Construction and Demolition Residuals as Raw Materials for the Production of Novel Geopolymer Building Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. X-ray Fluorescence Analysis
2.3. X-ray Diffraction Analysis
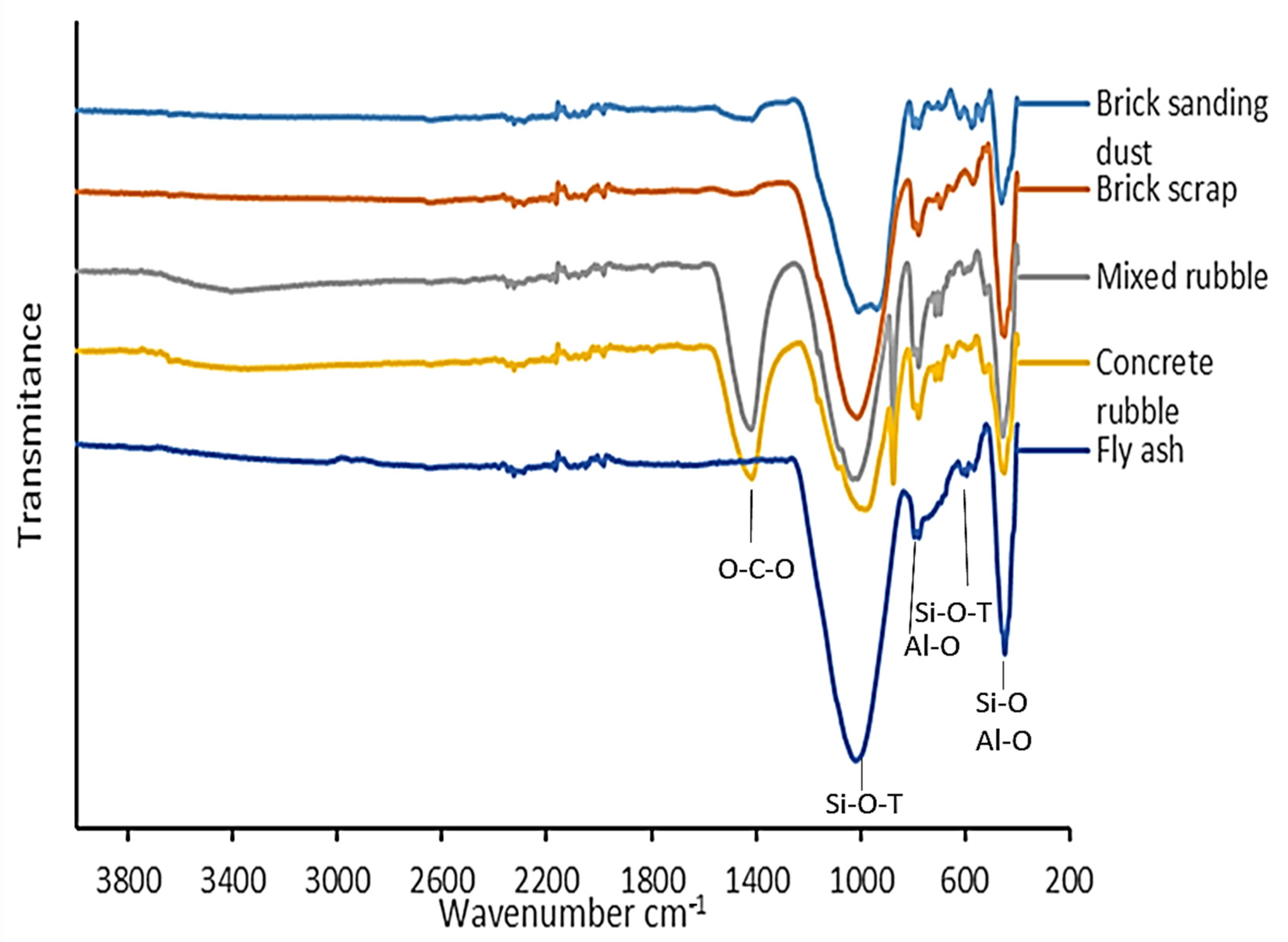
2.4. FTIR-Spectroscopy
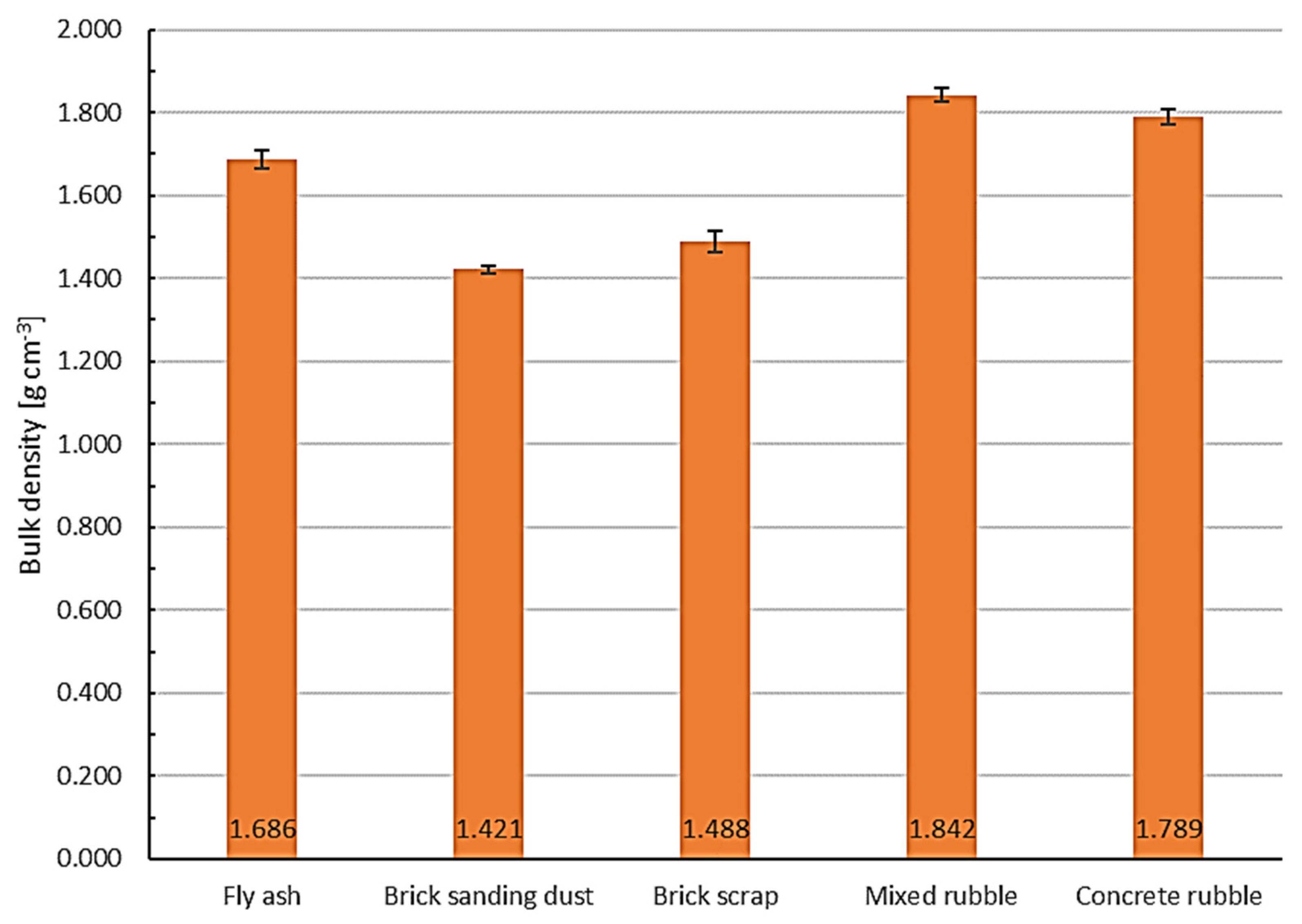
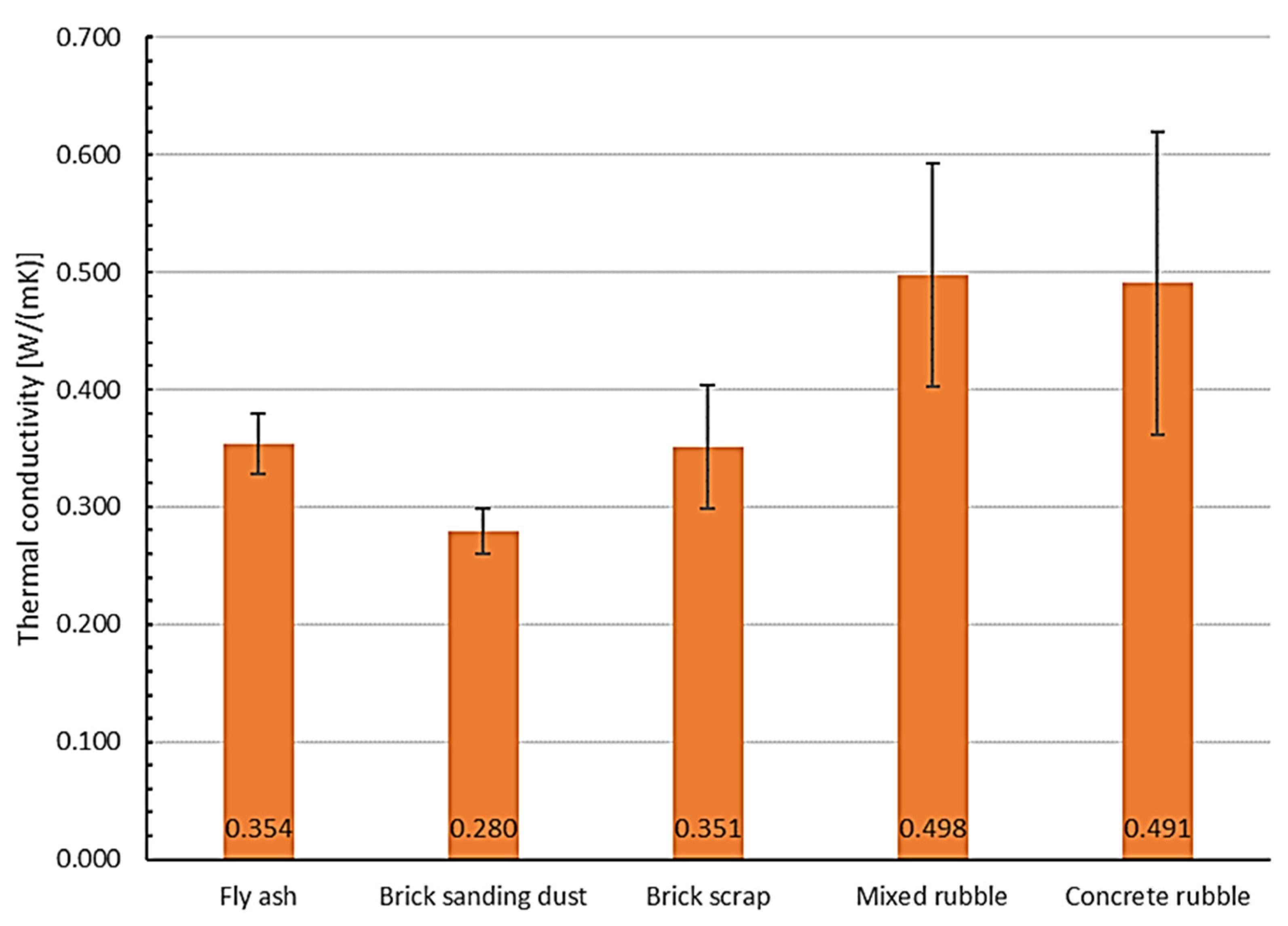
2.5. Determination of the Material Properties
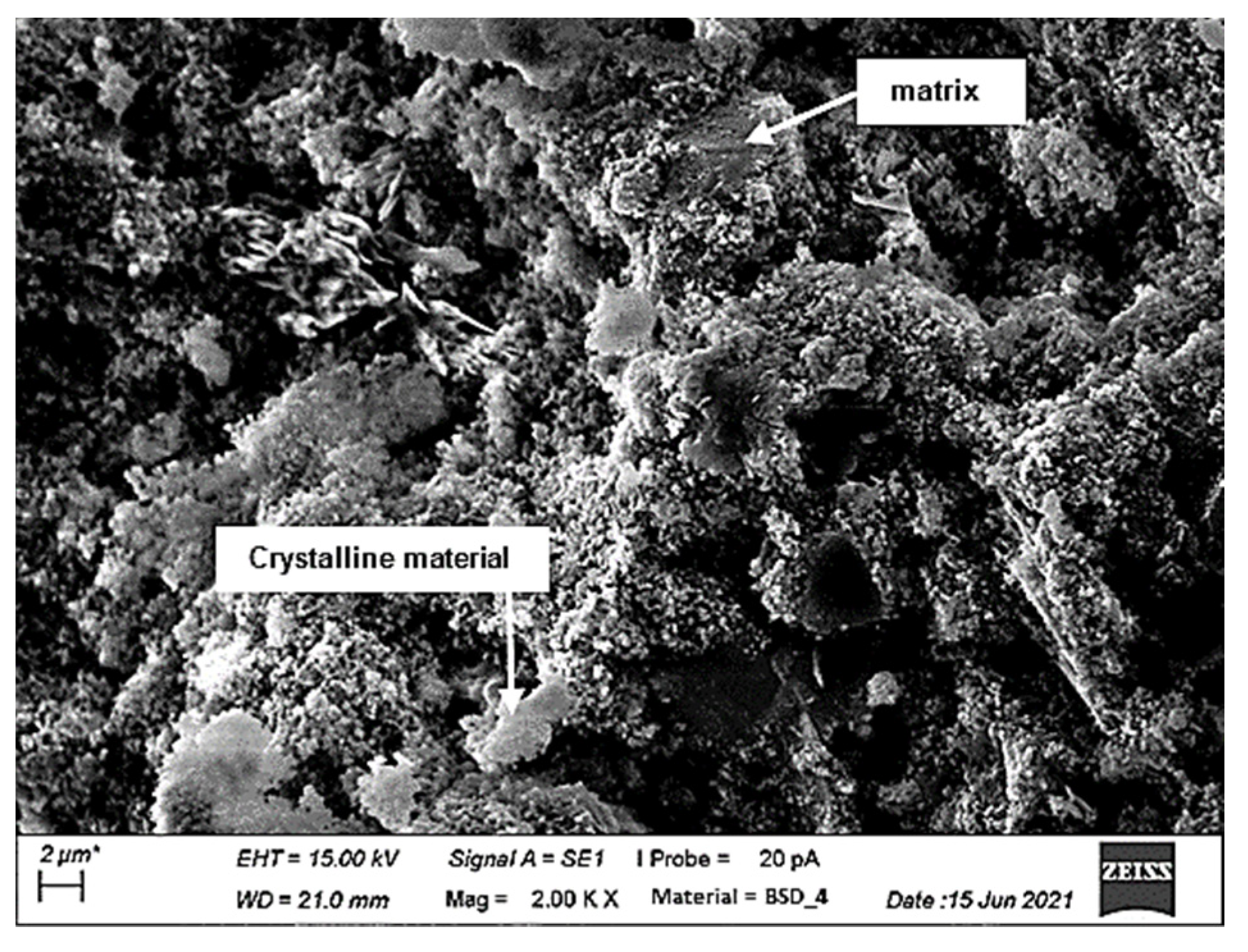
2.6. Scanning Electron Microscopy
3. Results
3.1. X-ray Fluorescence Analysis
3.2. X-ray Diffraction Analysis
3.3. FTIR-Spectroscopy
3.4. Determination of the Material Properties
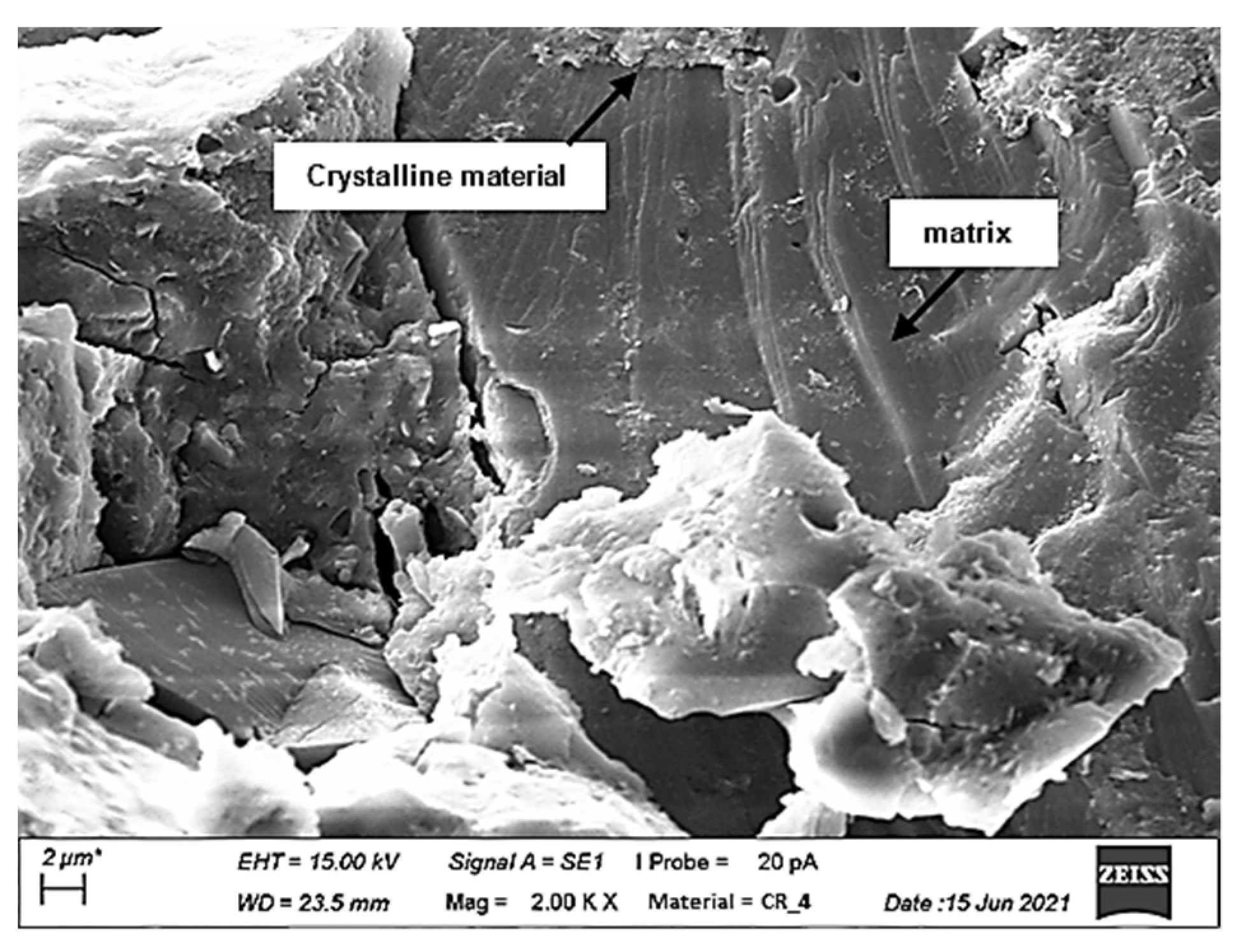
3.5. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistisches Bundesamt. Bautätigkeit—Fachserie 5 Reihe 1; Statistisches Bundesamt: Wiesbaden, Germany, 2019. [Google Scholar]
- Statistisches Bundesamt. Abfallbilanz (Abfallaufkommen/-Verbleib, Abfallintensität, Abfallaufkommen Nach Wirtschaftszweigen); Statistisches Bundesamt: Wiesbaden, Germany, 2018. [Google Scholar]
- Fehn, T.; Teipel, U. Werkstoffliche Aufbereitung von Wärmedämmverbundsystemen. Chem. Ing. Tech. 2020, 92, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Fehn, T.; Kugler, F.; Tübke, B.; Schweppe, R.; Mebert, P.; Krcmar, W.; Teipel, U. Charakterisierung und Störstoffanalyse von rückgewonnenen Stoffströmen aus Wärmedämmverbundsystemen. Chem. Ing. Tech. 2021, 93, 771–780. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Bai, T.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Fernández-Pereira, C. Coal fly ash-slag-based geopolymers: Microstructure and metal leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef]
- Weil, M.; Buchwald, A.; Dombrowski-Daube, K. How to Assess the Environmental Sustainability of Geopolymers? A Live Cycle Perspective. Adv. Sci. Technol. 2010, 69, 186–191. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Gado, R.A.; Feltin, N.; Ducourtieux, S.; Devoille, L. Recycling and utilization assessment of waste fired clay bricks (Grog) with granulated blast-furnace slag for geopolymer production. Process Saf. Environ. Prot. 2016, 103, 237–251. [Google Scholar] [CrossRef]
- Mohajerani, A.; Suter, D.; Jeffrey-Bailey, T.; Song, T.; Arulrajah, A.; Horpibulsuk, S.; Law, D. Recycling waste materials in geopolymer concrete. Clean Technol. Environ. Policy 2019, 21, 493–515. [Google Scholar] [CrossRef]
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Černý, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Kugler, F.; Fehn, T.; Sandner, M.; Krcmar, W.; Teipel, U. Microstructural and mechanical properties of geopolymers based on brick scrap and fly ash. Int. J. Ceram. Eng. Sci. 2022, 4, 92–101. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Telle, R.; Salmang, H.; Scholze, H. (Eds.) Keramik: 7, Vollst. Neubearb. und Erw. Aufl; Springer: Berlin, Germany, 2007. [Google Scholar]
- Koenders, E.; Weise, K.; Vogt, O. Werkstoffe im Bauwesen; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2020. [Google Scholar]
- Bohra, V.K.J.; Nerella, R.; Madduru, S.R.C.; Rohith, P. Microstructural characterization of fly ash based geopolymer. Mater. Today Proc. 2020, 27, 1625–1629. [Google Scholar] [CrossRef]
- Ambroise, J.; Maximilien, S.; Pera, J. Properties of Metakaolin blended cements. Adv. Cem. Based Mater. 1994, 1, 161–168. [Google Scholar] [CrossRef]
- Reig, F. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Ascensão, G.; Beersaerts, G.; Marchi, M.; Segata, M.; Faleschini, F.; Pontikes, Y. Shrinkage and Mitigation Strategies to Improve the Dimensional Stability of CaO-FeOx-Al2O3-SiO2 Inorganic Polymers. Materials 2019, 12, 3679. [Google Scholar] [CrossRef] [Green Version]
- Nath, D.C.D.; Bandyopadhyay, S.; Gupta, S.; Yu, A.; Blackburn, D.; White, C. Surface-coated fly ash used as filler in biodegradable poly(vinyl alcohol) composite films: Part 1—The modification process. Appl. Surf. Sci. 2010, 256, 2759–2763. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative study of the reactivity of fly ash in geopolymerization by FTIR. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer—Chemistry and Applications; Institut Géopolymère: Saint-Quentin, France, 2020. [Google Scholar]
- Alvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Klinowski, J. Nuclear magnetic resonance studies of zeolites. Prog. Nucl. Magn. Reson. Spectrosc. 1984, 16, 237–309. [Google Scholar] [CrossRef]
- Motorwala, A.; Shah, V.; Kammula, R.; Nannapaneni, P.; Raijiwala, D.B. ALKALI Activated FLY-ASH Based Geopolymer Concrete. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 159–166. [Google Scholar]
- REES, C.A. Mechanisms and Kinetics of Gel Formation in Geopolymers; The University of Melbourne: Melbourne, VIC, Australia, 2007. [Google Scholar]
- Nayak, P.S.; Singh, B.K. Instrumental characterization of clay by XRF, XRD and FTIR. Bull. Mater. Sci. 2007, 30, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Ascensão, G.; Marchi, M.; Segata, M.; Faleschini, F.; Pontikes, Y. Reaction kinetics and structural analysis of alkali activated Fe–Si–Ca rich materials. J. Clean. Prod. 2020, 246, 119065. [Google Scholar] [CrossRef]
- Kränzlein, E.; Harmel, J.; Pöllmann, H.; Krcmar, W. Influence of the Si/Al ratio in geopolymers on the stability against acidic attack and the immobilization of Pb2+ and Zn2+. Constr. Build. Mater. 2019, 227, 116634. [Google Scholar] [CrossRef]
- Kränzlein, E.; Pöllmann, H.; Krcmar, W. Metal powders as foaming agents in fly ash based geopolymer synthesis and their impact on the structure depending on the Na/Al ratio. Cem. Concr. Compos. 2018, 90, 161–168. [Google Scholar] [CrossRef]
- Criado, M.; Vicent, M.; García-Ten, F.J. Reactivation of alkali-activated materials made up of fly ashes from a coal power plant. Clean. Mater. 2022, 3, 100043. [Google Scholar] [CrossRef]









| Oxide | Brick Sanding Dust | Brick Scrap | Mixed Rubble | Concrete Rubble | Fly Ash |
|---|---|---|---|---|---|
| LOI | 1.30 | 0.92 | 11.01 | 14.47 | 3.86 |
| SiO2 | 61.48 | 57.43 | 63.43 | 59.50 | 52.35 |
| Al2O3 | 17.90 | 18.57 | 5.31 | 3.12 | 25.19 |
| CaO | 5.39 | 8.61 | 13.38 | 17.85 | 4.17 |
| Fe2O3 | 5.98 | 5.91 | 2.34 | 1.33 | 6.22 |
| TiO2 | 083 | 0.86 | 0.24 | 0.11 | 1.15 |
| MgO | 2.75 | 3.08 | 1.48 | 1.42 | 1.88 |
| SO3 | 0.33 | 0.18 | 0.43 | 0.59 | 0.23 |
| K2O | 3.04 | 2.81 | 1.81 | 1.18 | 2.09 |
| Na2O | 0.90 | 0.77 | 0.26 | 0.20 | 1.09 |
| P2O5 | 0.14 | 0.16 | 0.10 | 0.06 | 1.15 |
| V2O5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| Cr2O3 | 0.02 | 0.02 | 0.01 | 0.01 | 0.03 |
| Mn3O4 | 0.10 | 0.10 | 0.06 | 0.04 | 0.07 |
| ZnO | 0.02 | 0.02 | 0.01 | 0.01 | 0.05 |
| SrO | 0.01 | 0.02 | 0.03 | 0.04 | 0.14 |
| ZrO2 | 0.03 | 0.03 | 0.02 | 0.01 | 0.05 |
| BaO | 0.16 | 0.14 | 0.06 | 0.05 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugler, F.; Aumüller, J.; Krcmar, W.; Teipel, U. Construction and Demolition Residuals as Raw Materials for the Production of Novel Geopolymer Building Materials. Crystals 2022, 12, 678. https://doi.org/10.3390/cryst12050678
Kugler F, Aumüller J, Krcmar W, Teipel U. Construction and Demolition Residuals as Raw Materials for the Production of Novel Geopolymer Building Materials. Crystals. 2022; 12(5):678. https://doi.org/10.3390/cryst12050678
Chicago/Turabian StyleKugler, Felix, Jessica Aumüller, Wolfgang Krcmar, and Ulrich Teipel. 2022. "Construction and Demolition Residuals as Raw Materials for the Production of Novel Geopolymer Building Materials" Crystals 12, no. 5: 678. https://doi.org/10.3390/cryst12050678





