Abstract
Perovskite solar cells (PSC) are considered promising next generation photovoltaic devices due to their low cost and high-power conversion efficiency (PCE). The perovskite material in the photovoltaic devices plays the fundamental role for the unique performances of PSC. Formamidinium based perovskite materials have become a hot-topic for research due to their excellent characteristics, such as a lower band gap (1.48 V), broader light absorption, and better thermal stability compared to methylammonium based perovskite materials. There are four phases of perovskite materials, named the cubic α-phase, tetragonal β-phase, orthorhombic γ-phase, and δ-phase (yellow). Many research focus on the transition of α-phase and δ-phase. α-Phase FA-based perovskite is very useful for photovoltaic application. However, the phase stability of α-phase FA-based perovskite materials is quite poor. It transforms into its useless δ-phase at room temperature. This instability will lead the degradation of PCE and the other optoelectronic properties. For the practical application of PSC, it is urgent to understand more about the mechanism of this transformation and boost the stability of α-Phase FA-based perovskite materials. This review describes the strategies developed in the past several years, such as mixed cations, anion exchange, dimensions controlling, and surface engineering. These discussions present a perspective on the stability of α-phase of FA-based perovskite materials and the coming challenges in this field.
1. Introduction
Nowadays, due to the shortage of the non-renewable energy sources and many environmental issues from the consumption of fossil fuel, renewable energy sources will play a more and more important role in society. As one important renewable and clean energy source, solar energy has been a hot spot for research concerning various new energy sources [1]. Especially, the solar cells based on halide perovskite materials have attracted much attention due to their unique characteristics such as their proper bandgap (Eg), low cost, long carrier diffusion length, and high power conversion efficiency (PCE) [2,3]. They are considered as the promising candidate materials for the next generation photovoltaic devices. It was first reported by Miyasaka and coworkers and 3.8% PCE was achieved [4]. Up to date, PCE of photovoltaic devices based on halide perovskite have been reached a best efficiency of 25.8% in 2021 [5,6]. However, the poor stability of the halide perovskite is still a bottleneck for application in solar cells. In fact, the stability of the perovskite materials mainly depends upon the structures and compositions of perovskite materials. For example, MAPbX3 (X = I, Cl, Br) are typical hybrid perovskite materials and have been thoroughly studied in solar cells. However, its Eg is 1.55 eV, beyond the range of the optimal bandgap for solar cell (1.1–1.4 eV) and formamidinium based perovskite materials (FAPVs), which have a smaller bandgap, were reported by Eperon and coworkers [7]. FAPVs have more advantages and fascinating properties over methylammonium based perovskite materials (MAPVs), such as broader adsorption range, smaller bandgap (1.48 eV), higher efficiency, longer hole-diffusion length (~813 nm), better thermal, and light stability [8,9,10,11,12,13,14,15]. FAPVs have a typical perovskite chemical formula ABX3 (A = organic cation, B = metal cation, X = halide anion). The Goldschmidt tolerance factor (t) was defined to predict the phase stability of perovskite materials. The Goldschmidt tolerance factor is calculated by the following equation:
In this equation, RA, RB, RX are the ionic radii of A, B, and X, respectively [16]. When t ranged between 0.8 and 1.0, the stable cubic structure of perovskite materials can be obtained. When only FA+ is used as the cation, it suffers from a thermal instability that its trigonal structures (α-phase, black color) transform into unwanted hexagonal structure (δ-phase, yellow color) gradually at room temperature since it is bigger than 1 [16,17]. Hence, improving the stability of α-phase FAPVs is very important to boost the commercialization of FAPVs.
Many methods, such as mixed cations (anions) [18], additive engineering [19], surface engineering [20,21], and epitaxial growth [22,23] have been used to improve the stability of FAPVs. The stability of α-phase FAPVs is affected by environment (temperature, humidity) or its instinct structures. Environmental stability is an open challenge. Lee et al. reported the stability of FA0.9Cs0.1PbI3 perovskite against the light and humidity was improved due to partial substitution of Cs+ for FA+. PCE was improved from 14.9% to 16.5% due to the suppressed charge recombination [24]. In addition, many researchers reported α-phase FAPVs was stable at room temperature in humid or desiccated air ranges from a few hours to a few days [25,26,27,28,29,30,31]. Moreover, the stability over months also has been reported by using surface engineering [32]. But a thorough understanding of the phase transition of FAPVs is also lack and the stabilization mechanism is not clear.
Herein, we review different strategies, such as mixed cations or anions, dimensional controlling, surface engineering and additives to improve the stability of FAPVs. Meanwhile, the structures and properties are also reviewed. We discuss crystal structures with respect to investigate the phase transition mechanism and stabilization mechanism in a molecular-level structure. Particularly, we reviewed the hydrogen bonding for stabilizing the α-phase. Finally, we will discuss the challenges and future outlook for the stability of α-FAPVs.
2. The Strategies Applied to Stabilize the α-FAPVs
2.1. Mixed Cations Hybrid Perovskites
As mentioned, mixed cations are used to improve the stability of α FAPVs. Since the large size of FA+, partially replacement of FA+ with organic (such as MA+) or inorganic cations (Cs+, Rb+) can adjust the value of t, and occupy the A-site in APbI3 devices for enhancing the stability of α-phase FAPVs [33].
We mainly discuss APbX3 perovskites materials. A is mixed cations (Cs+, Rb+, MA+ or FA+). Pb is lead. X is iodine, bromine or mixed anions. The ratio of mixed cations can affect the phase stability of perovskites materials. For example, FAxMA1−xPbI3 film was synthesized at room temperature. In FAxMA1−xPbI3 materials, the stable α-phase can be formed with the ratio and FA+ was partially replaced by MA+. The lattice parameters and optical bandgap of FAxMA1−xPbI3 () at 298 K varied in accordance with Vegard’s law [34]. Kim et al. studied the effect of Cs/FA ratio in CsxFA1−xPb (I0.94Br0.06)3 perovskites. When the molar ratio of Cs/FA was 17:83, the stability is excellent and PCE can be achived to 16.5% [35]. Li et al. reported FA1−xCsxPbI3 alloy has lower δ-to-α-phase transition temperature than pure FAPbI3 and CsPbI3 [36]. FA1−xCsxPbI3 showed the stability phase under high humidity conditions. FA1−xCsxPbI3 solar cells have the best PCE of 16.1%. Kawachi et al. reported that α-phase and heat capacity of high-quality single crystals of CsxFA(1−x)PbI3 [37]. The multiple first-order transitions disappeared and phase transitions emerged at 300 and 149 K after doping with 10% Cs+ [37]. In addition, Rb+ have the similar properties with Cs+. Rb+ is hard to form a stable RbPbI3 perovskite due to too small of a size, but it can stabilize phase of FAPbI3 by reducing enthalpy [38]. Rb0.05FA0.95PbI3 own superior stability under high humidity condition (85%) and the complete phase conversion of α FAPbI3 by incorporating 5% Rb to FAPbI3. The PCE of Rb0.05FA0.95PbI3 based solar cell was 17.16% [39].
In addition to the above single ion substitution, double mixed-cations incorporated into FAPbI3 is also an effective method to improve the phase stability. In 2016, the PCE and outputs of Csx(MA0.17FA0.83)(100−x)Pb(I0.83Br0.17)3 were first investigated by Saliba and coworkers [40]. The PCE of high efficiency perovskite solar cells reached 21.1% and the output could be achieved at 18% under operational conditions after 250 h when different ratios of Cs/MA/FA cation were used. The XRD spectra illustrated that the yellow δ phase and PbI2 peak would disappear completely with different amounts of Cs+ (5, 10, 15%) (Figure 1a,b). The absorption and photoluminescence spectra proved that blue-shift occurred with the substitution of Cs+ from 0 to 15%. The processing conditions of thermal stability and film formation have been investigated (Figure 1c,f). The tolerance factor was tuned to a cubic lattice structure which matches the α-phase [40]. Gallardo et al. reported that two and three cation-doped hybrid perovskite nanopowders have different characterization [41]. The optical Eg values of the MAPbI3 and FAPbI3 were 1.53 eV and 1.45 eV, respectively. The Eg values would decrease when MA+ was introduced into FAPVs. In FAxCsyMA(1−x−y)PbI3-type perovskites, the perovskite structure would be more stable with addition 10% Cs+ to substitute the FA+ and Eg was 1.47 eV. X-ray diffraction showed that the phases of MAPbI3 or FAPbI3 changed after 4.5 months, the phases of FAxMA(1−x) PbI3-type perovskites were stable over time, cubic phases of FAxCsyMA(1−x−y) PbI3-type perovskites were also stable over time with 10% Cs+ [41]. Derbali et al. studied the structure, morphological and properties of K+ based mixed-halide perovskite materials [42]. The ionic radius of K+ is 133 pm. KxFA0.80−xMA0.20PbI2.8Cl0.2 (FAMA-K10%) perovskite films was fabricated by incorporation K+ into FAMA. The tolerance factor of FAMA and FAMA-K10% were 1.02 and 0.99, respectively. XRD indicated K+ can enhance the intensities of the photoactive α-phase and suppress the δ-phase. K+-doped demonstrated better crystallinity and reduced the bandgap [42]. Zhang’s group studied the effect of cation substitution with MA+, Cs+, Rb+ by First-principles calculations [43]. The basic building unit of α-phase and δ-phase of FAPbI3 was the PbI6 octahedrons. The cubic α-phase has been formed when PbI6 connected to each other in the form of a corner to corner. When three cations were doped, the transition temperature of FAPbI3 was affected. This effect can be studied by an approximate relation (see the formula 2). This indicated that α-phase was stabilized at low temperature and three cations doped have the lowest transition temperatures at the high concentration of 25%. In addition, the band structure was also studied (Figure 2a,g). The Eg were 1.57 eV, 1.61 eV and 1.69 eV in Cs+, Rb+ and MA+ doped system. These cations can stabilize the black phase of FAPbI3, but this was only short-term stability. So the mixing energies of these multication systems were also calculated (see Equation (3)). Moreover, Park et al. also investigated the effect of the A-site cation on the phase stability of the perovskite structure by using machine-learning models. They proposed triple cation phase diagrams can emphasize the concept of effective cation radius based on the mixture of cations [44].
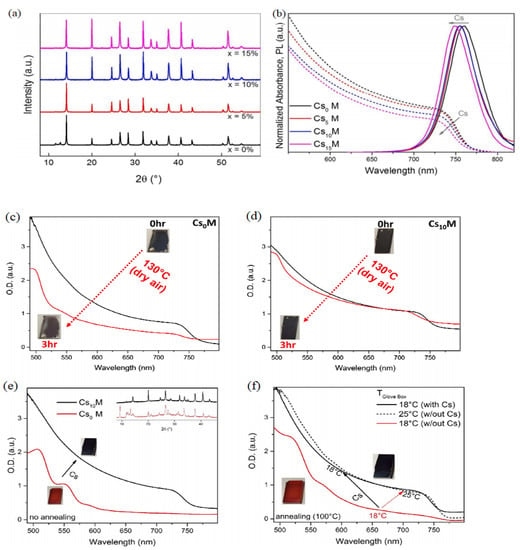
Figure 1.
Different spectrum of perovskite with different amounts of Cs (a) XRD, (b) Absorbance and PL spectra; UV–vis absorption of (c) Cs0M and (d) Cs10M films which were annealed at 130 °C for 3 h in dry air; (e) absorption spectra of as-fabricated materials which were not annealed at room temperature. The perovskite characters of Cs10M can be shown in the inset XRD data; (f) absorption spectra showed perovskite phase of Cs0M formed at 25 °C (dashed black line) and perovskite phase of Cs10M formed at 18 °C (solid black line) by using the spin coating. Reprinted with permission from Ref. [40]. Copyright 2016 Royal Society of Chemistry.
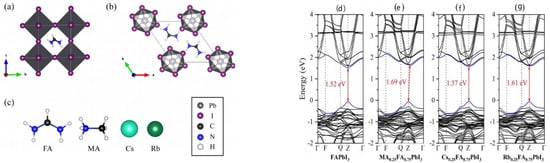
Figure 2.
Structures of α-phase (a,b) δ-phase of pure FAPbI3; (c) The structures of four cations (FA+, MA+, Cs+, and Rb+); Band structure of α-FAPbI3 (d), α-MA0.25FA0.75PbI3 (e), α-Cs0.25FA0.75PbI3 (f) and α-Rb0.25FA0.75PbI3 (g). Reprinted with permission from Ref. [43]. Copyright 2019 AIP Publishing.
2.2. Anion-Exchanged
Anion exchange, such as substitution I− with Br−, is another positive method on tuning the bandgap and improving the stability of FAPVs. The interaction between Br− and surrounding ions is stronger than that of I− [45].
In 2018, Li et al. used Cs+ and Br− to improve stability of α-FAPbI3 [46]. Deep research found that Cs+ doping was not enough to stabilize black-phase and Br− anion can stabilize the α-phase of FAPbI3. Finally, Cs+ and Br− co-doped FAPbI3 have some advantages, such as higher performance optoelectronics, good stability and a long carrier lifetime of 1.23 µs (Figure 3a,c) [46]. Jeon et al. researched the stability of (FAPbI3)1−x(MAPbBr3)x when the value of x ranged between 0 and 0.3 [47]. MAPbBr3 can stabilize the α-FAPbI3 and improve PCE. According to XRD spectra, the pure FAPbI3 has a hexagonal non-perovskite (P63mc) after annealing at 100 °C. And then a strong (111) diffraction peak at 13.9°C of the trigonal perovskite phase (P3m1) appeared when MA+ cation and Br− ions (15 mol%) were added to FAPbI3 (Figure 3d). A complete bilayer on a mesoporous TiO2 was successfully fabricated by using solvent-engineering technology. (FAPbI3)1−x(MAPbBr3)x has many advantages compared to the pure FAPbI3 (Figure 3e). The PCE of (FAPbI3)1−x(MAPbBr3)x can be achieved to 18.4% (Figure 3f) [47]. Long and co-workers developed a halid-exchange process (HEP) reaction (Figure 3g,h) [48]. XRD exhibited the structure stability of HPbI2 (Figure 3i). The large-grained and compact FAPbI3−xBrx thin films can be deposition without using antisolvent. The stability of FAPbI3−xBrx can be enhanced by reduced bromide. Br-doping can inhibit the α-phase conversion into δ-phase and stabilize the final α-phase. The reaction was described as:
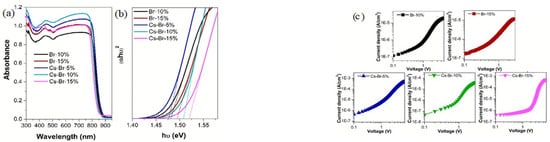
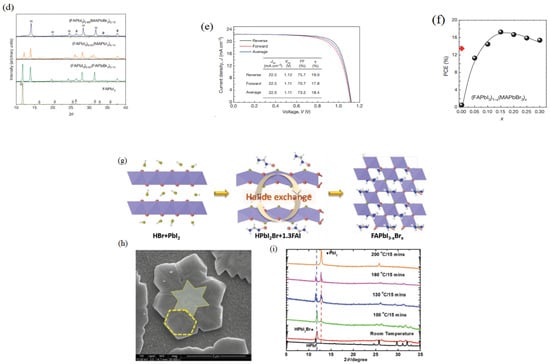
Figure 3.
(a) Absorbance spectra of stable FA-based halide perovskite and Tauc plot (b); (c) The I-V curves of doped formamidinium-lead-halide-based hole-only devices with the different structures (Reprinted with permission from Ref. [46]. Copyright 2018 Royal Society of Chemistry). (d) XRD spectra of four FA-based perovskites annealed at 100 °C for 10 min; (e) The red line and the black line indicated PCE values of pure FAPbI3 and (FAPbI3)1−x(MAPbBr3)x annealed at different temperature (100 °C, 150 °C), respectively. And then the α-phase was formed; (f) J–V curves of (FAPbI3)0.85(MAPbBr3)0.15 perovskite material. (Reprinted with permission from Ref. [47]. Copyright 2015 Nature). (g) Schematic diagram of intermediate synthesis of HPbI2Br and its halide exchange processing; (h) SEM of HPbI2Br; (i) XRD spectra of HPbI2Br material at different temperature. Reprinted with permission from Ref. [48]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Finally, the efficiency of PSC device was 19.0% without appreciable hysteresis. Rehman et al. analyzed the charge-carrier dynamics and mobilities across the rang of compositions of FAPb(BryI1−y)3. The valence band varied from 4p to 5p and the bandgap energy decreased by exchanging I− with Br−. The PL emission of FAPb (Br0.67I0.33)3 can record the laser excitation with an intensity of 7.2 W cm−2 following 5 min [49]. The PL have shift to a new low-energy PL feature at around 785 nm (1.58 V). XRD spectra showed the crystalline phases were formed with different contents of y. It was found that the bandgap can be tunable between 1.48 and 2.23 eV [50]. MA1−xFAxPbI3−yCly was firstly prepared through annealing at temperature of 80–110 °C. When Cl was doped, the average Jsc and Voc have increased to 19.04 mA/cm2 and 1.120 V, respectively. And then the PCE achieved to 15.95% [51].
In addition, the stabilization mechanism of FAPVs was also studied by Nan and co-workers [52]. The deep research found that 8H-FAPbX3 intermediate phase. Its structure and phase transition are shown in Figure 4. The locations and effects on phase stability of cations and anions were investigated. Smaller halides increased the thermodynamic stability due to their being located at the corner-sharing sites. The smaller cations stabilized the 3C phase due to occupying cubic cages (especially smaller Cs+). We can understand the intrinsic stabilization mechanism of FAPVs at molecular-level [52].
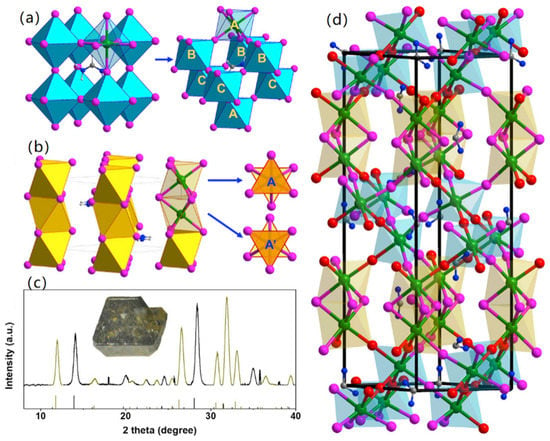
Figure 4.
(a) Structures of α-phase FAPbI3 along different axis ([100] and [111]); (b) Structures of δ-phase of FAPbI3 along the [0001] axis and A and A′ were the two types of [PbI6] octahedrons. (c) XRD of a 3C-FAPbI3 single crystal with a phase transition; (d) Structure of 8H-FAPbX3. Br (Red); I (purple); Pb (green); N (blue); C (gray); H (white). Reprinted with permission from Ref. [52]. Copyright 2021 Elsevier.
In a word, anion-exchange is an essential method to stabilize α-FAPbI3, and it can further improve phase stability since X sites and A/B-sites have strong interaction [16].
2.3. Control the Dimensions of Perovskites
3D perovskite materials have intrinsic instability. In order to improve the stability of FAPVs, some strategies, such as reducing dimension, forming multidimensions (mixing 3D perovskites with low-dimensional or formation of Ruddlesden-Popper type perovskite), can be used [53]. A systematic evolution of the dimensionality of the perovskite structure can be formed. The bandgap and binding energy were increasing when the dimensionality was decreasing. In other words, the dimensionality can affect the Eg of the perovskite materials. For example, the Eg values of multidimensions were affected by the 2D confinement effect [53].
Low-dimensional perovskites have higher stability, excellent optoelectronic properties and better moisture resistance than three-dimensional perovskites [54,55]. Reducing the dimensionality can tune the optoelectronic properties at morphological and molecular levels [56,57]. Meanwhile, lowering the dimensions also have some disadvantages. Since low-dimensional perovskites have some drawbacks, such as strong exciton-phonon interaction, intrinsic quantum-confinement, lower device performance [58]. The crystal structural dimensionalities of 2D, 1D and 0D are shown in Figure 5a,b [59]. Zero-dimensional perovskites are usually composed of isolated inorganic octahedral clusters and small cations and they are connected by H bonding. Photovoltaic performance of zero-dimensional perovskites always were limited due to their strong intrinsic quantum-confinement. 1D perovskites can be called perovskite nanowires and are <110> oriented perovskites. 1D perovskite (A′2AmBmX3m+2) can be formed when the value of m is 1. And it has BX6 octahedral unites which can interconnected with strong interaction by corner-sharing to form the 3D framework [59]. Huang et al. reported a new 1D inorganic-organic hybrid perovskite has high stability and humidity response [60]. The structure was composed of 1D inorganic perovskite-like chain, [(Me3)DAB(Me3)]2+ dications and water molecules. In compared to 3D perovskite materials, 2D halide perovskite materials are environmentally stable. The 2D perovskites include <100>, <110>, <111> orientated families. For example, the formula of (RNH3)2BX4 or (NH3RNH3) BX4 is typical <100> orientate 2D perovskites. (C16H13N3)PbBr4 is a typical <110> orientated 2D perovskites. This perovskite has layered structure due to the functional cation [59,60,61]. There are many synthesis methods, such as solution-phase growth, chemical vapor deposition. Lee et al. synthesized the phase-pure FAPbI3 films. 2D phenylethylammonium lead iodide was added to the precursor solution and then FAPbI3 was fabricated. The PCE was achieved to 20.64% [62]. 2D perovskite materials have good optical and electronic properties due to direct band-gaps. This also affect device performances and stability [63]. The stable 2D-IBA2FAPb2I7 has been formed [64]. The further research exhibited that the level of PbI2 and δ-FAPbI3 near the surface have been reduced due to IBAI treatment and electronic trap states caused by impurities at or near the surface of the perovskite can be passivated [64]. In addition, there are quasi 2D perovskites which is a hybrid dimensionality between 3D and 2D. This family have the Ruddlesden-Ropper crystal structure [65]. Perumallapelli et al. investigated the morphology, structure, optical properties of OA-based 2D and quasi-2D perovskites. Compared to the 3D counterpart, the low binding energy of Pb indicated the more electron-rich environment around the Pb centers [66]. In 2016, the reduce-dimensionality (quasi-2D) has been reported [67]. These materials have good stability. The origins of instability of RABX were explained by computational methods and the stability of dimensionally-tuned perovskites were investigated by DFT [67]. The DFT simulation of energetics was shown in Figure 5c. Dimensionality of perovskite have been tuned by changing the value of n in the series PEA2(CH3NH3)n−1PbnI3n+1. The structure is 2D (n = 1), quasi-2D (n > 1) and 3D (n = ∞).
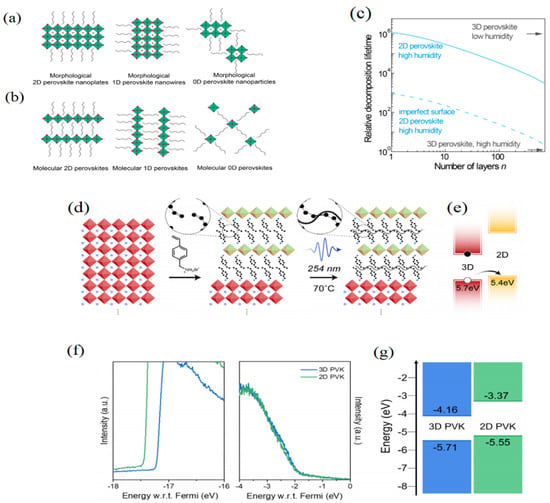
Figure 5.
(a) The different morphological perovskites, and (b) different molecular perovskites. (Reprinted with permission from Ref. [59]. Copyright 2018 WILEY-VCH Verlag GmbH &Co. KGaA, Weinheim). (c) DFT simulation of the formation energy of 3D and 2D perovskite with different n values. (Reprinted with permission from Ref. [67]. Copyright 2016 Royal Society of Chemistry). 2D/3D heterostructures have formation and optical properties by using the ligand VBABr. (d) Schematic diagram of processing which cross-linked 2D perovskites were formed by using the VBABr; (e) Energy level of the 2D/3D interface. (Reprinted with permission from Ref. [68]. Copyright 2019 ACS Publication). (f) UP spectra of the 3D perovskite and the 2D perovskite. (g) Energy levels of the 3D and 2D perovskites. Reprinted with permission from Ref. [69]. Copyright 2021 Science.
Multi-dimensional have been developed since it has outstanding photophisical properties, such as higher PCE [70,71], longer diffusion lengths [71,72], low exciton binding energies [73,74], low-temperature solution processibility [75]. 2D perovskites sometimes do not have the ideal photovoltaic performances due to their larger bandgap, weaker carrier transport [76,77]. 2D/3D perovskites have attracted much attention due to their long-term stability and high photo conversion efficiency. The 2D/3D multidimensional perovskite can be defined as M2An−1BnX3n+1, where M is a large cation; A is FA, MA or Cs; B is Pb of Sn; X is I, Br, Cl; and n is the number of metal halide sheets. The structures of 2D or 3D are determined by the value of n. The 3D perovskite have been formed when n is ∞; the 2D perovskite can be occurred when n is 1. However, it is different to obtain phase-pure crystals of higher n-value perovskites. Quasi-2D (i.e., n = 30, 40, 50, 60) and quasi-3D (n ≤ 3) are usually represented as 3D/2D perovskites [77,78]. Meanwhile, the optical Eg of M2An−1BnX3n+1 increased when the value of n decreased. In 2014, Smith et al. reported the Mesoscopic PSCs with a PCE of 4.73% using quasi-2D perovskites (PEA2MA2Pb3I10; n = 3). It has large open-circuit voltage (VOC) of 1.18V due to the increased bandgap of 2.06 eV [79]. Grancini et al., in 2017, showed one-year stable perovskite devices were formed by using an ultra-stable 2D/3D perovskite junction [80].
In addition to mixture 2D/3D perovskites, the other 2D/3D perovskite type has the bilayer structure. The bilayer structure of 2D/3D perovskite was first formed in 3D MAPbI3. Proppe’s group selected the ligand 4-vinylbenzylammonium (VBA), which has similar structure to phenethylammonium, to build the 2D/3D bilayer structure of (VBA)2PbI 4/(MAPbBr3)0.15(FAPbI3)0.85 (Figure 5d,e) [68]. The 2D perovskites were formed only at the top surface of the 3D layer. 2D/3D film exhibited faster decay and lower trap state densities. PCE was 20.4% and its initial efficiency (90%) was also retained after 2300 h. Recently, Cai et al. reported that the 2D/3D structure of (CF3-PEA)2FA0.85MA0.15Pb2I7/FA0.85MA0.15PbI3 have better chemical and thermal stability. PCE achieved 23.1% [81]. The structure, optoelectronic properties, and stability of 3D perovskite can be affected by 2D perovskite. The surface of the 3D perovskite can be passivated and the grain size can be increased by adding 2D perovskite. Besides, it also provided a hydrophobic umbrella to reduce the water-induced degradation, which result the stability of 3D perovskite was enhanced and did not compromise the optoelectronic properties [81]. In 2021, improving the stability of 3D perovskite materials were reported by Zhang and co-workers. Its surface was treated by using 2D layered perovskites [69]. The energetics for hole transport can be changed due to surface treatment (shown in Figure 5f,g). This energetics can increase out-of-plane transport rates and improve stability of PSCs. The PCE achieved 24.7% and 90% of PCE was still retained after 1000 h under 1-sun operation conditions [69].
Many methods have been used to prepare mixture 2D/3D perovskites. The CsPbI3·xEDAPbI4 was synthesized by a one-step method [82]. A-CsPbI3 perovskite films were stabilized by introducing the 2D perovskite of EDAPbI4. According to UV-vis and PL spectra, the 2D/3D perovskite of CsPbI3·0.05EDAPbI4 were formed. Ke et al. reported the stability of single-junction solar cells could be improved by using mixed 2D/3D perovskite composite absorbers deliver and PCE was 20.09% [83]. The mixture 2D/3D perovskites were prepared by another process, the two-step method. Stable 2D/3D hydrid perovskite solar cells were demonstrated by Zhou and coworkers [84]. A 2D/3D hydrid structure was formed due to the incorporation of 2-thiophenemethylammonium (ThMA). XRD spectra showed the orientation and crystalline growth of 2D/3D perovskite have been induced by ThMA spacer cations. The PCE can be achieved to 21.49% and a stabilized PCE was 21.1% since a high open circuit voltage (VOC) was 1.16V and a very notable fill factor (FF) could reach to 81% [84]. These processes mentioned above are called solution process. Of course, there are the other strategies to synthesize 2D/3D perovskite. For instance, the vapor process was been used. This process has some advantages, such as large scale and well-controlled reaction rates. In 2019, 2D/3D perovskite materials were prepared with a solid-vapor reaction method [85,86]. Similar to 2D/3D perovskites,1D/3D multidimensional perovskites have been studied. Yu and co-workers reported that HA+ was added to FAPbI3-based perovskite and 1D/3D structure of FAPbI3 were formed [87]. HA+ has strong hydrogen bond, which can increase the grain size and crystallinity of perovskite materials. HA+ has abundant H bond donor and acceptor sites. Thus, reactions between HA+ and [PbX6]4− occurred and 1D-HA-phase formed. Moreover, H bond interaction formed between 1D-HA-crystal and FAPbI3 due to N-H and I-H [87]. FTIR spectra made clearly that the stretching vibration of N-H in FAHA (~3397 cm−1) was red-shifted. The deeper research revealed that the HA based 1D-phase has the “rivet”-like structure which passivated the grain boundary of 3D perovskite and can improve the stability of FAPbI3-based perovskites [87]. The PCE can be achieved to 21.20% when HA+ was added.
2.4. Surface Engineering and Growth Control
Surface Engineering has been playing an important role in improving the efficient and stable FAPVs. The situation of the materials surfaces influences the surface defect density, the charge transport and injection between the functional layers. Surface confinement is one of surface engineering. It includes two aspects: controlling the growth of the perovskite crystal, confining the charge carriers [88,89]. The pure cubic-FAPbI3 was synthesized at room temperature by using surface functionalization [90]. Long-chain alkyl (LA) or aromatic ammonium cations can be used to stabilize the phase since it was important in the surface functionalization. The surface functionalization can decrease the surface energy of the α-phase. The origin of the surface LA-induced phase stabilization can be understanded by DFT and the formation energy was calculated by Equitation 6 [89,90].
where is the total energy, is the energy of PbI2, is the energy calculated from gas phase PFAI or FAI, respectively. , , , are the calculated energy of (PEA or EA)2(FA)2PbnI3n+1 PbI2, FAI, PEAI and EAI, respectively. The calculated formation energy (E) of δ-FAPbI3 and α-FAPbI3 were −4.93 eV and −4.48 eV, respectively. The δ-phase was more stable than α-phase at room temperature (RT).
However, the surface energy can be reduced by the surface PEA termination. This confirmed that replacement of surface FA with PEA cation was thermodynamically favorable regardless of the n value (shown in Figure 6a,c). The schematic of the proposed growth processes under various LA/FA ratios (α) is shown in Figure 6d,f. Under low α values, the amount of LA cations was not enough to stabilize the perovskite lattice. When α value was higher, LA cations were sufficient and occupied all surface sites. Under proper α values, the surface ligand cations were equilibrium with the free cations in the solution and LA cations were used to modify the perovskite surface in order to stabilize it. Moreover, this FAPbI3 perovskite stabilized at RT over several months has excellent photophysical properties. So the surface chemistry on the thermodynamical of perovskite materials is very important. Recently, Lu et al. investigated the conversion from δ- to α-FAPbI3 at 100 °C by the MASCN vapor treatment method [91]. SCN− ions can absorb on the surface of δ-FAPbI3 and replace iodide ions. Then the top layer of face-sharing octahedra was disintegrated and the corner-sharing architecture of α-FAPbI3 has been changed (shown in Figure 6g,h). The PCE of this perovskite solar cell was more than 23% and this perovskite was thermally stable. PSCs remained at ~90% of initial value after 500 h.
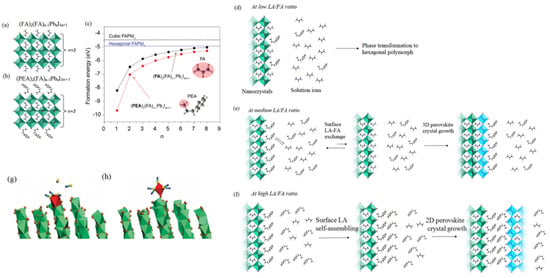
Figure 6.
Structures of the (a) (FA)2(FA)n−1PbnI3n+1 and (b) (PEA)2(FA)n−1PbnI3n+1; and (c) Corresponding the calculated formation energies. The growth processes with different α ratios. (d) the hexagonal phase perovskite was formed at a low α value; (e) A new 3D cubic perovskite was formed at a proper α value; (f) layered perovskites were formed by self-assembly at a high α value. (Reprinted with permission from Ref. [90]. Copyright 2017 ACS Publications). MD simulations displayed the initial face-sharing octahedra (g) conversion into the corner-sharing octahedra (h). Pb-I (green); iodideis (orange balls); octahedra (red); S (yellow); C (light blue); N (dark blue). Reprinted with permission from Ref. [91]. Copyright 2020 Science.
Zhang et al. firstly reported the surface layer of 3D perovskitoid (Me-PDA) Pb2I6 was modified by using simple surface treatment with MePDAI2. This resulted that surface texture was smooth, charge-carrier mobility was higher and surface-defect density was reduced [92]. (Me-PDA)Pb2I6 and MAPbI3 have different crystal structures (Figure 7a). 3D corner-sharing octahedra dimers () of (Me-PDA) Pb2I6 can replace every octahedron with an edge-sharing octahedra dimer as the basic motif. Figure 7b,c revealed that the octahedra dimers formed along the b direction and 3D framework formed by corner-sharing along a, b, and c directions. Moreover Me-PDA2+ occupied the rectangular A-site cavities [92]. Deng et al. used anti-solvent free spin-coating method to fabricate the higher performance FAPVs at low temperature. In that method, a small amount of MABr was dropped after spinning the perovskite precursor without using the anti-solvents [93]. The δ to α-phase transformation energy was significantly reduced due to MABr application. PCE could be up to 18.5% [94]. Majeed’s group reported that Cs0.05(FA0.95MA0.05)0.95Pb(I0.95Br0.05)3 perovskite was synthesized by using anti-solvent method [95]. KBr in the CB anti-solvent, which was added to this perovskite, inhibited nonradiative recombination and was beneficial to charge extraction at interfaces. As a result, the PCE was significant boosted from 15.36% to 18.29% and a long-term stability of 1080 h under 30–50% was obtained. Moreover, anti-solvents are crucial impact on the perovskite crystal growth kinetics and formation of α-FAPbI3 phase [95]. Our group used carboxylates to stabilize the α-phase of FAPbI3 by surface engineering method. The effect of different amount of Ac− was studied. When AC− was introduced into the system, the preference of crystal growth orientation (012) and the barrier energy of two phase (α-phase and δ-phase) have been changed. The mechanism of AC− on stabilizing the α-phase is shown in Figure 7d,g [96].
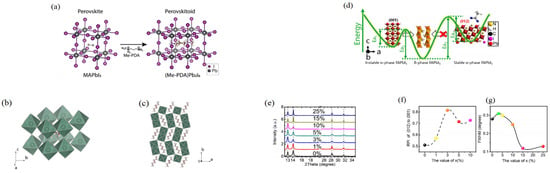
Figure 7.
(a) The structures of (Me-PDA)Pb2I6 and MAPbI3; Side view (b) and top view (c) of crystal structure of (Me-PDA)Pb2I6. (Reprinted with permission from Ref. [92]. Copyright 2021 Elsevier Inc.). The stability of the α-FAPbI3 was affected by adding Ac−. (d) Schematic illustration; (e) The calibrated XRD spectra of the perovskite materials with different amount of AC−; (f) The evolution of RPI of XRD diffraction peak (102) to peak (001); (g) FWHM of (001) with different amount of AC−. Reprinted with permission from Ref. [96]. Copyright 2021 Wiley-VCH GmbH.
Another method of surface engineering is surface passivation. Surface passivation can reduce the density of surface traps and enhance the device’s luminescence efficiency. Current surface passivation methods include forming a 2D perovskite layer by the reaction of ammonium halides and introducing a monolayer of small molecules without forming the 2D perovskites on the surface of 3D perovskite. In 2019, atomic-level passivation mechanism of ammonium salts was reported. Different ammonium salt, such as ethylammonium iodide, imidazolium iodide and guanidinium iodide were used to modify the surface of FA0.9Cs0.07MA0.03Pb(I0.92Br0.08)3 perovskite. α-FAPbI3 was formed when 10 mol% [(C2H5) NH3]I was doped. In this process, the formation of the mixed FA/MA structure was the main factor and this structure was similar to 1D/2D or 2D/3D perovskite materials. Meanwhile, Cs/EA, Cs/EA/FA were also formed. Finally, PCE could be controlled from 20.5% to 22.3%, 22.1%, and 21.0%, respectively [97]. Wang et al. demonstrated a nanocrystal-pinning method for defects passivation [98]. 2-bromoethyltrimethylammonium bromide (BETAB) was used to modify FA1–xMAxPbI3 films. Nanocrystal was correlation with the charge sites on perovskite surface [97,98]. The generation of metallic Pb (0) was retarded and multiple types of trap density were decreased due to the introduction of BETAB. Another ammonium salt benzyltriethylammonium chloride([BATAm]Cl) was used as modifier and can make the surface passivation. The PCE was 20.45% and retained 84% after 30 days. The stability of the device was good [99].
In addition, using additive is another important way to stabilize α-phase of FA based perovskite materials. Some additives will affect the hydrogen bonding. In 2016, Lee et al. reported that H bonding was important in stabilizing octahedral tilts in hybrid perovskites [100]. Direction of the H bonding between the organic molecule and the anion framework was also important in optimizing solar cell efficiency. The Figure 8a,b show the Octahedral tilting (Ground-state, GS) and no Octahedral tilting (High-symmetry, HS), respectively. The lattice parameters, the effective ionic radii and the tolerance factors (shown in Figure 8c) were calculated by first-principles calculations. The rotation of the staggered MA cation around the C-N bond was hindered due to the strong hydrogen bonding [100,101]. The ground-state structure has the three strong hydrogen bonding interactions, HN (1)-IA(1), HN (2)-IE(2), HN (2′)-IE(2′). The high-symmetry structure has only one weaken hydrogen bonding, HN (1)-IA(1). Aleksandra et al. investigated the chemical bonding of FAPbX3. Its chemical bonding was different from MA. FAPbX3 has stronger hydrogen bonding, the π-anion bonding and more hindered motion inside the perovskite inorganic cage [102]. Hong et al. reported that a new additive (guaninium, G) can stabilize the FAPVs [103]. They used it to tune the properties of α-FAPbI3 due to its special functional units. The novel perovskite structure can be formed, which has low dimensional G2PbI4. It has been established by solid-state NMR that G interacted with the perovskite lattice at the atomic level. The NMR displayed that the microstructure of bulk mechanochemical FAPbI3 was formed when 10 mol% GI doped. NMR spectrum contained four G peaks and these were different from the peaks of neat GI or G2PbI4, which illustrated the new G/FA-based iodoplumbate phase formed. The structural changes of FAPbI3 were investigated by a sensitice diagnostic tool (14N NMR). The narrower 14N SSB manifolds manifested that GI affected the crystallographic symmetry of the 3D perovskite due to the narrower 14N SSB manifolds (See Figure 8f). TEM images also illustrated that new G environments modified by the proximity of the α-phase of 3D perovskite was formed (shown in Figure 8g,h). According to Figure 8i, the mixed hybrid perovskite structures were formed due to a number of different tautomeric forms and their propensity to dimerization by H bonding [103]. Methylenediammonium dichloride (MDACl2) was used as additive to stabilize the α-phase FAPbI3 since MDA has more H groups [104]. Larger number of H bonds were formed between MDA and I−. So smaller amount of MDA can structurally stabilize the α-FAPbI3. The XRD illustrated that there was no any impurity peak originating from FACl or MDACl2, when MDACl2 was added to the FAPbI3 (shown in Figure 8d). This result implied that the FAPbI3 perovskite lattices included MDACl2, and the number of H bonds between PbI2 and MDA increased (shown in Figure 8e). The band gap has been changed due to introduction of the MDACl2. It was confirmed by DFT and compared for two cases as Equations (7) and (8).
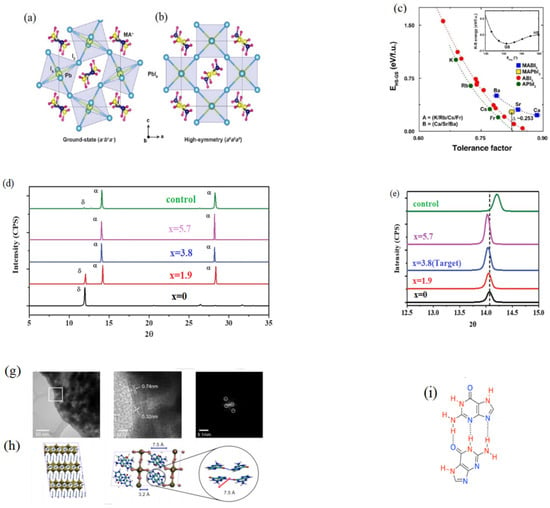
Figure 8.
(a) The ground state of o-MAPbI3; (b) High symmetry structures of o-MAPbI3; (c) Corresponding the energy of o-ABI3 (red), o-APbI3 (green), o-MABI3 (blue), and o-MAPbI3 (yellow). The difference of t can be seen according to the horizontal error bars of the o-MABI3 hybrid structures. (Reprinted with permission from Ref. [100]. Copyright 2016 ACS Publications). XRD patterns of FAPbI3:xMDACl2 perovskite. (d) The perovskite was exposed to 80% humidity for 24 h and annealed at 150 °C for 10 min. (e) The perovskite synthesized with different x values (x = 0, 1.9, 3.8, and 5.7 mol%) and (001) orientation peaks was magnified. Reprinted with permission from Ref. [102]. Copyright 2019 Science. (f) 14N NMR spectra of α-FAPbI3 (top) and G-containing α-FAPbI3 (bottom). (g) A lower magnification TEM image and high-resolution TEM image of G2FAn−1PbnPbI3n+1 (n = 20) perovskite; (h) The structure of G2FAPb2I7 optimized by DFT. (i) The structure of H bond (HB) dimer, including H bond donating (red) and accepting sites (blue). Reprinted with permission from Ref. [103]. Copyright by 2019 Angewandte Chemie International Edition.
FA vacancy:
Cl insertion:
According to Equations (7) and (8), the FA cation vacancies and the insertion of Cl− with a small ionic radius can be assumed. The band gap (1.47 eV) with FA vacancy was bigger than that of origin FAPbI3 (1.45 eV). That of the Cl insertion has been increased to 1.69 eV. PCE of the related device could reach to 23%. In 2020, the impact of strain relaxation on performance of α-FAPbI3 was studied by Kim and coworkers [105]. They found that 0.03 mol fraction of both MDA and Cs cations could decrease lattice strain, increase carried lifetime, reduced Urbach energy and defect concertation. PCE could achieve to 24.4%. The dual replacement of FA+ sites with MDA2+ and Cs+ can relax the lattice strain of MDA-stabilized α-FAPbI3.
In this paper, we mainly introduce Pb-based perovskite. However, the toxicity of lead is high and this can affect their practical application. Some Pb free perovskite solar cells based on FA have also been investigation [106,107]. For example, Li et al. used MA/FA exchange to form MAxFA1−xSnI3 perovskites materials with 9.11% PCE. The devices of these materials demostrated good stabilty [107]. If the stability of Sn-base FA perovskites materials can be improved in the future, these materials will be widely applicated.
3. Summary and Future Outlook
FAPVs materials have been studied in the last few years. FAPVs materials have two phases, α-phase (black) and δ-phase (yellow). α-phase FAPVs tend to transform into δ-phase at room temperature. Inherent intrinsic instability of α-phase FAPVs is a very bothering problem. Improving the stability of α-phase perovskite is a key point. In this article, we have reviewed recent strategies to stabilize the α-phase of FAPVs materials including cation-doped (alkali metal cations, MA+), anion-exchanged, multi-dimensional perovskites (2D perovskites, 2D/3D perovskites) and surface-engineering. At first, cation-doped and anion-exchanged are very useful methods to stabilize FAPVs. For example, alkali metal cation-doped perovskite, especially Cs+ cation, have been used to improve the stability and PCE of perovskite materials. Furthermore, controlling the dimensions of perovskites is another strategy to stabilize the phase. 2D/3D bilayer structure has higher PCE and trap state densities. The 2D perovskites were formed only at the top surface of the 3D layer, it is effectively stabilized the α-phase beneath though. Controlling the surface composition is an important strategy to form bilayer structure. Surface engineering including surface confinement and surface passivation, has become an emerging effective technology to stabilize α-phase FAPVs. Finally, we discussed the influence of some additives. These additives mainly influence the H bonds in the materials. Direction of the H bonding between the anion framework and the organic molecule was also important to boosting the solar cell efficiency.
Although many research have been reported to improve the stability of FAPVs, a deep understanding of the mechanisms of surface engineering and additives are still not clear. We accentuate the importance to improve the stability of α-phase FAPVs and the key effect of hydrogen bonding in the FAPVs. For the practical application of FAPVs in solar cells, there are still a lot of works related to phase stability to carry out. We hope that more stable α-FAPVs and deeper understanding of the related stabilizing mechanisms will be revealed in the coming future.
Author Contributions
Writing—original draft preparation, H.Z., J.H. and L.Z.; writing—review and editing, H.Z. and J.H. Collect informations, S.L., Y.C., Y.S., B.X., J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from the National Natural Science Foundation of China (12064050/21965038); the Major Project of Science and Technology of Yunnan Province under Grant (202002AE090010). This work is also supported by a grant from Key Laboratory for Crop Production and Smart Agriculture of Yunnan Province, Agricultural Union Youth Program of Yunnan Provincial Science and Technology Department (2018FG001-102), Scientific Research Fund of Yunnan Education Department (2021J0114).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, N.S.; Naidu, K.C.B. A review on perovskite solar cell (PSCs), materials and applications. J. Mater. 2021, 7, 940–956. [Google Scholar]
- Mayengbam, R.; Mazumder, J.T.; Tripathy, S.K. Structural, electronic and mechanical properties of formamidinium lead halideperovskites, [HC(NH2)2]PbX3(X = I, Br) from first-principles calculations. Mater. Today Proc. 2021, 43, 3627–3630. [Google Scholar] [CrossRef]
- Xu, Q.L.; Yang, D.W.; Lv, J.; Sun, Y.Y.; Zhang, L.J. Perovskite Solar Absorbers: Materials by Design. Small Methods 2018, 2, 1700316. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.X.; Qi, W.J.; Zhou, X.; Li, J.L.; Cheng, J.; Zhao, Y.; Li, Y.L.; Zhang, X.D. Passivation of defects in perovskite solar cell: From a chemistry point of view. Nano Energy 2020, 77, 105237–105264. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Lee, G.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; Shin, T.J.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.C.; Seo, J.W.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Gratzel, M.; Angelis, F.D. Cation-Induced Band-GapTuningin Organohalide Perovskites: Interplay of Spin−Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Zhou, Y.Y.; Zhen, L.; Zhang, L.; Ju, M.G.; Luo, D.Y.; Yang, Y.; Yang, M.J.; Kim, D.H.; Yang, W.Q.; et al. Stable Formamidinium-Based Perovskite Solar Cellsvia In Situ Grain Encapsulation. Adv.Energy Mater. 2018, 8, 1800232–1800241. [Google Scholar] [CrossRef]
- Koh, T.M.; Fu, K.W.; Fang, Y.N.; Chen, S.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G.; Boix, P.P.; Baikie, T. Formamidinium-Containing Metal-Halide: An Alternative Material for Near-IR Absorption Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16458–16462. [Google Scholar] [CrossRef]
- Jannat, F.; Ahmed, S.; Alim, M.A. Performance analysis of cesium formamidinium lead mixed halide based perovskite solar cell with MoOx as hole transport material via SCAPS-1D. Int. J. Light Electron Opt. 2021, 228, 166202–166211. [Google Scholar] [CrossRef]
- He, X.; Guo, P.F.; Wu, J.H.; Tu, Y.G.; Lan, Z.; Lin, J.M.; Huang, M.L. Hybrid perovskite by mixing formamidinium and methylammonium leadiodides for high-performance planar solar cells with efficiency of 19.41%. Sol. Energy 2017, 157, 853–859. [Google Scholar] [CrossRef]
- Qiu, Z.W.; Li, N.; Huang, Z.J.; Chen, Q.; Zhou, H.P. Recent Advances in Improving Phase Stability of Perovskite Solar Cells. Small Methods 2020, 4, 1900877. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Li, G.; Zhang, T.; Guo, N.; Xu, F.; Qian, X.; Zhao, Y. Ion-exchange-induced 2D–3D conversion of HMA1−xFAxPbI3Cl perovskite into a high-quality MA1−xFAxPbI3 perovskite. Angew. Chem. Int. Ed. 2016, 55, 13460–13464. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive engineering for efficient and stable perovskite solar cells. Adv. Energy Mater. 2020, 10, 1902579. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhu, L.; Zhao, B.G.; Song, J.; Gu, X.Q.; Qiang, Y.H. Surface engineering of perovskite films for efficient solar cells. Sci. Reorts 2017, 7, 14478–14486. [Google Scholar] [CrossRef] [Green Version]
- Prochowicza, D.; Tavakolic, M.M.; Wolska-Pietkiewicze, M.; Jędrzejewskae, M.; Trivedi, S.; Kumar, M.; Zakeeruddina, S.M.; Lewińskib, J.; Graetzel, M.; Yadav, P. Suppressing recombination in perovskite solar cells via surface engineering of TiO2 ETL. Sol. Energy 2020, 197, 50–57. [Google Scholar] [CrossRef]
- Masi, S.; Echeverra-Arrondo, C.; Salim, K.M.M.; Ngo, T.T.; Mendez, P.F.; Lopez-Fraguas, E.; Macias-Pinilla, D.F.; Planelles, J.; Climente, J.I.; Mora-Seró, I. Chemi-structural stabilization of formamidinium lead iodide perovskite by using embedded quantumdots. ACS Energy Lett. 2020, 5, 418–427. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, Y.S.; Li, Y.H.; Yu, Y.G.; Cai, J.; Chiu, M.H.; Rao, R.; Gu, Y.; Wang, C.F.; Choi, W.; et al. Strain engineering and epitaxial stabilization of halid perovskites. Nature 2020, 577, 209–215. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310–1501318. [Google Scholar] [CrossRef]
- Zhou, Y.; Kwun, J.; Garces, H.F.; Pang, S.; Padture, N.P. Observation of Phase-Retention Behavior of the HC(NH2)2PbI3 Black Perovskite Polymorph upon Mesoporous TiO2 Scaffolds. Chem. Commun. 2016, 52, 7273–7277. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.T.; Weber, O.J.; Frost, J.M.; Walsh, A. Cubic perovskite structure of black formamidinium lead iodide, α-[HC-(NH2)2]PbI3, at 298k. J. Phys. Chem. Lett. 2015, 6, 3209–3212. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved Phase Stability of Formamidinium Lead Triiodide Perovskite by Strain Relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Zhu, X.; Zuo, S.; Yang, Z.; Feng, J.; Wang, Z.; Zhang, X.; Priya, S.; Liu, S.F.; Yang, D. In Situ Grain Boundary Modication via Two Dimensional Nanoplates to Remarkably Improve Stability and Efficiency of Perovskite Solar Cells. Interfaces 2018, 10, 39802–39808. [Google Scholar]
- Chen, T.; Foley, B.J.; Park, C.; Brown, C.M.; Harriger, L.W.; Lee, J.; Ruff, J.; Yoon, M.; Choi, J.J.; Lee, S.H. Entropy-Driven Structural Transition and Kinetic Trapping in Formamidinium Lead Iodide Perovskite. Sci. Adv. 2016, 2, e1601650–e1601656. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Zhang, D.; Kam, M.; Zhang, Q.; Poddar, S.; Fu, Y.; Mo, X.; Fan, Z. Significantly Improved Black Phase Stability of FAPbI3 Nanowires via Spatially Confined Vapor Phase Growth in Nano-porous Templates. Nanoscale 2018, 10, 15164–15172. [Google Scholar] [CrossRef]
- Cordero, F.; Cracium, F.; Trequattrini, F.; Generosi, A.; Paci, B.; Paoletti, A.M.; Pennesi, G. Stability of Cubic FAPbI3 from X-ray Diffraction, Anelastic, and Dielectric Measurements. J. Phys. Chem. Lett. 2019, 10, 2463–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Wu, Y.Z.; Zhang, S.; Wu, T.H.; He, T.; Zhu, W.H.; Han, L.Y. Stabilizing Formamidinium Lead Iodide Perovskite bySulfonyl-Functionalized Phenethylammonium Salt via Crystallization Control and Surface Passivation. Sol. RRL. 2020, 4, 2000069–2000076. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.; Jin, S. Nanowire Lasers of Formamidinium Lead Halide Perovskites and Their Stabilized Alloys with Improved Stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Weber, O.J.; Charles, B.; Weller, M.T. Phase behaviour and composition in the formamidinium-methylammonium hybrid lead iodide perovskite solid solution. J. Mater. Chem. A 2016, 4, 15375–15382. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.C.; Seok, H.J.; Kim, S.K.; Kim, D.H.; Hou, B.; Kim, H.K. The effect of Cs/FA ratio on the long-term stability of mixed cation perovskite solar cells. Sol. RPL 2021, 5, 2100660–2100687. [Google Scholar]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Kawachi, S.; Atsumi, M.; Saito, N.; Ohashi, N.; Murakami, Y.; Yamaura, J. Structural and Thermal Properties in Formamidinium and Cs-Mixed Lead Halides. J. Phys. Chem. Lett. 2019, 10, 6967–6972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Cui, W.R.; Zang, Z.H.; Ti, F.Y.; Li, X.D.; Qin, G.G. Quantitative phase analysis on Cs-and Rb-doped FAPbI3 and corresponding solar cell efficiency simulations. Sol. Energy 2019, 188, 224–229. [Google Scholar] [CrossRef]
- Park, Y.H.; Jeong, I.; Bae, S.; Son, H.J.; Lee, P.; Lee, J.; Lee, C.H.; Ko, M.J. Inorganic Rubidium Cation as an Enhancer for Photovoltaic Performance and Moisture Stability of HC(NH2)2PbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1605988–1605997. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, J.J.; Sepúlveda, M.B.; Aguilar, T.; Alcántara, R.; Fernández-Lorenzo, C.; Navas, J. Intrinsic stability analysis of perovskite nanopowder with double andt ripple cation in a site, FAxMA(1−x)PbI3 and FAxCsyMA(1−x−y)PbI3. Mater. Res. Bull. 2019, 119, 110528–110539. [Google Scholar] [CrossRef]
- Derbalia, S.; Nouneha, K.; Floreac, M.; Leonatc, L.N.; Stancuc, V.; Tomulescuc, A.G.; Galcac, A.C.; Secud, M.; Pintiliec, L.; Ebn Touhami, M. Potassium-containing triple-cation mixed-halide perovskite materials: Toward efficient and stable solar cells. J. Alloy. Compd. 2020, 858, 158335. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xie, C.G.; Li, G.; Dai, P.P.; Yang, L.; Liu, R.Y.; Pan, B.C. Effect of cation replacement on the phase stability of formamidinium lead iodide perovskite. J. Chem. Phys. 2019, 151, 134104–134111. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Mall, R.; Alharbi, F.H.; Sanvito, S.; Tabet, N.; Bensmail, H.; El-Mellouhi, F. Learn-and-Matche Molecular Cations for Perovskites. J. Phy. Chem. A 2019, 123, 7323–7334. [Google Scholar] [CrossRef]
- Gao, P.; Gratzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Li, Q.X.; Liu, Y.C.; Zhang, Y.X.; Hu, M.X.; Yang, Z.; Liu, S.Z. Synergistic Enhancement of Cs and Br Doping in Formamidinium Lead Halide Perovskites for High Performance Optoelectronics. Cryst. Eng. Comm. 2018, 20, 5510–5518. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–481. [Google Scholar] [CrossRef]
- Long, M.Z.; Zhang, T.K.; Xu, W.Y.; Zeng, X.L.; Xie, F.Y.; Li, Q.; Chen, Z.F.; Zhou, F.R.; Wong, K.S.; Yan, K.Y.; et al. Large-Grain Formamidinium PbI3–xBrx for High-Performance Perovskite Solar Cells via Intermediate Halide Exchange. Adv. Energy Mater. 2017, 7, 1601882–1601890. [Google Scholar] [CrossRef]
- Rehman, W.; Milot, R.L.; Eperon, G.E.; Wehrenfenning, C.; Boland, J.L.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Charge-Carrier Dynamics and Mobilities in Formamidinium Lead Mixed-Halide Perovskites. Adv. Mater. 2015, 27, 7938–7944. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Chemical Management for Colorful, Efficient, and Stable Inorganic-Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Li, B.; Zhu, H.; Xu, Q.; Ouyang, J. High Performance Planar Perovskite Solar Cells with A Perovskite of Mixed Organic Cations and Mixed Halides, MA1−xFAxPbI3−yCly. J. Mater. Chem. A 2016, 4, 12543–12553. [Google Scholar] [CrossRef] [Green Version]
- Nan, Z.A.; Chen, L.; Liu, Q.; Wang, S.H.; Chen, Z.X.; Kang, S.Y.; Ji, J.B.; Tan, Y.Y.; Hui, Y.; Yan, J.W.; et al. Revealing phase evolution mechanism for 36 for mamidinium-based lead halide perovskites by a key intermediate phase. Chem 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Gao, P.; Bin Mohd Yusoff, A.R.; Nazeeruddin, M.K. Dimensionality engineering of hybrid halide perovskite light absorbers. Nat. Commun. 2018, 9, 528–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.G.; Ma, L.F.; Yan, D.P. Facile Synthesis of 1D Organic–Inorganic Perovskite Micro-Belts with High Water Stability for Sensor and Photonic Applications. Chem. Sci. 2019, 10, 4567–4572. [Google Scholar] [CrossRef] [Green Version]
- Pious, J.K.; Katre, A.; Muthu, C.; Chakraborty, S.; Krishna, S.; Nair, V.C. Zero-Dimensional lead-free hybrid perovskite-like material with a quantum-well structure. Chem. Mater. 2019, 31, 1941–1945. [Google Scholar] [CrossRef]
- Kahwagi, R.F.; Thornton, S.T.; Smith, B.; Koleilat, G.I. Dimensionality engineering of metal halide perovskites. Front. Optoelectron. 2020, 13, 196–224. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.H.; Ma, B. Low dimensional metal halide perovskites and hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–653. [Google Scholar] [CrossRef]
- Ju, M.G.; Dai, J.; Ma, L.; Zhou, Y.Y.; Zeng, X.C. Zero-Dimensional Organic-Inorganic Perovskite Variant: Transition between Molecular and Solid Crystal. J. Am. Chem. Soc. 2018, 140, 10456–10463. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Lyu, M.Q.; Yun, J.H.; Hao, M.M.; Wang, L.Z. Progress and Perspective in Low-Dimensional Metal Halide Perovskites for Optoelectronic Applications. Sol. RRL 2018, 2, 1700186–1700213. [Google Scholar] [CrossRef]
- Huang, G.Q.; Zhou, H.L.; Wang, C.P.; Kashi, C.; Ye, X.L.; Li, W.H.; Wang, G.; Xu, G. A new 1D inorganic–organic hybrid perovskite-like semiconductor with high stability and humidity response. Inorg. Chem. Commun. 2021, 28, 108581–108587. [Google Scholar] [CrossRef]
- Li, Y.Y.; Lin, C.K.; Zheng, G.L.; Cheng, Z.Y.; You, H.; Wang, W.D.; Lin, J. Novel <110>-Oriented Organic−Inorganic Perovskite Compound Stabilized by N-(3-Aminopropyl)imidazole with Improved Optical Properties. Chem. Mater. 2006, 18, 3463–3469. [Google Scholar] [CrossRef]
- Lee, J.W.; Dai, Z.H.; Han, T.H.; Choi, C.; Chang, S.Y.; Lee, S.J.; Marco, N.D.; Zhao, X.X.; Sun, P.Y.; Huang, Y.; et al. 2D perovskite stabilized phase-pure formamidinium perovskite solar cell. Nat. Commun. 2018, 9, 3021–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.B.; Akhtar, M.S.; Shin, H.S.; Ameen, S.; Nazeeruddin, M.K. A review on two-dimensional (2D) and 2D-3D multidimensional perovskite solar cells: Perovskites structures, stability, and photovoltaic performances. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100405–100427. [Google Scholar] [CrossRef]
- Liu, Y.H.; Akin, S.; Hinderhofer, A.; Eickemeyer, F.T.; Zhu, H.W.; Seo, J.Y.; Zhang, J.H.; Schreiber, F.; Zhang, H.; Zakeeruddin, S.M.; et al. Stabilization of highly efficient and stable phase-pure FAPbI3 Perovskite Solar Cells by Molecularly Tailored 2D-Overlayers. Angew. Chem. Int. Ed. 2020, 59, 15688–15694. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef]
- Perumallapellia, G.R.; Tsudaa, T.; Formaneka, P.; Kiriya, N.; Bakulevd, V.; Simona, F.; Voita, B.; Mannsfelde, S.C.B.; Kiriya, A. New insights into the structure of two-dimensional lead iodide-based perovskites. Org. Electron. 2020, 87, 105935–105951. [Google Scholar] [CrossRef]
- Quan, L.N.; Yuan, M.G.; Comin, R.; Voznyy, O.; Beauregard, E.M.; Hoogland, S.; Buin, A.; Kirmani, A.R.; Zhao, K.; Amassian, A.; et al. Ligand-Stabilized Reduced-Dimensionality Perovskites. J. Am. Chem. Soc. 2016, 138, 2649–2655. [Google Scholar] [CrossRef] [Green Version]
- Proppe, A.H.; Wei, M.Y.; Chen, B.; Quintero-bermudez, R.; Kelley, S.O.; Sargent, E.H. Photochemically Cross-Linked Quantum Well Ligands for 2D/3D Perovskite Photovoltaics with Improved Photovoltage and Stability. J. Am. Chem. Soc. 2019, 141, 14180–14189. [Google Scholar] [CrossRef]
- Zhang, F.; Park, Y.; Yao, C.L.; Liu, H.P.; Dunfield, S.P.; Xiao, C.X.; Yan, Y.F.; Larson, B.W.; Zhu, K. Metastable Dion-Jacobson 2D structure enables efficient and stable perovskite solar cells. Science 2021, 375, 71–76. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Saidaminov, M.I.; Haque, M.A.; Alarousu, E.; Sarmah, S.P.; Murali, B.; Dursun, I.; Miao, X.H.; Abdelhady, A.L.; Wu, T.; et al. Formamidinium lead halide perovskite crystals with unprecedented long carrier dynamics and diffusion length. ACS Energy Lett. 2016, 1, 32–37. [Google Scholar] [CrossRef]
- Yettapu, G.R.; Talukdar, D.; Sarkar, S.; Swarnkar, A.; Nag, A.; Ghosh, P.; Mandal, P. Terahertz conductivity within colloidal CsPbBr3 perovskite nanocrystals: Remarkably high carrier mobilities and large diffusion lengths. Nano Lett. 2016, 16, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.J.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.W.; Stranks, S.D.; Snaith, S.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Even, J.; Pedesseau, L.; Katan, C. Analysis of multivalley and multibandgap absorption and enhancement of free carriers related to exciton screening in hybrid perovskites. J. Phys. Chem. C 2014, 118, 11566–11572. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, K. Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef]
- Cao, F.R.; Zhang, P.; Li, L. Multidimensional perovskite solar cells. Fundam. Res. 2021, 2, 237–253. [Google Scholar] [CrossRef]
- Zhang, F.; Kim, D.; Zhu, K. 3D/2D Multidimensional perovskites: Balance of highper formance and stability for perovskite solar cells. Curr. Opin. Electrochem. 2018, 11, 105–113. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A Hole-Conductor-Free, Fully Printable Mesoscopic Perovskite Solar Cell with High Stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; Angelis, D.F.; Graetzel, M.; et al. One-year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684–15691. [Google Scholar] [CrossRef]
- Cai, Y.; Wen, J.L.; Liu, Z.; Qian, F.; Duan, C.Y.; He, K.; Zhao, W.J.; Zhan, S.; Yang, S.M.; Cui, J.; et al. Graded 2D/3D (CF3-PEA)2FA0.85MA0.15Pb2I7/FA0.85MA0.15PbI3 heterojunction for stable perovskite solar cell with an efficiency over 23.0%. J. Energy Chem. 2022, 65, 480–489. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Dar, M.I.; Li, G.; Xu, F.; Guo, N.J.; Gratzel, M.; Zhao, Y.X. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017, 3, e1700841–e1700846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, W.J.; Chen, C.; Spanopoulos, I.; Mao, L.L.; Hadar, I.; Li, X.T.; Hoffman, J.M.; Song, Z.N.; Yan, Y.F.; Kantzidis, M. Narrow-Bandgap Mixed Lead/Tin-Based 2D Dion−Jacobson Perovskites Boost the Performance of Solar Cells. J. Am. Chem. Soc. 2020, 142, 15049–15057. [Google Scholar] [CrossRef]
- Zhou, T.; Lai, H.T.; Liu, T.T.; Lu, D.; Wan, X.J.; Zhang, X.D.; Liu, Y.S.; Chen, Y.S. Highly Efficient and Stable Solar Cells Based on Crystalline Oriented 2D/3D Hybrid Perovskite. Adv. Mater. 2019, 31, 1901242–1901250. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.T.; Zhang, J.; Wang, J.M.; Long, M.Z.; Xie, F.Y.; Chen, J.; Wu, B.J.; Shi, T.T.; Yan, K.Y.; Xie, W.G.; et al. Stable and scalable 3D-2D planar heterojunction perovskite solar cells via vapor deposition. Nano. Energy 2019, 59, 619–625. [Google Scholar] [CrossRef]
- LaPlaca, M.G.; GilEscrig, L.; Guo, D.; Palazon, F.; Savenije, T.J.; Sessolo, M.; Bolink, H.J. Vacuum-deposited 2D/3D perovskite heterojunctions. ACS Energy Lett. 2019, 4, 2893–2901. [Google Scholar] [CrossRef]
- Yu, S.S.; Liu, H.L.; Wang, S.; Zhu, H.W.; Dong, X.F.; Li, X.G. Hydrazinium cation mixed FAPbI3-based perovskite with 1D/3D hybrid dimension structure for efficient and stable solar cells. Chem. Eng. J. 2021, 403, 125724–125732. [Google Scholar] [CrossRef]
- Yang, K.Y.; Li, F.S.; Hu, H.L.; Guo, T.L.; Kim, T.W. Surface engineering towards highly efficient perovskite light-emitting diodes. Nano Energy 2019, 65, 104029–104042. [Google Scholar] [CrossRef]
- Lu, H.A.; Krishna, A.; Zakeeruddin, S.M.; Gratzel, M.; Hagfelds, A. Compositional and Interface Engineering of Organic-Inorganic Lead Halide Perovskite Solar Cells. Iscience 2020, 23, 101359–101372. [Google Scholar] [CrossRef]
- Fu, Y.P.; Wu, T.; Wang, J.; Zhai, J.Y.; Shearer, M.J.; Zhao, Y.Z.; Hamers, R.J.; Kan, E.; Deng, K.M.; Zhu, X.Y.; et al. Stabilization of the Metastable Lead Iodide Perovskite Phase via Surface Functionalization. Nano Lett. 2017, 17, 4405–4414. [Google Scholar] [CrossRef]
- Lu, H.Z.; Liu, Y.L.; Ahlawat, P.; Mishra, A.; Tress, W.R.; Zhan, Y.Q.; Zheng, L.; Hagfeldt, A.; Grätzel, M. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells. Science 2020, 370, eabb8985–eabb8993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, H.P.; Larson, B.W.; Xiao, C.X.; Dunfield, S.P.; Reid, O.G.; Chen, X.H.; Yang, M.J.; Berry, J.J.; Beard, M.C.; et al. Surface lattice engineering through three-dimensional lead iodide perovskitoid for high-performance perovskite solar cells. Chem 2021, 7, 744–785. [Google Scholar] [CrossRef]
- Deng, W.B.; Li, F.M.; Li, J.Y.; Wang, M.; Hu, Y.C.; Liu, M.Z. Anti-solvent free fabrication of FAPVs at low temperature towards to high performance flexible perovskite solar cells. Nano Energy 2020, 70, 104505–104512. [Google Scholar] [CrossRef]
- Majeed, S.M.; Ahmed, D.S.; Mohammed, M.A. Anti-solvent engineering via potassium bromide additive for highly effcient and stable perovskite solar cells. Org. Electron. 2021, 99, 106310–106317. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Que, L.; Wang, Y.; Wang, F.; Wu, J.; Li, S. Optimization of anti-solvent engineering toward high performance perovskite solar cells. J. Mater. Res. 2019, 34, 2416–2424. [Google Scholar] [CrossRef]
- He, J.J.; Chu, Y.F.; Sun, Y.C.; Zhang, R.; Li, J.; Zhao, L.; Zhao, H.M.; Liu, P.F.; Li, S. Beyond the Limit of Goldschmidt Tolerance Factor: Crystal Surface Engineering to Boost the α-phase Stability of Formamidinium-Only Hybrid Inorganic-Organic Perovskites. Sol. RRL 2021, 5, 2100188–2100200. [Google Scholar] [CrossRef]
- Alharbi, E.A.; Alyamani, A.Y.; Kubicki, D.J.; Uhl, A.R.; Walder, B.J.; Alanazi, A.Q.; Luo, J.; Burgos-Caminal, A.; Albadri, A.; Albrithen, H.; et al. Atomic-level passivation mechanism of ammonium salts enabling highly efficient perovskite solar cells. Nat. Commun. 2019, 10, 3008–3016. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qiu, Y.K.; Wang, L.Y.; Zhang, T.K.; Zhu, L.; Shan, T.; Wang, Y.; Jiang, J.K.; Kong, L.T.; Zhong, H.L.; et al. Organic nanocrystals induced surface passivation towards high-efficiency and stable perovskite solar cells. Nano Energy 2021, 89, 106445–106453. [Google Scholar] [CrossRef]
- Azam, M.; Khan, A.A.; Liang, G.X.; Li, G.J.; Chen, S.; Zheng, Z.H.; Farooq, U.; Ishaq, M.; Fan, P.; Wang, Z.J.; et al. Examing the Interfacial Defect Passivation with Chlorinated Organic Salt for Highly Efficient and Stable Perovskite Solar Cells. Sol. RPL 2020, 4, 2000358–2000366. [Google Scholar]
- Lee, J.H.; Bristowe, N.C.; Lee, J.H.; Lee, S.H.; Bristowe, P.D.; Cheetham, A.K.; Jang, H.M. Resolving the Physical Origin of Octahedral Tilting in Halide Perovskites. Chem. Mater. 2016, 28, 4259–4266. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Bristowe, N.C.; Bristowe, P.D.; Cheetham, A.K. Role of hydrogen-bonding and its interplay with octahedral tilting in CH3NH3PbI3. Chem. Commun. 2015, 51, 6434–6437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oranskaia, A.; Schwingenschlogl, U. Suppressing X-Migrations and Enhancing the Phase Stability of Cubic FAPbX3 (X = Br, I). Adv. Energy Mater. 2019, 9, 1901411–1901422. [Google Scholar] [CrossRef]
- Hong, L.; Milić, J.V.; Ahlawat, P.; Mladenović, M.; Kubicki, D.J.; Jahanabkhshi, F.; Ren, D.; Gélvez-Rueda, M.C.; Ruiz-Preciado, M.A.; Ummadisingu, A.; et al. Guanine-Stabilized Formamidinium Lead Iodide Perovskites. Angew. Chem. Int. Ed. 2019, 59, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Kim, M.; Lee, S.U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S. Efficient, stable solar cells by using inherent bandgap of a-phase formamidinium lead iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Min, H.; Lee, K.S.; Yoon, S.M.; Seok, S. Impact of strain relaxation on performance of a-formamidinium lead iodide perovskite solar cells. Science 2020, 370, 108–112. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.P.; Zhang, X.T.; Shen, X.Y.; Lu, M.; Wu, J.L.; Shi, Z.F.; Colvin, V.L.; Hu, J.H.; Bai, X.; et al. Lead-Free Halide Perovskites for Light Emission: Recent Advances and Perspectives. Adv. Sci. 2021, 8, 2003334. [Google Scholar] [CrossRef]
- Li, F.J.; Hou, X.Y.; Wang, Z.; Cui, X.X.; Xie, G.H.; Yan, F.; Zhao, X.Z.; Tai, Q.D. FA/MA Cation Exchange for Efficient and Reproducible Tin-Baded Perovskite Solar Cells. Appl. Mater. Interfaces 2021, 13, 40656–40663. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).