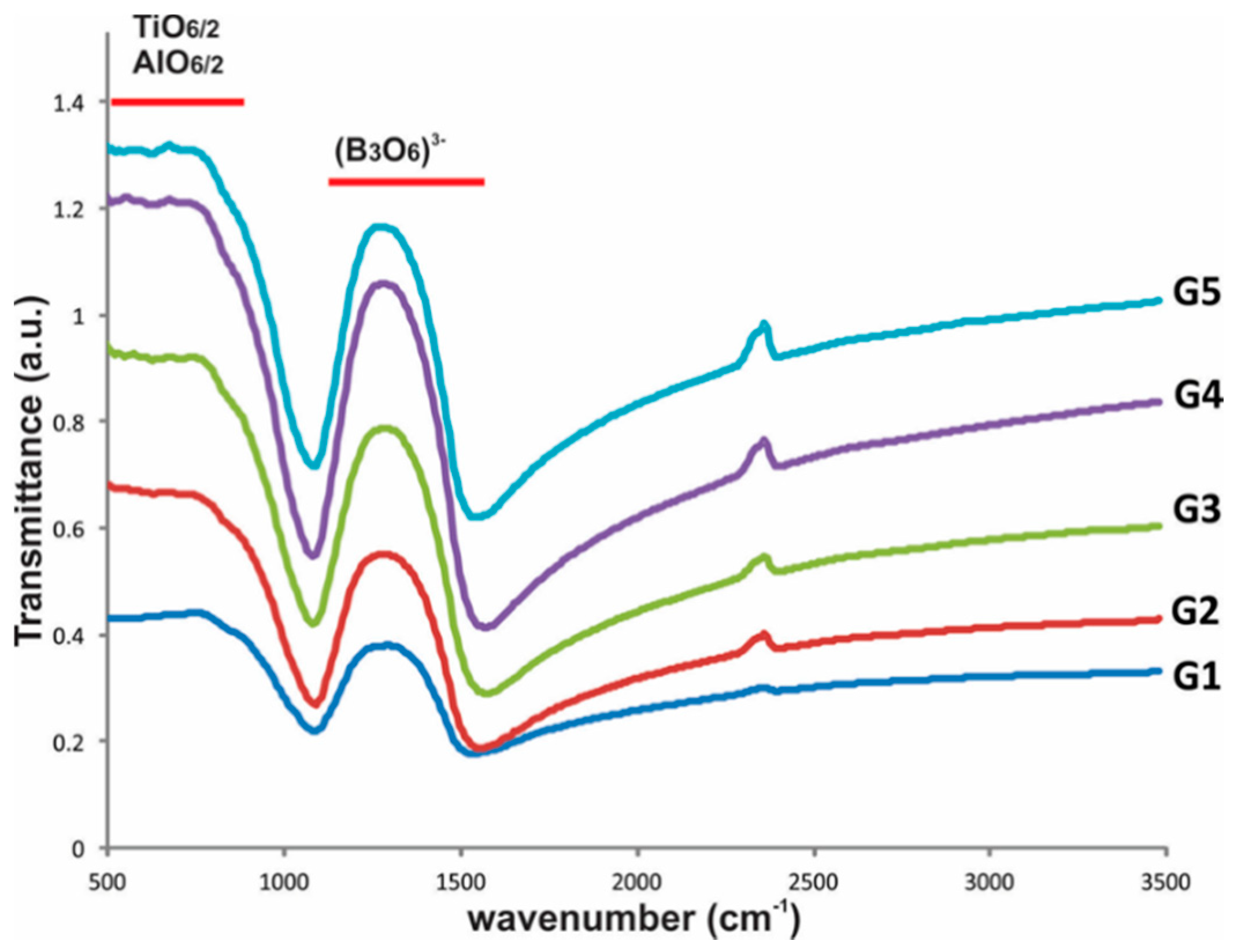
Glass characterization by FTIR, Figure 3, showed the presence of two absorption bands: the first at wave number range 1100–1500 cm−1, which is associated with the B-O bond stretching vibrations and B-O bridging between (B3O6)3− boroxol rings and BO3 triangles [32,33]. The second band appeared at wave number range 500–900 cm−1, attributed to the Ti-O stretching vibrations of the octahedral [TiO6/2] and [AlO6/2] units [4,20,29,34].
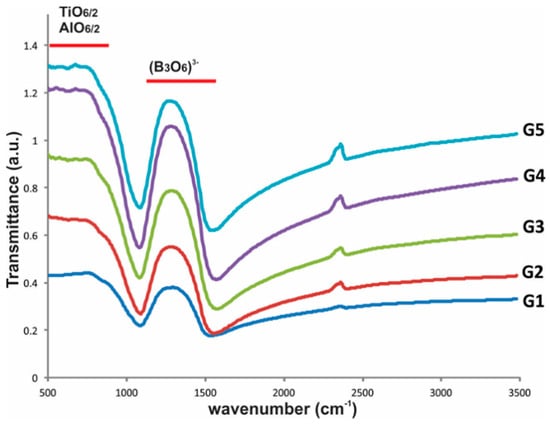
Figure 3.
FTIR results of the glasses.
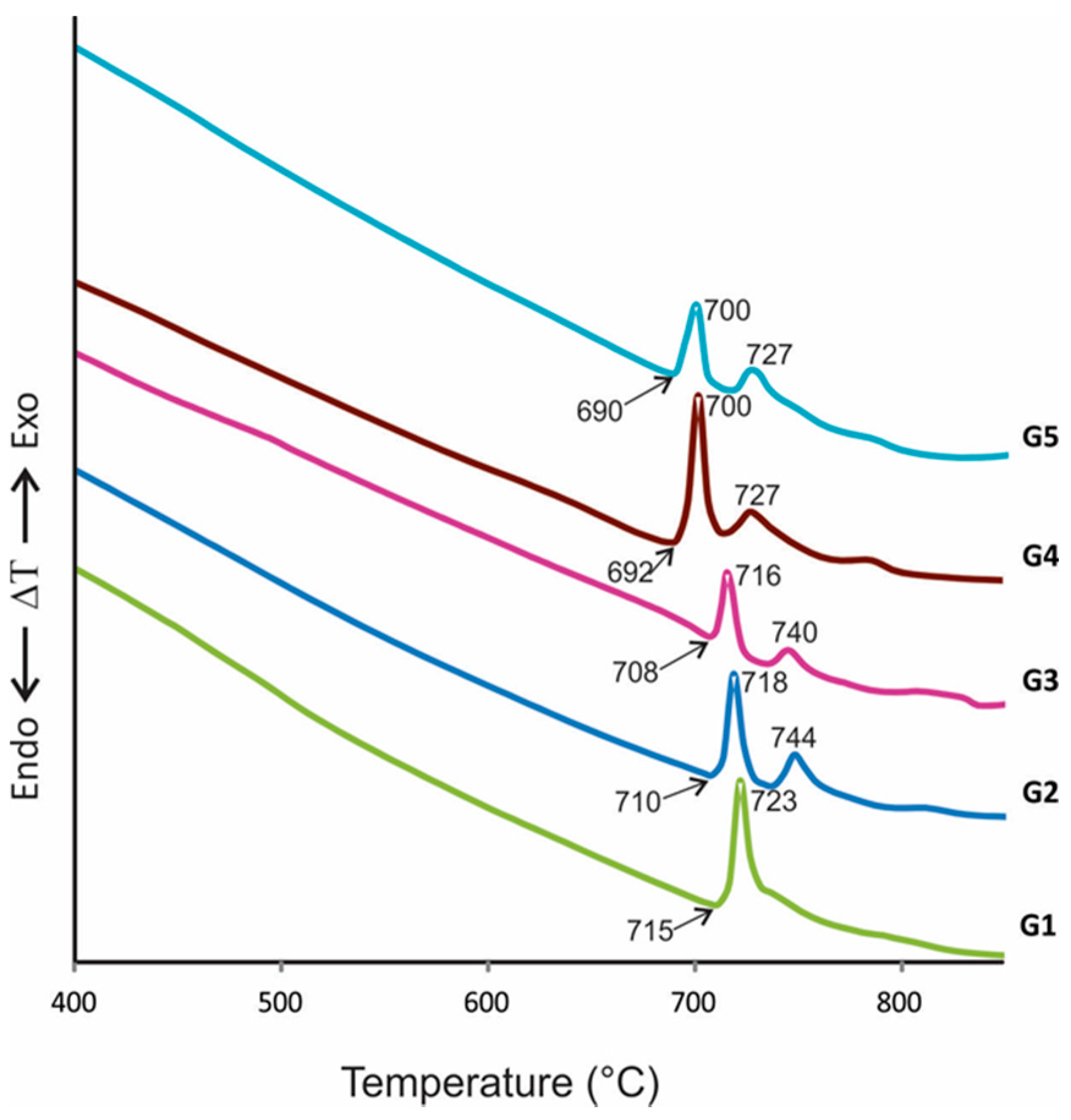
On the other hand, Figure 4 shows the DTA curves of glass samples. The curves observed one or two exothermic peaks at temperatures between 700 and 744 °C, attributed to crystallization events, indicating that all samples can crystallize to obtain glass-ceramics materials with at least one crystalline phase. Likewise, it can be appreciated that an increasing PbO content in starting compositions from 0.5% (G1) to 4.5% (G5) diminished Tg and Tc temperatures, which is essential for economic reasons related to energetic costs.
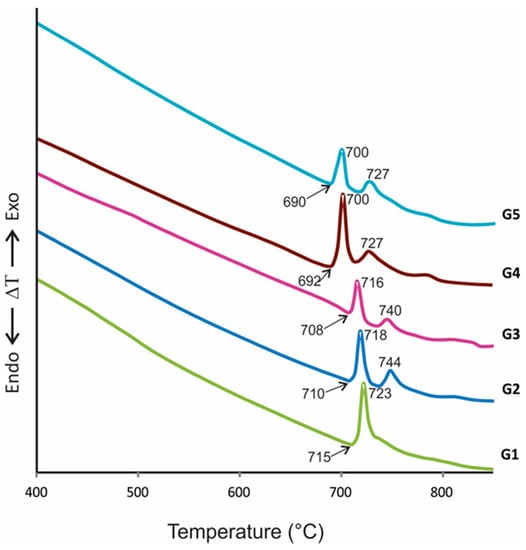
Figure 4.
DTA curves of the glasses show Tg (value marked whit arrow) and Tc temperatures.
3.2. Glass-Ceramic Characterization
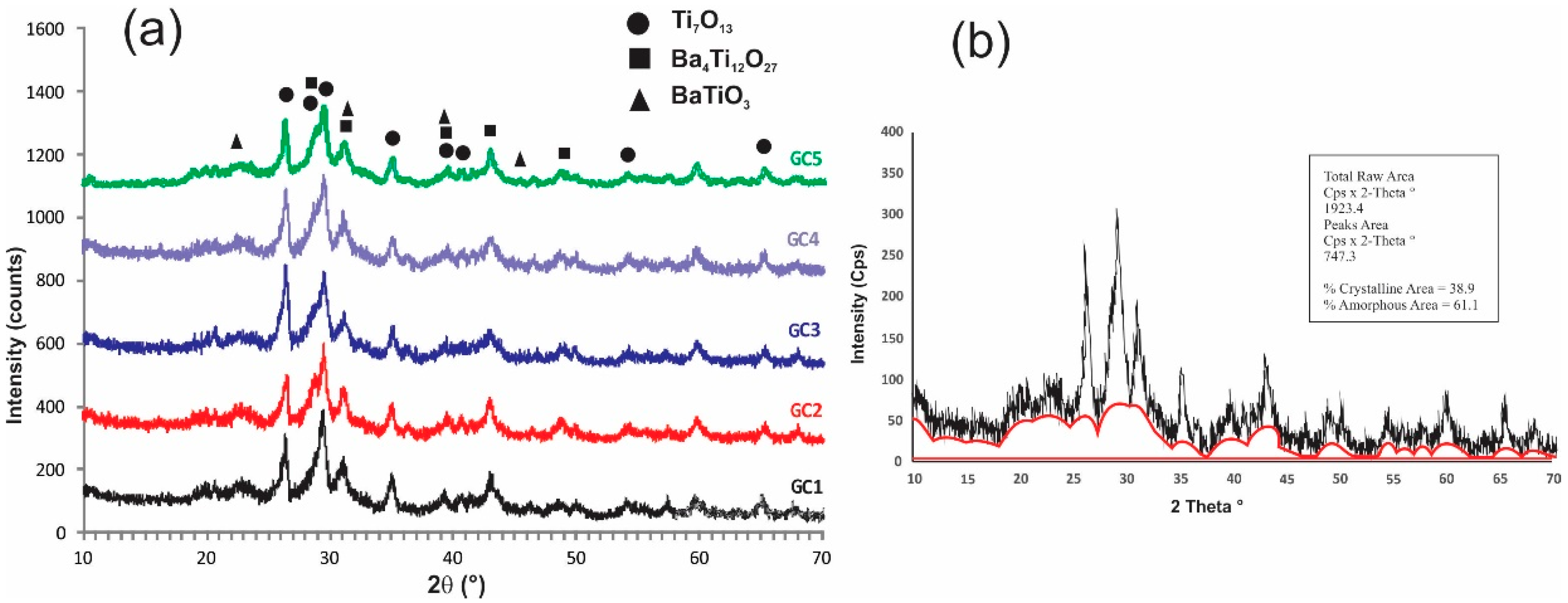
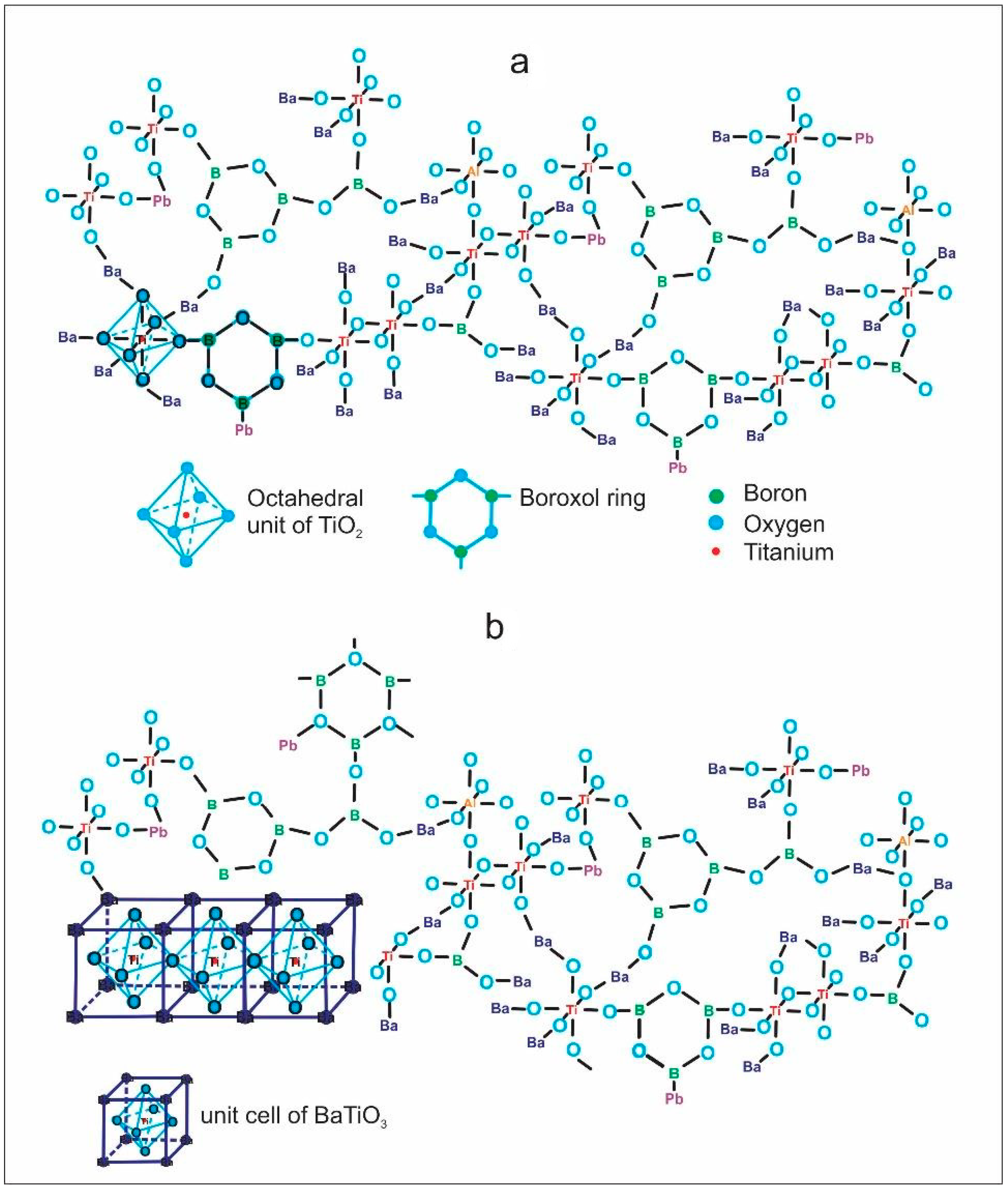
According to the DTA results, the glassy samples that were heat-treated at the first exothermic peak temperature were crystallized. Figure 5 and Table 2 show the XRD results and crystallite size of the obtained glass-ceramics, which are partially crystallized; the XRD patterns are also strongly distorted due to the materials’ poor crystallinity, which means they have disordered atoms in crystalline phases and there is a significant quantity of amorphous phase. The samples presented Ba4Ti12O27 (ICDD 00-044-0013) and Ti7O13 (ICDD 00-050-0789) as principal phases. In addition, BaTiO3 (ICDD 96-210-0859) was a minor phase, considering the intensities of diffraction peaks. Likewise, the increasing PbO concentration and decrease in BaO did not make a difference in the obtained crystalline phases since no phases of lead or potassium titanates were detected. The above suggests that these elements could join the residual vitreous matrix or substitute barium atoms in the crystalline phases to form solid solutions. In Figure 6a, the idealized structure of the glasses, formed by boroxol rings and octahedral Ti groups, can be principally appreciated; however, there is the possibility that the glassy network contains other minor functional groups such as the tetrahedral species 4Ti and 4Al, reported for similar glasses but with higher B2O3 content [4]. Figure 6b presents the glass with a small area with ordered atoms, which can act as a nucleus for the crystalline growth of BaTiO3 with perovskite structure, which is the simplest phase in the obtain glass-ceramics. In the same way, the octahedral units of [TiO6/2] become bonded by Ba ions to form more complex structures such as Ba4Ti12O27, as described by others researchers [35,36]. Additionally, according to the XRD patterns, there is a considerable quantity of remaining vitreous phase, which was calculated taking into consideration the areas under the curve of the diffraction peaks. The amount (Area %) of each sample’s crystalline and vitreous phase is shown in Table 2, and these results suggested that heat treatment time can be increased to obtain a significant amount of crystalline phase.
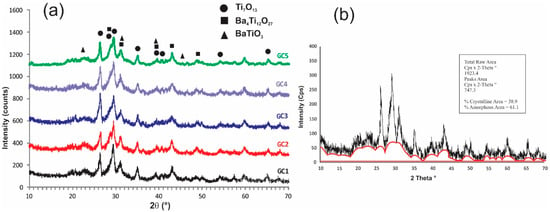
Figure 5.
XRD results: (a) X-ray patterns showing crystalline phases present in the glass-ceramics, (b) Glassy and crystalline phases calculated from XRD pattern of the GC5.

Table 2.
Results: % of glassy and crystalline phases, crystallite size, density, chemical resistance, and band-gap of glass-ceramics materials.
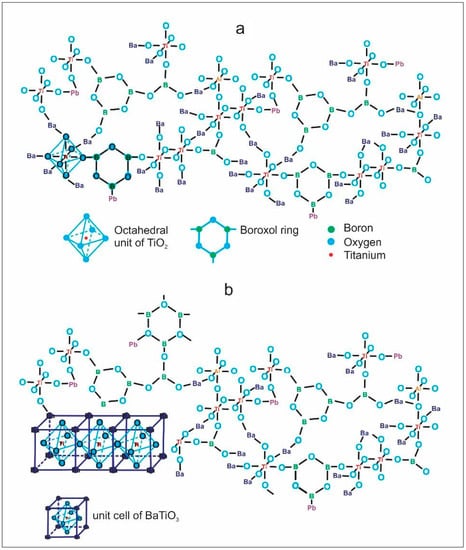
Figure 6.
(a) Idealized structure of the obtained glasses and (b) glass with a small area with ordered atoms forming BaTiO3 phase.
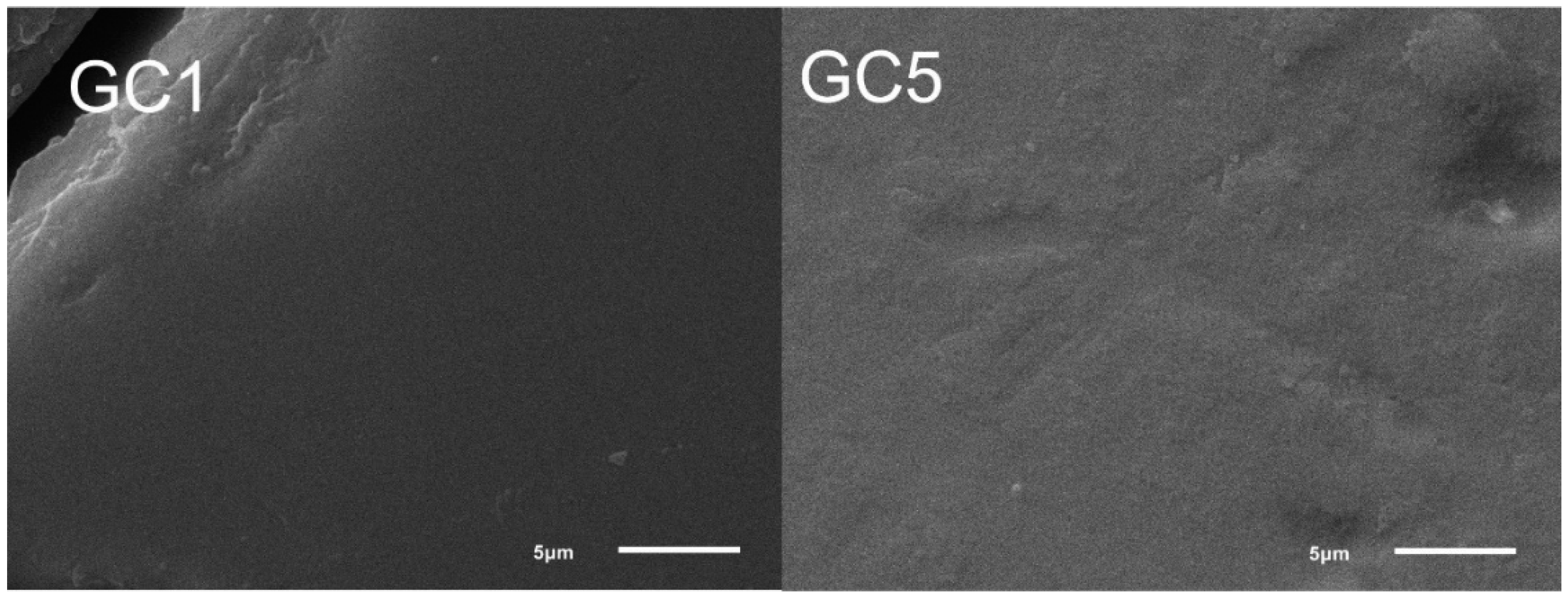
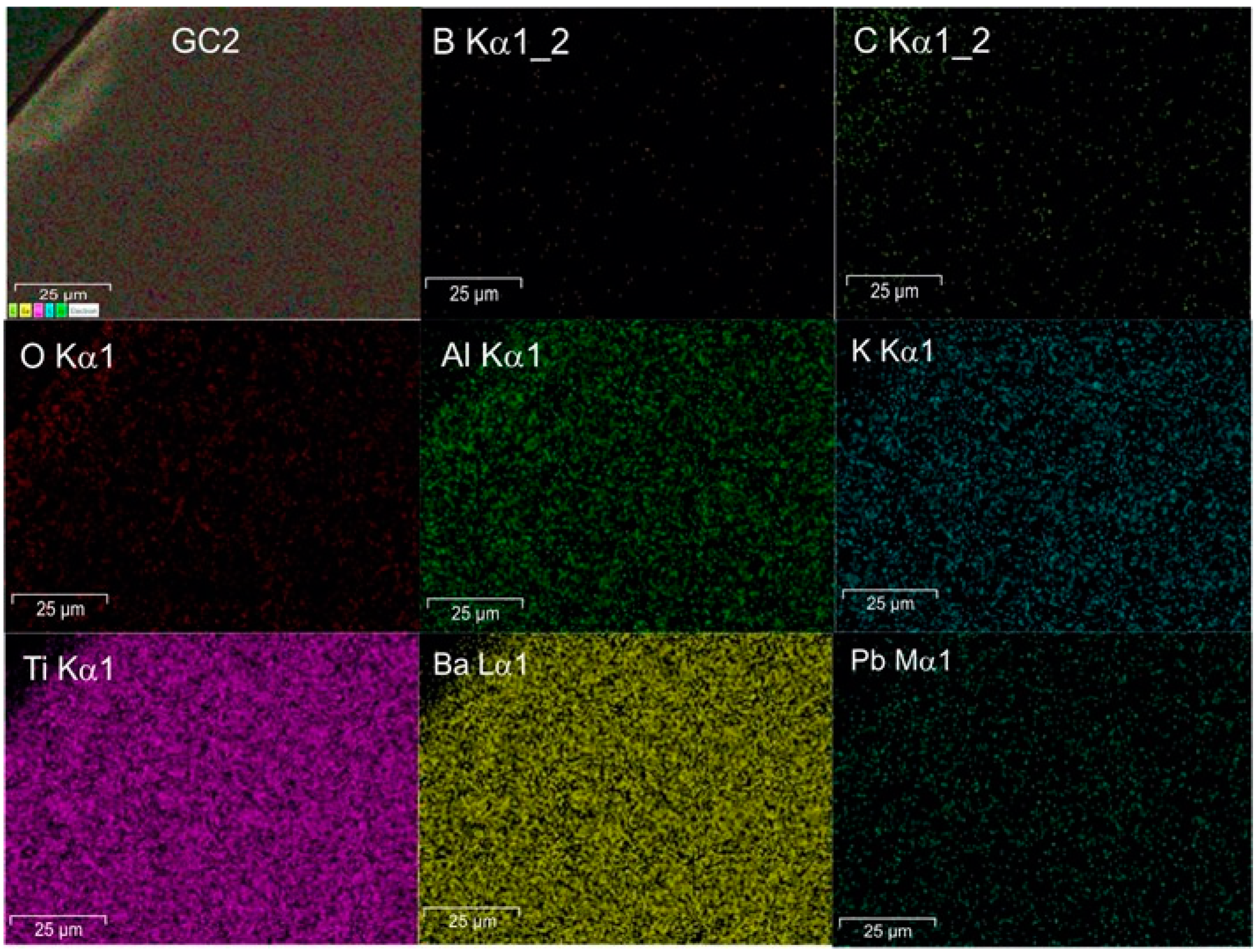
Figure 7 shows the micrographs of samples GC1 and GC5. All glass-ceramics presented a uniform microstructure without pores and cracks. The microstructure is proposed to be formed by crystals with a fine grain size embedded in the abundant residual vitreous phase and homogeneously distributed throughout the material. The above is confirmed by the XRD results showing the presence of crystalline phases with nanometric size in the range of 10.14 to 22.35 nm, as well as a uniform elemental distribution according to the EDS mapping, as seen in Figure 8.
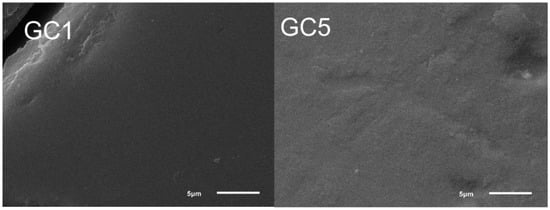
Figure 7.
SEM micrographs of the samples GC1 and GC5.
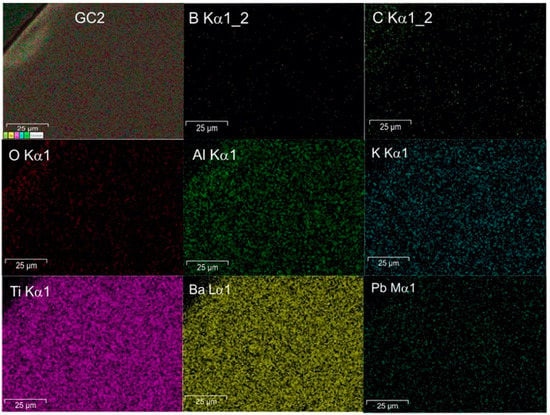
Figure 8.
EDS mapping of the sample GC2.
3.3. Evaluation of Properties
Table 2 presents the results of properties evaluated for the glass-ceramics composites. The density values were between 2.80 and 3.55 g/cm3, which increased with the PbO content in the initial batch; this agrees with Fernandez Navarro’s statement that this oxide increases glass density [37].
In addition, the chemical durability of glass-ceramics was determined to estimate the chemical resistance in acid and alkaline environments, respectively. The chemical resistance (% weight losses) was 22.83 to 30.48 in the acid medium and 10.7 to 16.3 in the basic medium, Table 2. In this context, the chemical attack can be attributed mainly to the composition stability of crystalline and vitreous phases, each phase’s amount in the material, and the attack media. According to the standard, samples with values higher than 5% should not be used in humid atmospheres.
The DRUV-Vis results of samples GC1–GC5 are shown in Figure 9 and listed in Table 2. The values were in the range of 2.88 to 3.05 eV, and it can be seen that lower values correspond to the compositions GC4 and GC5, which have the higher contents of PbO (3.5 and 4.5%, respectively).
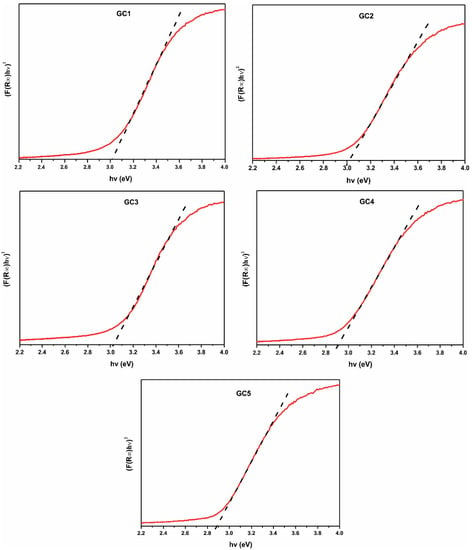
Figure 9.
UV-Vis spectra of the synthesized glass-ceramics.
4. Discussion
The melting technique obtained the free pore or cracked stable glasses. From DTA curves, it is observed that the glass transition (Tg) and the crystallization peak onset temperature (Tx) are very close, which means that the glasses contain structural units with high crystallization tendency [38,39], which is necessary for glass-ceramics obtaining. It is clear that both Tc and Tg decreased with increasing PbO content (comparing G1 and G5, Tc diminished 23 °C, approximately); this can be attributed to the flux effect of PbO and is related to the decrease in glass viscosity, which facilitated the pouring of glasses [37] and implies the reduction in the manufacturing cost of glass-ceramics.
On the other hand, the FTIR gives us information about the short-range ordering of the glasses. In this case, it can be deduced that boroxol rings (B3O6)3− were incorporated into the titanate glassy phase by Ba2+ and Pb2+ ions, giving rise to a single glassy phase (Figure 2), and EDS did not detect the presence of B2O3, but FTIR did. In addition, the principal identified Ba ions could link units [TiO6/2] to form crystalline structures such as Ba4Ti12O27 and BaTiO3, present in glass-ceramics after heat treatment, as a consequence of the rearrangement of these octahedral groups, which are essential in both structures [25,40,41].
Concerning the low chemical resistance in glass-ceramics (high weight losses), according to Barbieri et al., acid attack occurs by ionic diffusion due to proton domain H+ and is closely related to the ionic exchange with alkaline ions [40]. This medium is attributed principally to the dissolution of the glassy matrix, composed of boron, aluminum, and potassium oxides; therefore, ions are easily interchangeable as K+ can be responsible for the low resistance in acid solutions. It is reported in the literature that the incorporation of heavy metals can improve the chemical stability of borate glasses. This work observed no effect on the glass-ceramic behavior for the five compositions investigated [41,42].
On the other hand, the band-gap results of obtaining glass-ceramics are directly related to crystalline phases. Still, principally with Ba4Ti12O27 (the main phase detected), these results are in accordance with Kataoka K. et al., who established that this compound presents a conductivity at 300 K and behavior as a type-n semiconductor [36]. Glass-ceramics band-gaps are similar to those reported by Madheshiya et al. for glass-ceramics containing Bi2Ti2O7 as the principal crystalline phase [32]. Moreover, it can also appreciate that all samples presented values lower than TiO2 anatase (3–3.38 eV), the BaTiO3 (3.2 eV), and the Bismuth Barium titanates (2.94–2.99), which have been reported as photocatalyst [43,44,45,46,47]. The results show that glass-ceramics produced could be promissory as photocatalytic materials. Several applications such as hydrogen production or organic compounds degradation by heterogeneous photocatalysts using UV-Vis irradiation, especially GC4 and GC5 (with 3.5 and 4.5 PbO percent, respectively). The glass-ceramics mentioned above presented lower band-gap values than other similar materials and crystallite sizes of nanometers.
Finally, it can conclude that the introduction of (9) K2O and (0.5–4.5) PbO in glasses does not affect the composition of crystalline phases in glass-ceramics since potassium or lead titanates were obtained. However, glass-ceramics with higher content of PbO (GC4 and GC5) showed the lowest values of band-gap and Tc, making them more attractive for production.
Author Contributions
Writing—original draft preparation, M.A.G.-L.; glass and glass-ceramics preparation, P.P.-P.; band-gap analyses and interpretation, M.Á.E.-B.; chemical resistance and density analyses, D.M.N.-R.; characterization by DTA and XRD, A.R.-P.; FTIR analyses and explanation, Z.V.Q.J.; interpretation of results, M.P.; microstructural characterization of glasses and glass-ceramics, B.S.-R. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Mexican Program PRODEP.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, J.; Chen, W. Formation and structure of titanate glasses. J. Non-Cryst. Solids 1986, 80, 135–140. [Google Scholar]
- Takahashi, J.; Nakano, H.; Kageyama, K. Fabrication and dielectric properties of barium titanate-based glass-ceramics for tunable microwave LTCC application. J. Eur. Ceram. Soc. 2006, 26, 2123–2127. [Google Scholar] [CrossRef]
- Ruiz-Valdez, J.J.; Gorokhovsky, A.V.; Escalante-García, J.I.; Mendoza-Suarez, G. Glass-ceramics materials with regulated dielectric properties based in the system BaO-PbO-TiO2-B2O3-Al2O3. J. Eur. Ceram. Soc. 2004, 24, 1505–1508. [Google Scholar] [CrossRef]
- Ruiz-Valdez, J.J.; Gorokhovsky, A.V.; Escalante-García. Vitrification in the BaO-B2O3-Al2O3-TiO2 system containing small admixtures of PbO. J. Non-Cryst. Solids 2005, 351, 2036–2041. [Google Scholar] [CrossRef]
- Boroica, L.; Medianu, V.R.; Dinescu, M.; Borrica, I. Glass and glass-ceramics materials obtained by pulsed laser deposition in the BaO-TiO2-B2O3. Appl. Surf. Sci. 2005, 248, 381–387. [Google Scholar] [CrossRef]
- González, M.A.; Gorokhovsky, A.; Escalante, J.I.; Ponce, P.; Escobedo, M.A. Crystallization and properties of glass-ceramics of the K2O-BaO-B2O3-Al2O3-TiO2 System. Mat. Sci. Forum 2013, 755, 125–132. [Google Scholar] [CrossRef]
- Kokubo, T.; Tashiro, M. Dielectric properties of fine-grained PbTiO3 crystals precipitated in a glass. J. Non-Cryst. Solids 1974, 13, 328–340. [Google Scholar] [CrossRef]
- Mandal, R.K.; Prasad, C.D.; Parkash, O.; Kumar, D. Dielectric behaviour of glasses and glass ceramics in the system BaO-PbO-TiO2-B2O3-SiO2. Bull. Mater. Sci. 1987, 9, 255–262. [Google Scholar] [CrossRef]
- Gonzalez Lozano, M.A.; Gorokhovsky, A.; Escalante García, J.I.; Ponce Peña, P.; Escobedo Bretado, M.A.; López Chipres, E.; Mojica Marín, V. Glass-forming tendency in the K2O-BaO-B2O3-Al2O3-TiO2 system. Int. J. Phys. Sci. 2011, 6, 8164–8170. [Google Scholar] [CrossRef]
- Stojanovic, B.D.; Foschini, C.R.; Zaghete, M.A.; Cilense, M.; Varela, J.A. Microstructure of doped barium titanate prepared from polymeric precursors. Bol. Soc. Esp. Ceram. Vidr. 2002, 41, 90–193. [Google Scholar] [CrossRef]
- Shi, R.; Pu, Y.; Wang, W.; Shi, Y.; Li, J.; Guo, X.; Yang, M. Flash sintering of barium titanate. Ceram. Int. 2019, 45, 7085–7089. [Google Scholar] [CrossRef]
- Cai, Z.; Xing, X.; Yu, R.; Sun, X.; Liu, G. Morphology-controlled synthesis of lead titanate powders. Inorg. Chem. 2007, 46, 7423–7427. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.S.; Varma, K.B.R. Effect of the Addition of B2O3 and BaO-B2O3-SiO2 Glasses on the microstructure and dielectric properties of giant dielectric constant material CaCu3Ti4O12. J. Solid State Chem. 2007, 180, 1918–1927. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Feng, X.; Lu, X. High quality and yield in potassium titanate whiskers synthesized by calcination from hidrous titania. J. Am. Ceram. Soc. 2004, 87, 326–330. [Google Scholar] [CrossRef]
- Li, G.L.; Liu, M.; Wang, G.H. Microstructure studies of potassium hexatitanate whiskers. J. Mater. Res. 2001, 16, 3614–3620. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Performance of potassium titanate whiskers reinforced polyamide-6 composites. Polymer 1998, 39, 5461. [Google Scholar] [CrossRef]
- Li, J.; Wen, Z.; Xu, X.; Zhu, X. Lithium ion conduction in the anion substituted La2/3-xLi3x-yTiO3-yFy electrolyte with perovskite-type structure. Solid State Ion. 2005, 176, 2269–2276. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Sharma, M.L. Liquid phase sintering of BaTiO3 by boric oxide (B2O3) and lead borate (PbB2O4) glasses and its effect on dielectric strength and dielectric constant. Mat. Res. Bull. 1989, 24, 773–779. [Google Scholar] [CrossRef]
- Lee, J.-A.; Lee, J.-H.; Kim, J.-J. Effect of borate glass additives on the sintering behaviour and dielectric properties of BaTi4O9 ceramics. J. Eur. Ceram. Soc. 2006, 26, 2135–2138. [Google Scholar] [CrossRef]
- Huang, Y.X.; Senos, A.M.R. Effect of the powder precursor characteristics in the reaction sintering of aluminium titanate. Mat. Res. Bull. 2002, 37, 99–111. [Google Scholar] [CrossRef]
- Ponce-Peña, P.; González-Lozano, M.A.; Escobedo-Bretado, M.A.; De Lira-Gómez, P.; García-Sánchez, E.; Rivera, E.; Alexandrova, L. Synthesis and characterization of potassium hexatitanate using boric acid as the flux. Ceram. Int. 2015, 41, 10051–10056. [Google Scholar] [CrossRef]
- Escobedo Bretado, M.A.; González Lozano, M.A.; Collins Martínez, V.; López Ortiz, A.; Meléndez Zaragoza, M.; Lara, R.H.; Moreno Medina, C.U. Synthesis, characterization and photocatalytic evaluation of potassium hexatitanate (K2Ti6O13) fibers. Int. J. Hydrog. Energy 2019, 44, 12470–12476. [Google Scholar] [CrossRef]
- Manyu, H.; Yimin, L.; Chunguang, L.; Xia, L. Structural, electronic and elastic properties of potassium hexatitanate crystal from first-principles calculations. Phys. B Condens. Matter. 2012, 407, 2811–2815. [Google Scholar] [CrossRef]
- Tewatia, K.; Sharma, A.; Sharma, M.; Kumar, A. Factors affecting morphological and electrical properties of Barium Titanate: A brief review. Mat. Today Proc. 2021, 44, 4548–4556. [Google Scholar] [CrossRef]
- Wei, J.; Jiang, D.; Yu, W.; Shang, F.; Chen, G. The effect of Hf doping on the dielectric and energy storage performance of barium titanate based glass ceramics. Ceram. Int. 2021, 47, 11581–11586. [Google Scholar] [CrossRef]
- Shankar, J.; Deshpande, V. Effect of MgO addition on the properties of PbO–TiO2–B2O3 glass and glass–ceramics. Ceram. Int. 2013, 39, S15–S18. [Google Scholar] [CrossRef]
- Al-Assiri, M.S.; El-Desoky, M.M.; Al-Hajry, A.; Al-Shahrani, A.; Al-Mogeeth, A.M.; Bahgat, A.A. Study of nanostructural behavior and transport properties of BaTiO3 doped vanadate glasses and glass–ceramics dispersed with ferroelectric nanocrystals. Phys. B 2009, 404, 1437–1445. [Google Scholar] [CrossRef]
- Shweta; Gautam, C.; Tripathi, V.P.; Kumar, S.; Behera, S. Synthesis, physical and mechanical properties of lead strontium titanate glass ceramics. Physica B 2021, 615, 413069. [Google Scholar] [CrossRef]
- Pernice, P.; Esposito, S.; Aronne, A.; Sigaev, V.N. Structure and crystallization behavior of glasses in the BaO-B2O3-Al2O3 system. J. Non-Cryst. Solids. 1999, 258, 1–10. [Google Scholar] [CrossRef]
- GOST 10134-82. Inorganic and Glass-Ceramic Materials, Method for the Determination of Chemical Resistance, Russia, 1983.
- Heiskanen, J. Comparison of three methods for determining the particle density of soil with liquid pycnometers. Commun. Soil Sci. Plant Anal. 1992, 23, 841–846. [Google Scholar] [CrossRef]
- Madheshiya, A.; Dey, K.K.; Ghosh, M.; Singh, J.; Gautam, C. Synthesis, structural, optical and solid state NMR study of lead bismuth titanate borosilicate glasses. J. Non-Cryst. Solids. 2019, 503–504, 288–296. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Singh, A.K. A review on infrared spectroscopy of borate glasses with effects of different additives. ISRN Ceram. 2012, 2012, 428497. [Google Scholar] [CrossRef] [Green Version]
- Doweidar, H.; El-Egili, K.; Ramadan, R.; Al-Zaibani, M. Structural units distribution, phase separation and properties of PbO–TiO2–B2O3 glasses. J. Non-Cryst. Solids. 2017, 466, 37–44. [Google Scholar] [CrossRef]
- Currao, A. Ba4Ti12027: Rietveld refinement using X-ray powder diffraction data. Acta Cryst. 1999, 55, 2–4. [Google Scholar]
- Kataoka, K.; Hayakawa, H.; Iyo, A.; Ken-ichi, O.; Akimoto, J. Synthesis, crystal structure and physical properties of Ba4Ti12027. Key Eng. Mater. 2013, 566, 211–214. [Google Scholar] [CrossRef]
- Fernández Navarro, J.M. El Vidrio, 2nd ed.; CSIC: Madrid, Spain, 1985; pp. 142–143. [Google Scholar]
- Steimacher, A.; Astrath, N.G.C.; Novatski, A.; Pedrochi, F.; Bento, A.C.; Baesso, M.L.; Medina, A.N. Characterization of thermo-optical and mechanical properties of calcium aluminosilicate glasses. J. Non-Cryst. Solids 2006, 352, 3613–3617. [Google Scholar] [CrossRef]
- Hubrý, A. Evaluation of glass-forming tendency by means of DTA. Czech. J. Phys. B 1972, 22, 1187–1192. [Google Scholar]
- Barbieri, L.; Karamanov, A.; Corradi, A.; Lancellotti, I.; Pelino, M.; Rincon, J.M. Structure, chemical durability and crystallization behavior of incinerator-based glassy systems. J. Non-Cryst. Solids 2008, 354, 521–528. [Google Scholar] [CrossRef]
- Dwaikat, N.; Sayyed, M.I.; Mhareb, M.H.A.; Dong, M.; Alajerami, Y.S.M.; Alrammah, I.; Khalid, A.; Ashiq, M.G.B. Durability, optical and radiation shielding properties for new series of boro-tellurite glass. Optik 2021, 245, 167667. [Google Scholar] [CrossRef]
- Stalin, S.; Gaikwad, D.K.; Al-Buriahi, M.S.; Srinivasu, C.; Ahmmad, S.A.; Tekin, H.O.; Rahman, S. Influence of Bi2O3/WO3 substitution on the optical, mechanical, chemical durability and gamma ray shielding properties of lithium-borate glasses. Ceram. Int. 2021, 47, 5286–5299. [Google Scholar] [CrossRef]
- Avinash, B.S.; Chaturmukha, V.S.; Jayanna, H.S.; Naveen, C.S.; Rajeeva, M.P.; Harish, B.M.; Suresh, S.; Lamani, A.R. Effect of particle size on band gap and DC electrical conductivity of TiO2 nanomaterial. AIP Conf. Proc. 2016, 1728, 020426. [Google Scholar] [CrossRef]
- Savio, A.K.P.D.; Starikov, D.; Bensaoula, A.; Pillai, R.; de la Torre García, L.L.; Robles Hernández, F.C. Tunable TiO2 (anatase and rutile) materials manufactured by mechanical means. Ceram. Int. 2012, 38, 3529–3535. [Google Scholar] [CrossRef]
- Savio, A.K.P.D.; Fletcher, J.; Robles Hernández, F.C. Sonosynthesis of nanostructured TiO2 doped with transition metals having variable bandgap. Ceram. Int. 2013, 39, 2753–2765. [Google Scholar] [CrossRef]
- Cernea, M.; Secua, M.; Radu, R.; Ganea, P.; Surdu, V.A.; Trusca, R.; Vasile, E.T.; Secu, E.C. Structural, electrical properties and photoluminescence analyses of the terbium doped barium titanate. J. Alloy. Compd. 2021, 8781, 60380. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, V.; Kaur, A.; Singh, L. Study of the crystallization and structural behavior of bismuth barium titanate glass-ceramics. J. Non-Cryst. Solids. 2021, 557, 120563. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).