Influence of Polymer Latexes on the Properties of High Performance Cement–Based Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportioning and Paste/Mortar Preparation
2.3. Methods
3. Results and Discussion
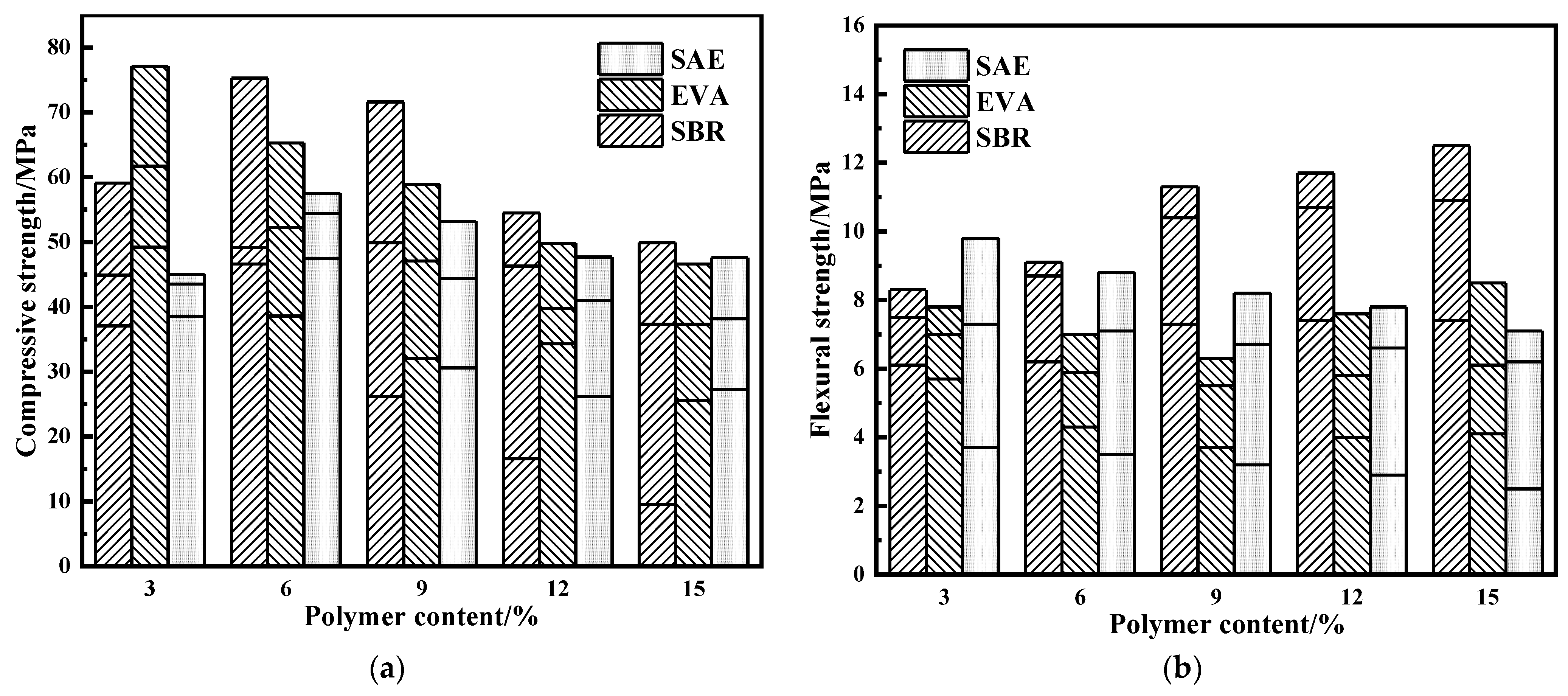
3.1. Mechanical Properties
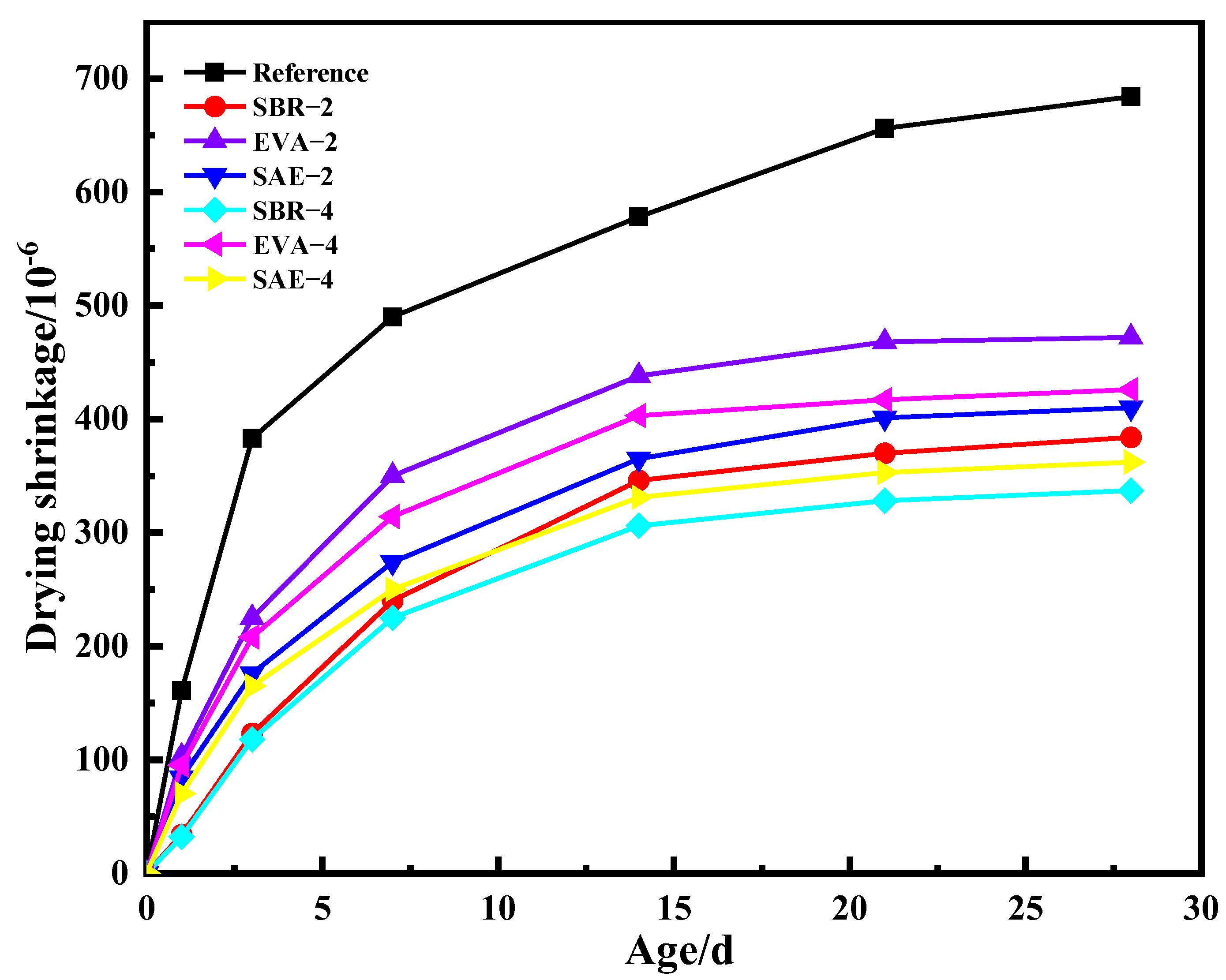
3.2. Shrinkage Properties
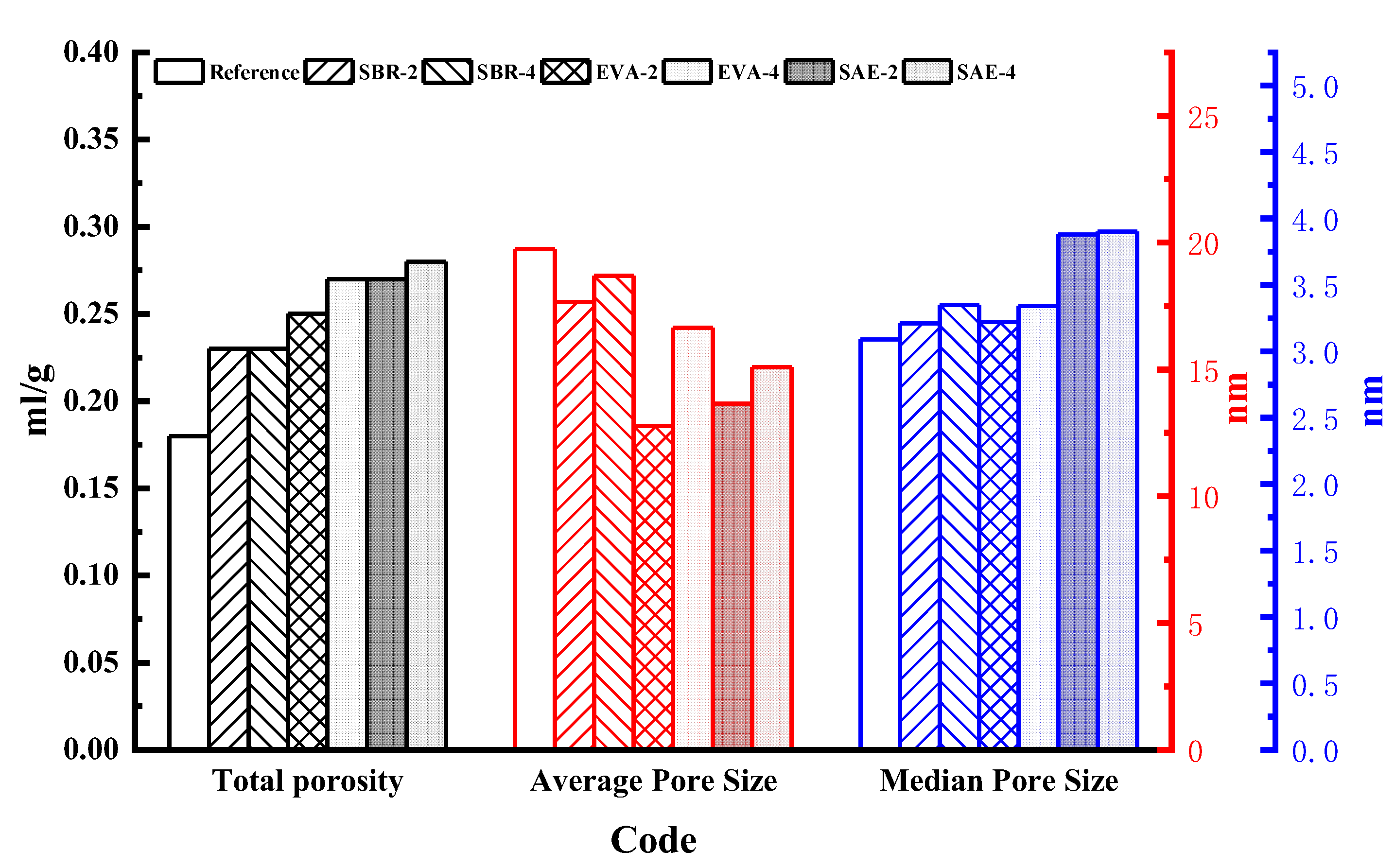
3.3. Pore Structure Analysis
3.4. X-ray Diffraction Analysis
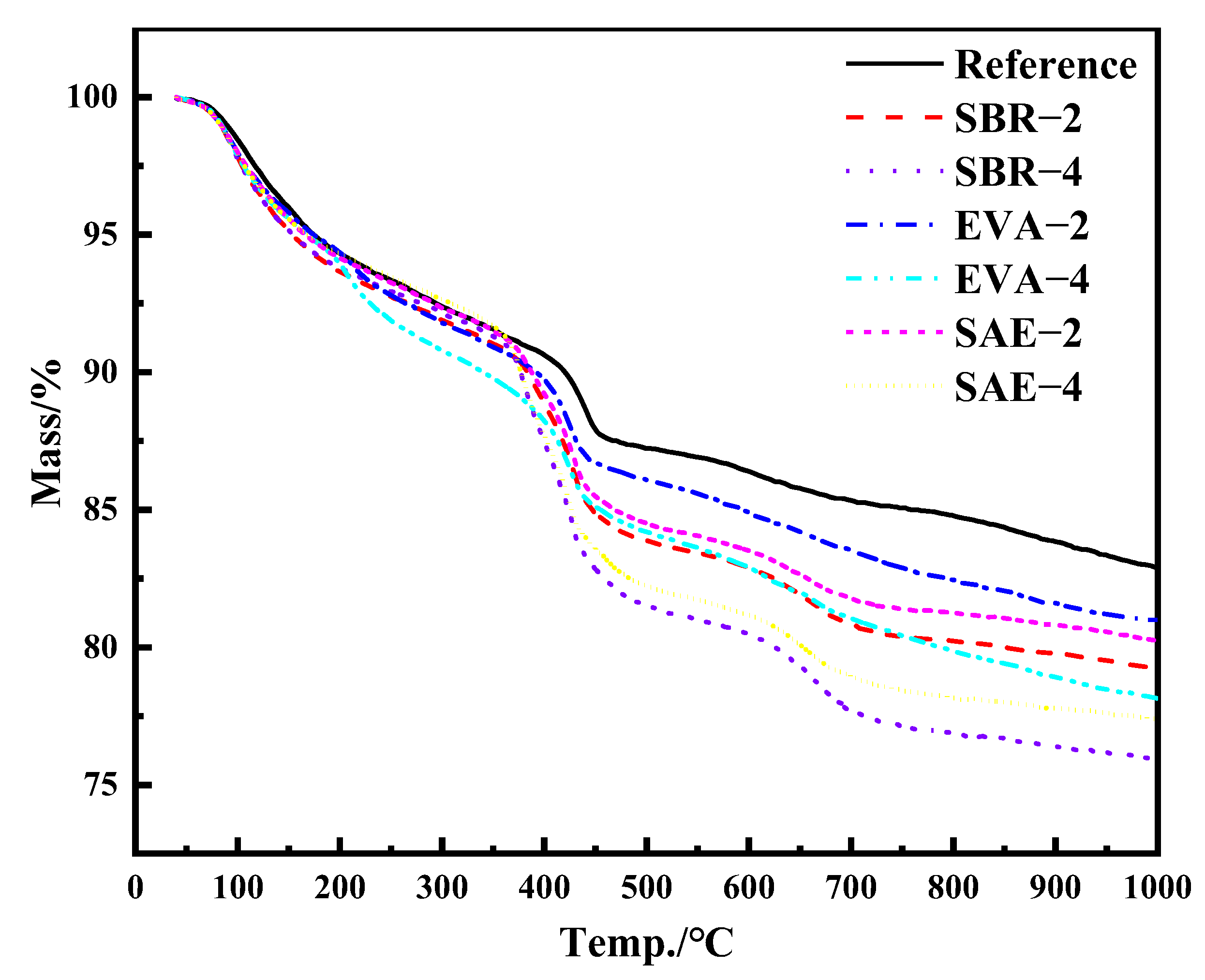
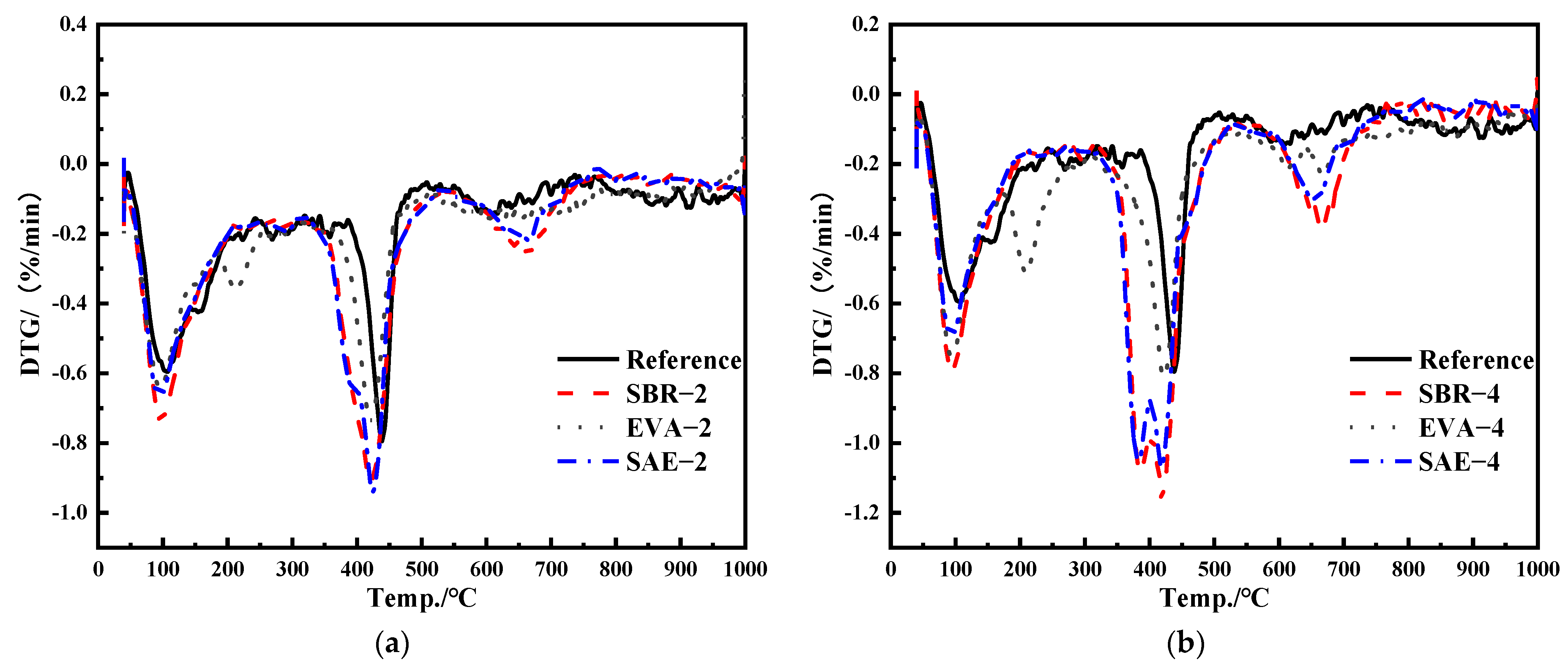
3.5. Simultaneous Thermal Analysis
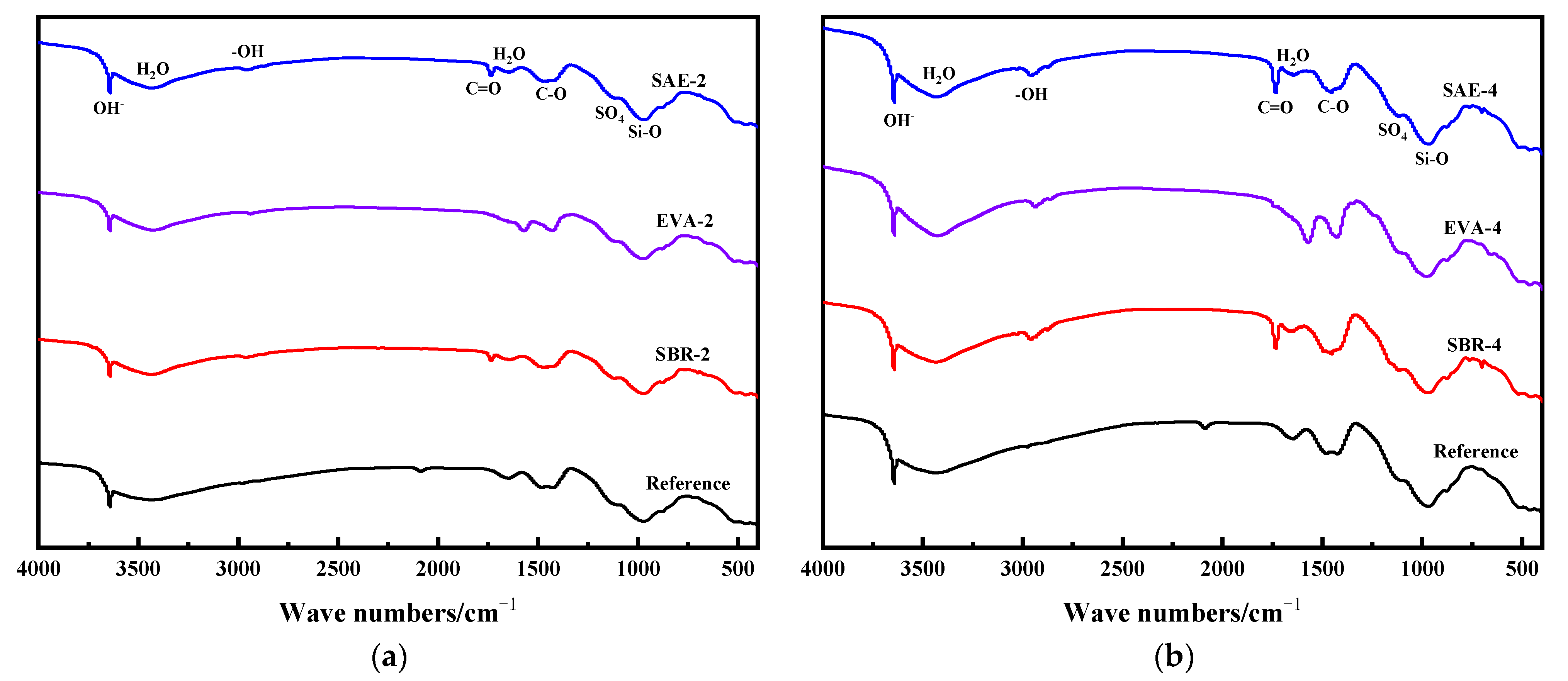
3.6. FTIR Analysis
4. Conclusions
- (1)
- The incorporation of a polymer latex induces a stronger diffraction peak of portlandite. With the increase of the relative content of the polymer latex, the weight loss of the modified samples increased; the weight loss of SBR–4 was 6.98% higher than that of the reference group. Polymer latexes retard conversion from ettringite to monosulfate, accompanied by the decline of the chemically bound water and promotion in portlandite. The relatively stronger stretching–vibration peak of SO4 in ettringite proves the stability of ettringite. In addition, polymer latex also changes the hydrogen bond strength between water molecules, resulting in the change of vibration frequency of water molecules.
- (2)
- Polymer latexes induce higher porosity with the average pore size reduced. The total porosity of the reference group was 0.18 mL/g. The total porosity of SBR–4 was 0.23 mL/g, whereas the total porosity of EVA–4 was 0.27 mL/g, and the total porosity of SAE–4 increased to 0.28 mL/g. The significance of polymer latexes on porosity exhibits the decreasing order of styrene–butadiene latex, ethylene vinyl acetate copolymer, and silicone acrylate latex. Polymer latexes remarkably hinder drying shrinkage development owing to its water retention effect.
- (3)
- Polymer latexes have no significant effect on the development of compressive strength. Although ethylene vinyl acetate copolymer and silicone acrylate latexes reduce the tensile strength, the tensile strength of the styrene–butadiene latex–modified samples is significantly improved. Styrene–butadiene latex increases the flexural strength at 28 days by 50% compared with reference group of 9.0 MPa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Description | Units |
| C | Quality of P.I 42.5 Portland cement | g |
| CH | Content of Ca(OH)2 in hydration products | % |
| DTG | Differential thermal gravity | |
| EVA | Ethylene–vinyl acetate copolymer latex | |
| FTIR | Fourier transform infrared reflection | |
| H | Content of bound water in hydration products | % |
| Lcem | The loss on ignition of cement | % |
| Lpol | The loss on ignition of polymer latex | % |
| m | Initial mass of sample | mg |
| Δm | The thermogravimetric loss in the calculation temperature range | mg |
| m40°C | Mass of sample at 40 °C | mg |
| m1000°C | Mass of sample at 1000 °C | mg |
| MCH | The molar mass of Ca(OH)2 | g/mol |
| Mloss | The molar mass of water | g/mol |
| MFT | Minimum film forming temperature of polymer latex | °C |
| P | Quality of polymer latex | g |
| pH | The expression of acidity and alkalinity of aqueous solution | |
| PS | Particle size of polymer latex | μm |
| SAE | Silicone–acrylic latex | |
| SBR | Styrene–butadiene latex | |
| SC | Mass percentage of the remaining part of the polymer latex in the total amount after drying under the specified conditions | % |
| Sp | Quality of Superplasticizer | g |
| St | The physical quantity of volume or length reduction degree caused by drying and water loss of the sample | % |
| TG | Thermal Gravity Analysis | |
| V | Resistance of polymer latex to flow | map·s |
| W | Quality of Water | g |
References
- Satish, C.; Yoshihiko, O. Polymers in Concrete; CRC Press: Boca Raton, FL, USA, 2020; Volume 8, p. 12. [Google Scholar]
- Ohama, Y. Recent progress in concrete–polymer composites. Adv. Cem. Based Mater. 1997, 5, 31–40. [Google Scholar] [CrossRef]
- Renuka, S.M.; Suraj, R.; Siva, A.; Mugahed, A.; Radhamanohar, A.; Nikolai, V.; Roman, F. The Effect of Superabsorbent Polymer and Nano–Silica on the Properties of Blended Cement. Crystals 2021, 11, 1394. [Google Scholar]
- He, Y.H.; Mo, L.W.; Mao, Z.Y.; Huang, F.F.; Han, Z.H. Influence of Polyvinyl Alcohol Powder on the Mechanical Performance and Volume Stability of Sulfoaluminate–Portland Cement Composite. Crystals 2021, 11, 692. [Google Scholar] [CrossRef]
- Guo, S.Y.; Zhang, X.; Chen, J.Z.; Mou, B.; Ren, J. Mechanical and interface bonding properties of epoxy resin reinforced Portland cement repairing mortar. Constr. Build. Mater. 2020, 264, 120715. [Google Scholar] [CrossRef]
- Bahranifard, Z.; Tabrizi, F.F.; Vosoughi, A.R. An investigation on the effect of styrene–butyl acrylate copolymer latex to improve the properties of polymer modified concrete. Constr. Build. Mater. 2019, 205, 175–185. [Google Scholar] [CrossRef]
- Bessaies, B.H.; Baumann, R.; Schmitz, M.; Radler, M.; Roussel, N. Effect of polyacrylamide on rheology of fresh cement pastes. Cem. Concr. Res. 2015, 76, 98–106. [Google Scholar] [CrossRef]
- Zhang, X.J.; Du, M.R.; Fang, H.Y.; Shi, M.S.; Zhang, C.; Wang, F.M. Polymer–modified cement mortars: Their enhanced properties, applications, prospects, and challenges. Constr. Build. Mater. 2021, 299, 124290. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, J.; Dong, Q.; Zhao, X. Meso–scale cracking behavior of Cement Treated Base material. Constr. Build. Mater. 2020, 239, 117823. [Google Scholar] [CrossRef]
- Maruyama, I.; Teramoto, A. Temperature dependence of autogenous shrinkage of silica fume cement pastes with a very low water–binder ratio. Cem. Concr. Res. 2013, 50, 41–50. [Google Scholar] [CrossRef]
- Tsigarida, A.; Tsampali, E.; Konstantinidis, A.A.; Stefanidou, M. On the Use of Confocal Microscopy for Calculating the Surface Microroughness and the Respective Hydrophobic Properties of Marble Specimens. J. Build. Eng. 2021, 33, 101876. [Google Scholar] [CrossRef]
- Macek, W. Fracture Surface Formation of Notched 2017A–T4 Aluminium Alloy under Bending Fatigue. Int. J. Fract. 2021, 934, 1–17. [Google Scholar] [CrossRef]
- Rottstegge, J.; Arnold, M.; Herschke, L.; Glasser, G. Solid state NMR and LVSEM studies on the hardening of latex modified tile mortar systems. Cem. Concr. Res. 2005, 35, 2233–2243. [Google Scholar] [CrossRef]
- Shi, C.; Zou, X.W.; Yang, L.; Wang, P.; Niu, M.D. Influence of humidity on the mechanical properties of polymer–modified cement–based repair materials. Constr. Build. Mater. 2020, 261, 119928. [Google Scholar] [CrossRef]
- Knapen, E.; Gemert, D.V. Cement hydration and microstructure formation in the presence of water–soluble polymers. Cem. Concr. Res. 2009, 39, 6–13. [Google Scholar] [CrossRef]
- Kong, X.M.; Emmerling, S.; Pakusch, J.; Rueckel, M.; Nieberle, J. Retardation effect of styrene–acrylate copolymer latexes on cement hydration. Cem. Concr. Res. 2015, 75, 23–41. [Google Scholar] [CrossRef]
- Ohama, Y.; Kan, S. Effects of specimen size on strength and drying shrinkage of polymer–modified concrete. Int. J. Cem. Compos. Lightweight Concr. 1982, 4, 229–233. [Google Scholar] [CrossRef]
- Sowoidnich, T.; Rachowski, T.; RößLER, C.; Völkel, A.; Ludwig, H.M. Calcium complexation and cluster formation as principal modes of action of polymers used as superplasticizer in cement systems. Cem. Concr. Res. 2015, 73, 42–50. [Google Scholar] [CrossRef]
- Lukowski, P.; Dębska, D. Effect of Polymer Addition on Performance of Portland Cement Mortar Exposed to Sulphate Attack. Materials 2020, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Ramli, M.; Akhavan, T.A. Effects of polymer modification on the permeability of cement mortars under different curing conditions: A correlational study that includes pore distributions, water absorption and compressive strength. Constr. Build. Mater. 2012, 28, 561–570. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, X.Y.; Jin, N.G.; Zhao, R.; Li, Z.J.; Ma, H.Y. Research on the microstructure formation of polyacrylate latex modified mortars. Constr. Build. Mater. 2013, 47, 1381–1394. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Lu, Z.Y.; Hu, X.S.; Chen, B.M.; Li, Z.J.; Liang, R.; Sun, G.X. A mechanical strong polymer–cement composite fabricated by in situ polymerization within the cement matrix. J. Build. Eng. 2021, 42, 103048. [Google Scholar] [CrossRef]
- Silva, D.A.; John, V.M.; Ribeiro, J.L.D.; Roman, H.R. Pore size distribution of hydrated cement pastes modified with polymers. Cem. Concr. Res. 2001, 31, 1177–1184. [Google Scholar] [CrossRef]
- Zhang, X.W.; Lu, C.X.; Shen, J.Y. Influence of tartaric acid on early hydration and mortar performance of Portland cement–calcium aluminate cement–anhydrite binder. Constr. Build. Mater. 2016, 112, 877–884. [Google Scholar]
- Nguyen, D.D.; Devlin, L.P.; Koshy, P.; Sorrell, C.C. Effects of acetic acid on early hydration of Portland cement. J. Therm. Anal. Calorim. 2016, 123, 489–499. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.C.; Lu, Z.B.; Zhang, Q.; Dong, B.Q.; Xing, F. Influence of triethanolamine on the hydration product of portlandite in cement paste and the mechanism. Cem. Concr. Res. 2016, 87, 64–76. [Google Scholar]
- Huang, Y.B.; Wang, R.Z.; Zhou, J.J.; Yu, F. Effects of eva emulsion addition on magnesium phosphate cement performances. J. Funct. Mater. 2014, 45, 11071–11080. [Google Scholar]
- Singh, N.B.; Sarvahi, R.; Singh, N.P.; Shukla, A.K. Effect of temperature on the hydration of ordinary Portland cement in the presence of a superplasticizer. Thermochim. Acta 1994, 247, 381–388. [Google Scholar] [CrossRef]
- Morozov, Y.; Castela, A.S.; Dias, A.P.S.; Montemor, M.F. Chloride–induced corrosion behavior of reinforcing steel in spent fluid cracking catalyst modified mortars. Cem. Concr. Res. 2013, 47, 1–7. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- JIE, Z.; George, W.S. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar]
- Lu, Z.C.; Kong, X.M.; Jansen, D.; Zhang, C.Y.; Wang, J.; Pang, X.F.; Yin, J.H. Towards a further understanding of cement hydration in the presence of triethanolamine. Cem. Concr. Res. 2020, 132, 106041. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Na2O | f–CaO | SO3 |
|---|---|---|---|---|---|---|---|
| 20.95 | 5.19 | 64.29 | 1.85 | 3.83 | 0.52 | 0.96 | 0.95 |
| Types | pH | PS (μm) | MFT * (°C) | SC (%) | V (map·s) |
|---|---|---|---|---|---|
| SBR | 7.0–9.0 | 0.112 | 15 | 48 | 50–150 |
| EVA | 9.6 | 2 | 0 | 54.5 | 2206 |
| SAE | 7.0–8.0 | 2 | 24 | 48 | 1000–3000 |
| Sample ID | P/C | W/C | Sp/C |
|---|---|---|---|
| Reference | 0% | 0.22 | 1.7% |
| SBR1–5 | 3%, 6%, 9%, 12%, 15% | 0.22 | 1.7% |
| EVA1–5 | 3%, 6%, 9%, 12%, 15% | 0.22 | 1.7% |
| SAE1–5 | 3%, 6%, 9%, 12%, 15% | 0.22 | 1.7% |
| Title | Reference | SBR–2 | SBR–4 | EVA–2 | EVA–4 | SAE–2 | SAE–4 |
|---|---|---|---|---|---|---|---|
| H2O | 20.7% | 20.35% | 19.2% | 17.8% | 17.9% | 19.4% | 20.1% |
| Ca(OH)2 | 14.0% | 18.7% | 20.8% | 13.8% | 14.3% | 17.8% | 19.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; Li, X.; Gao, X.; Fan, X.; Zhao, R.; Yang, T. Influence of Polymer Latexes on the Properties of High Performance Cement–Based Materials. Crystals 2022, 12, 789. https://doi.org/10.3390/cryst12060789
Cheng D, Li X, Gao X, Fan X, Zhao R, Yang T. Influence of Polymer Latexes on the Properties of High Performance Cement–Based Materials. Crystals. 2022; 12(6):789. https://doi.org/10.3390/cryst12060789
Chicago/Turabian StyleCheng, Daxiang, Xiaosheng Li, Xu Gao, Xiaochun Fan, Rui Zhao, and Tingli Yang. 2022. "Influence of Polymer Latexes on the Properties of High Performance Cement–Based Materials" Crystals 12, no. 6: 789. https://doi.org/10.3390/cryst12060789




