Facile Glycothermal Synthesis of KxNa(1−x)NbO3 Particles
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Glycothermal Synthesis
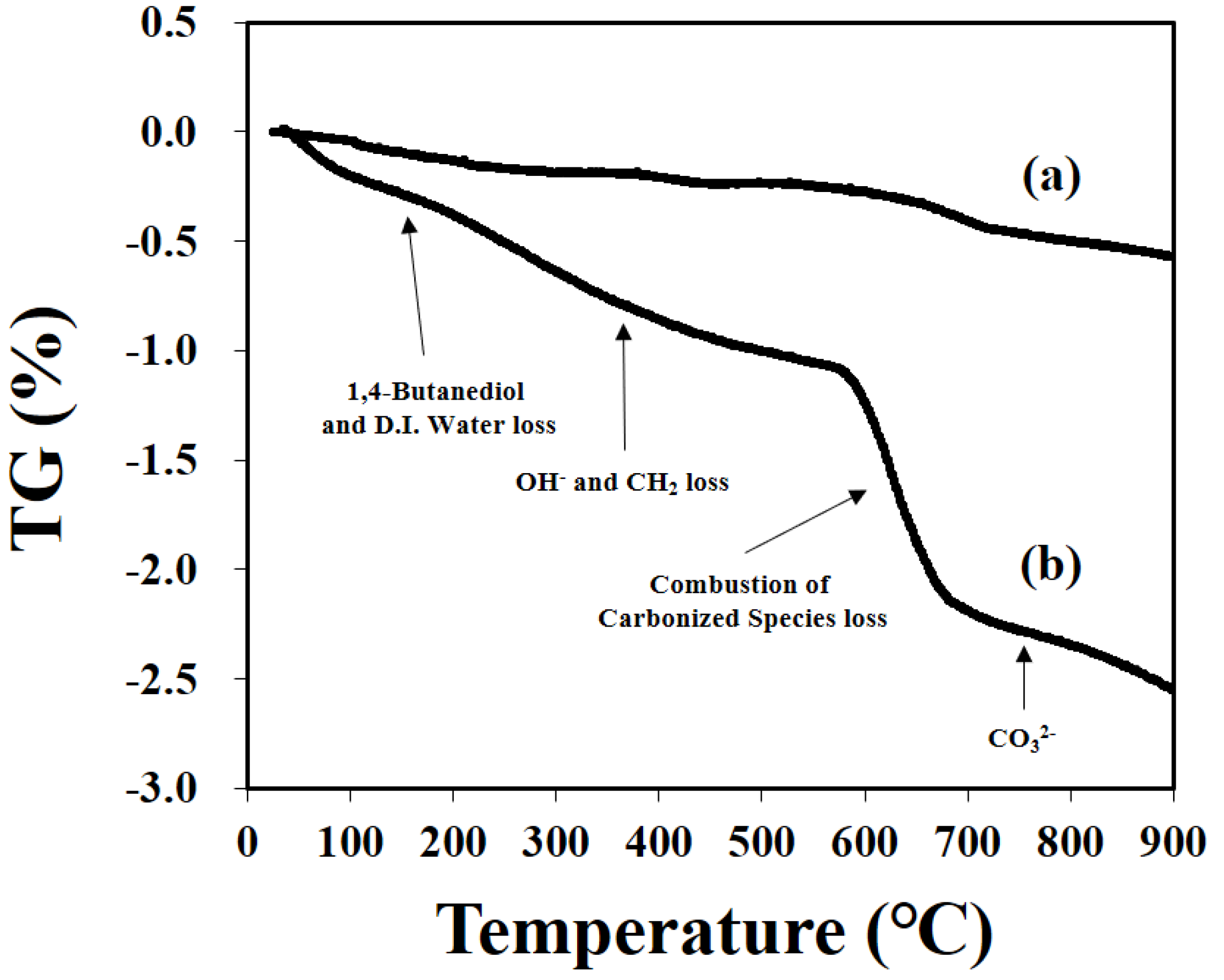
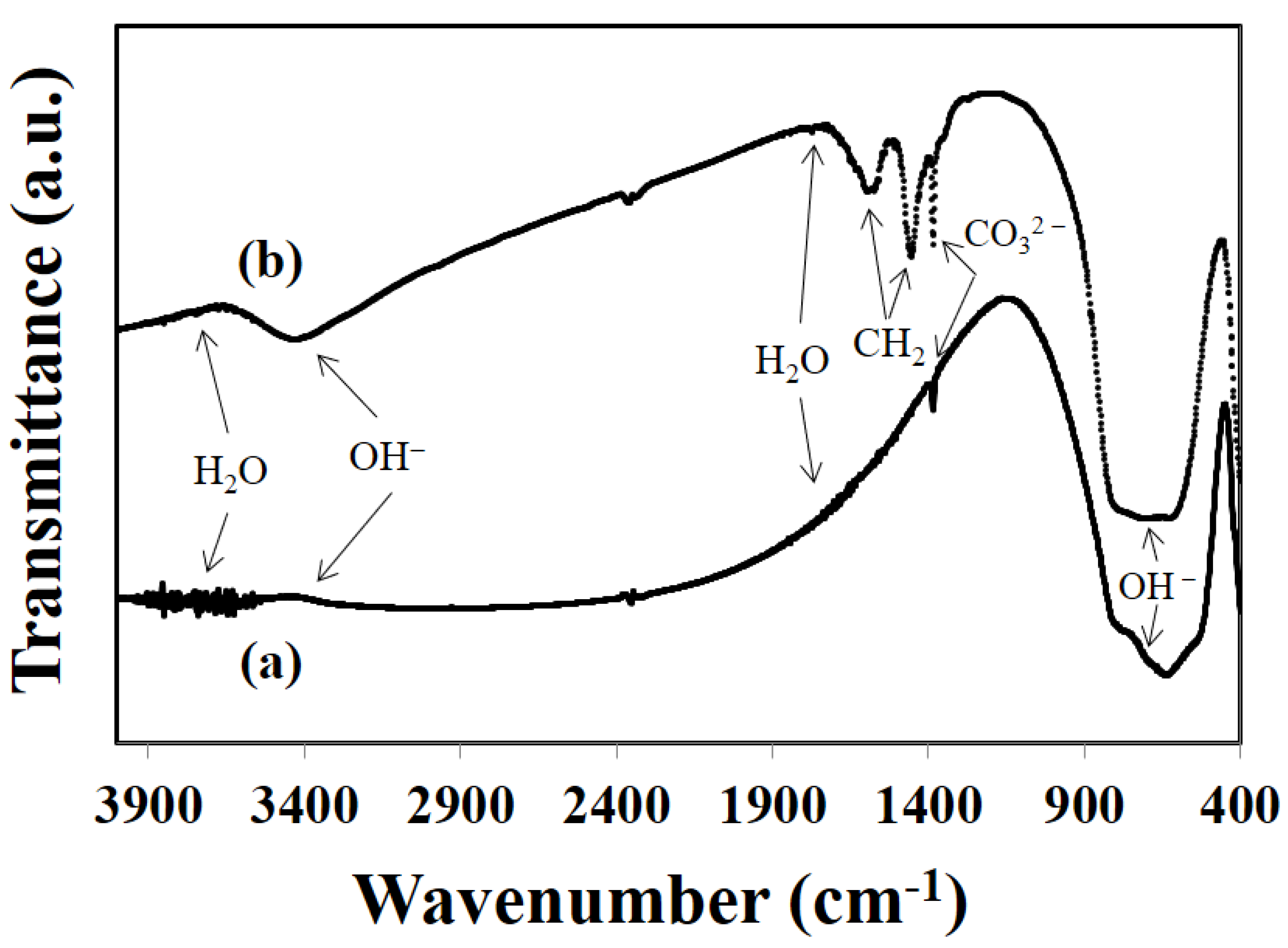
2.2. Characterization
3. Results and Discussion
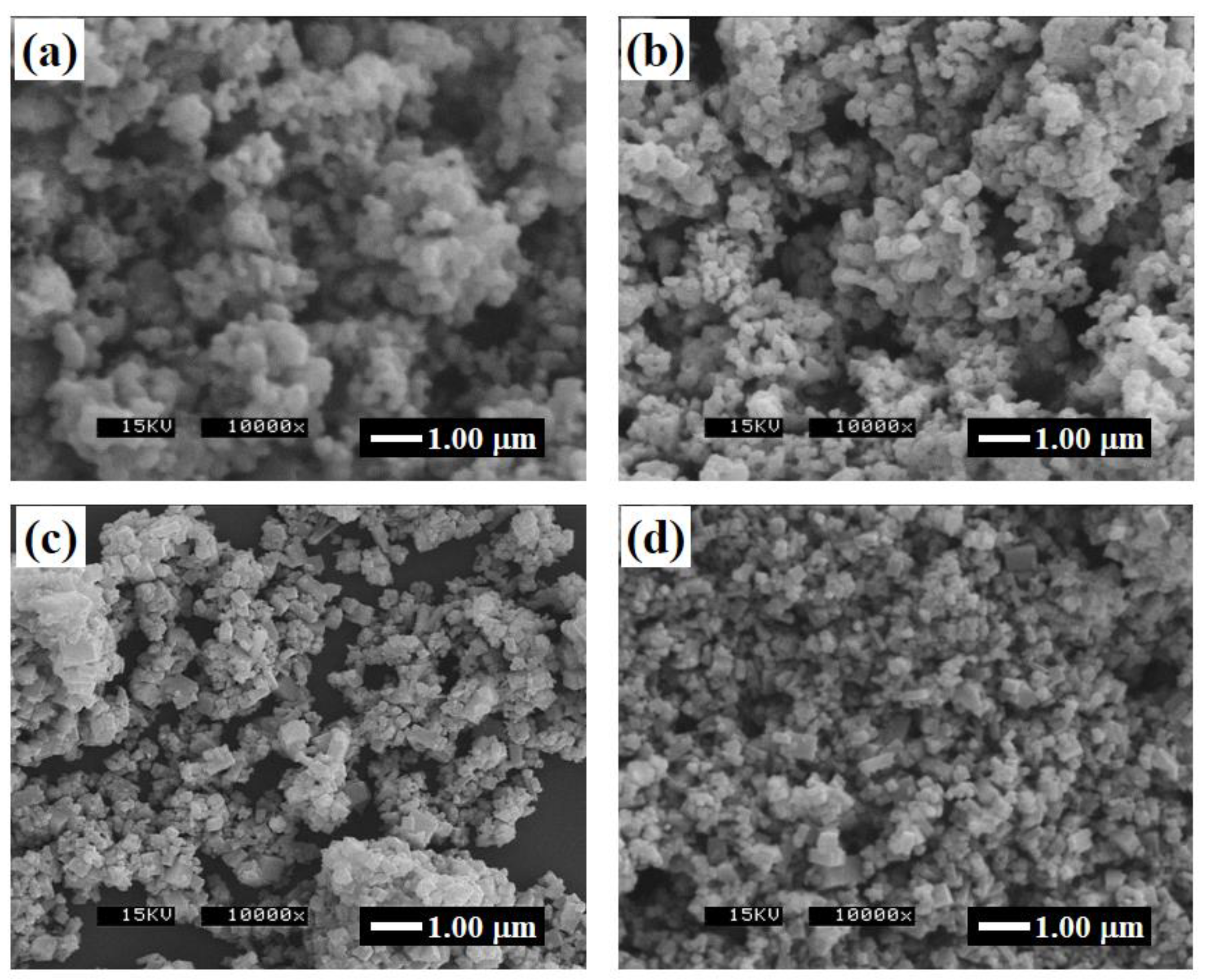
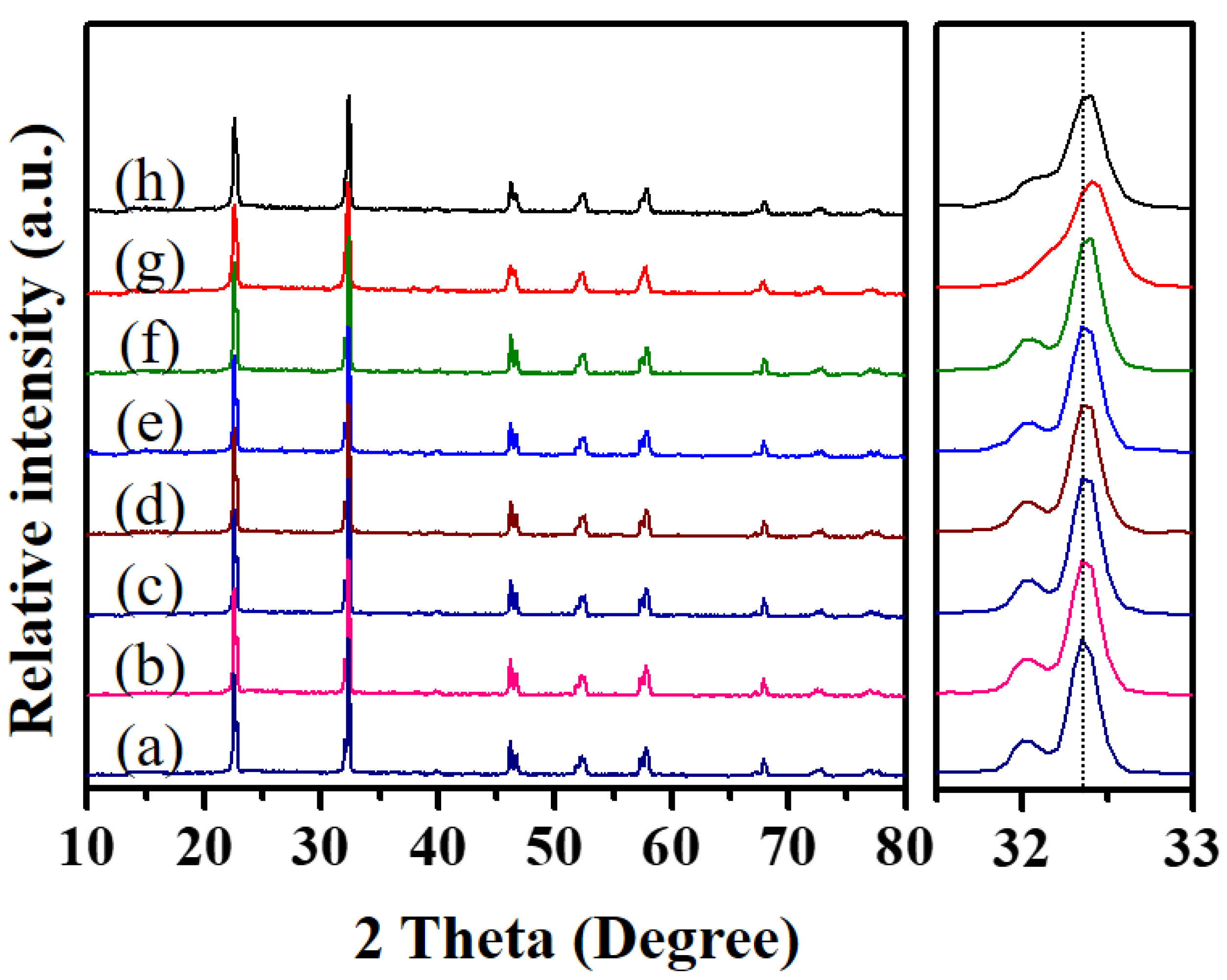
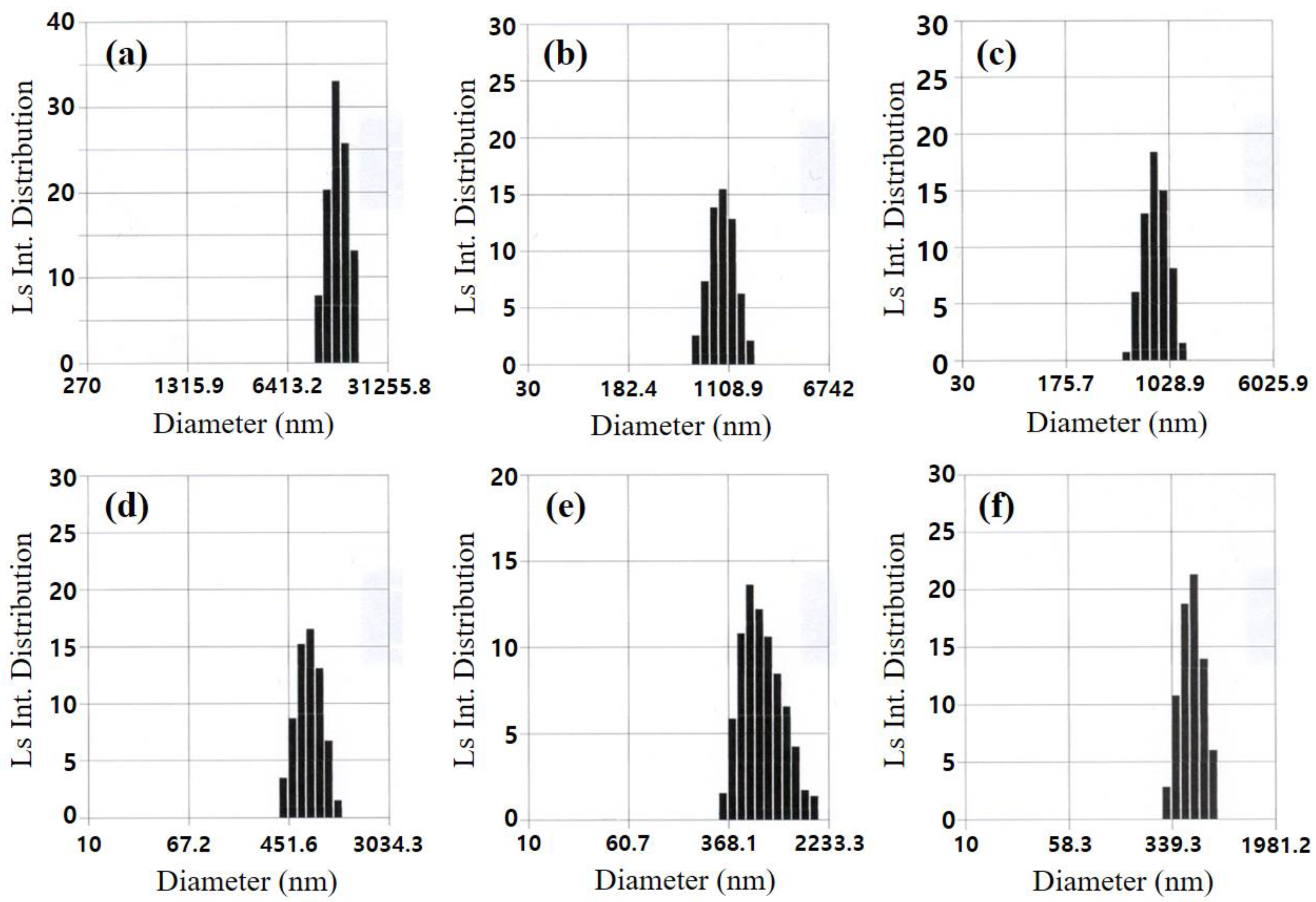
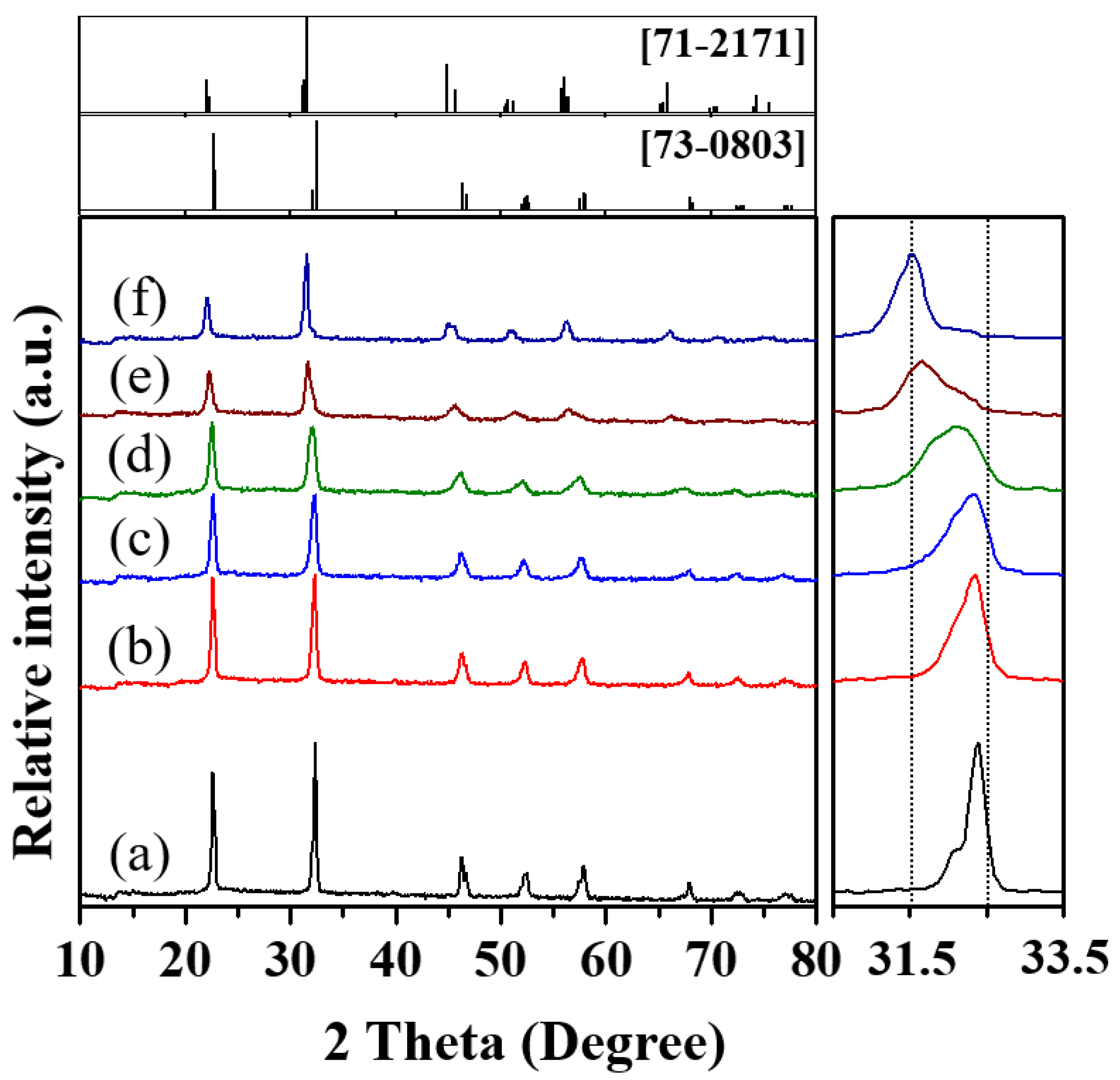
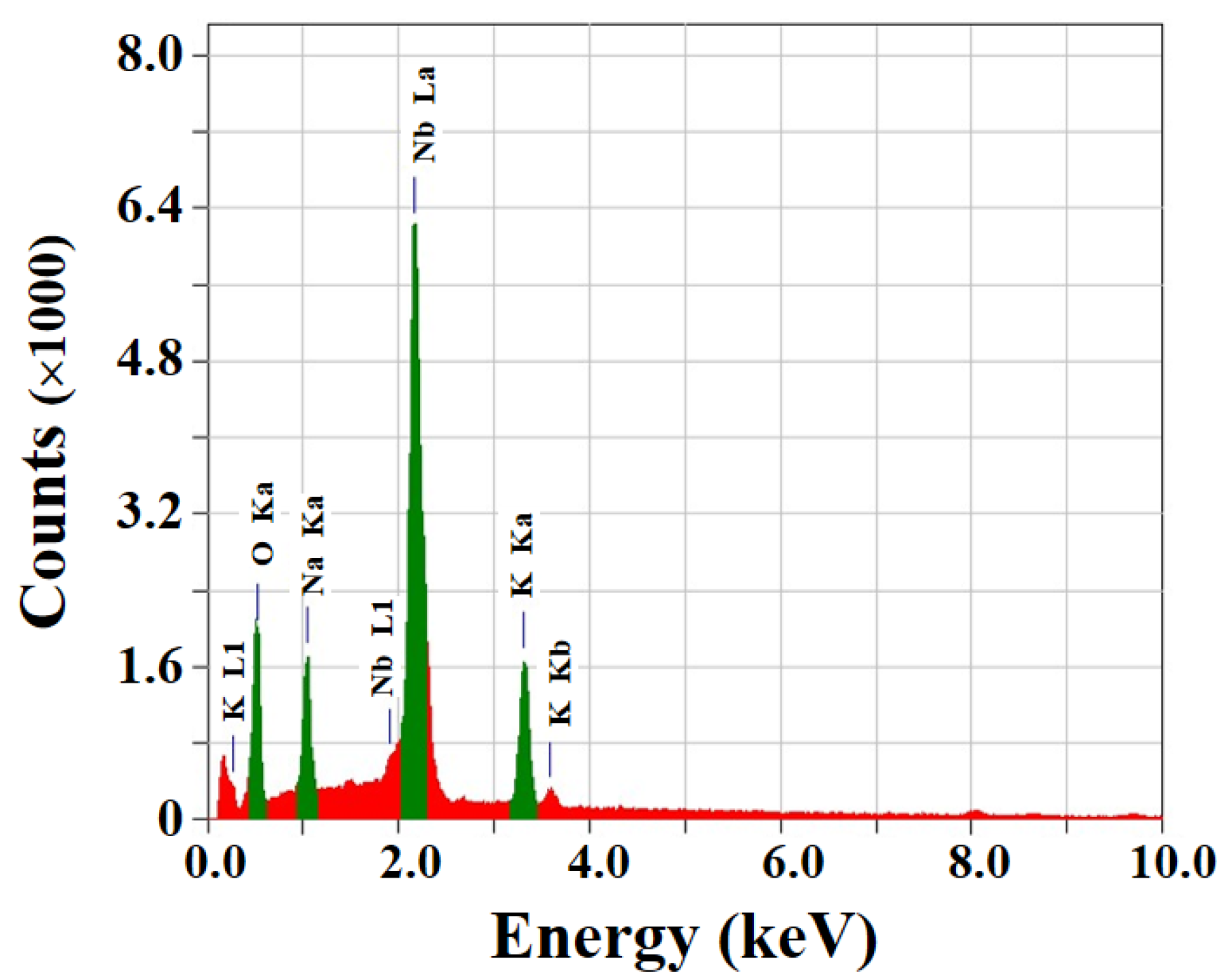
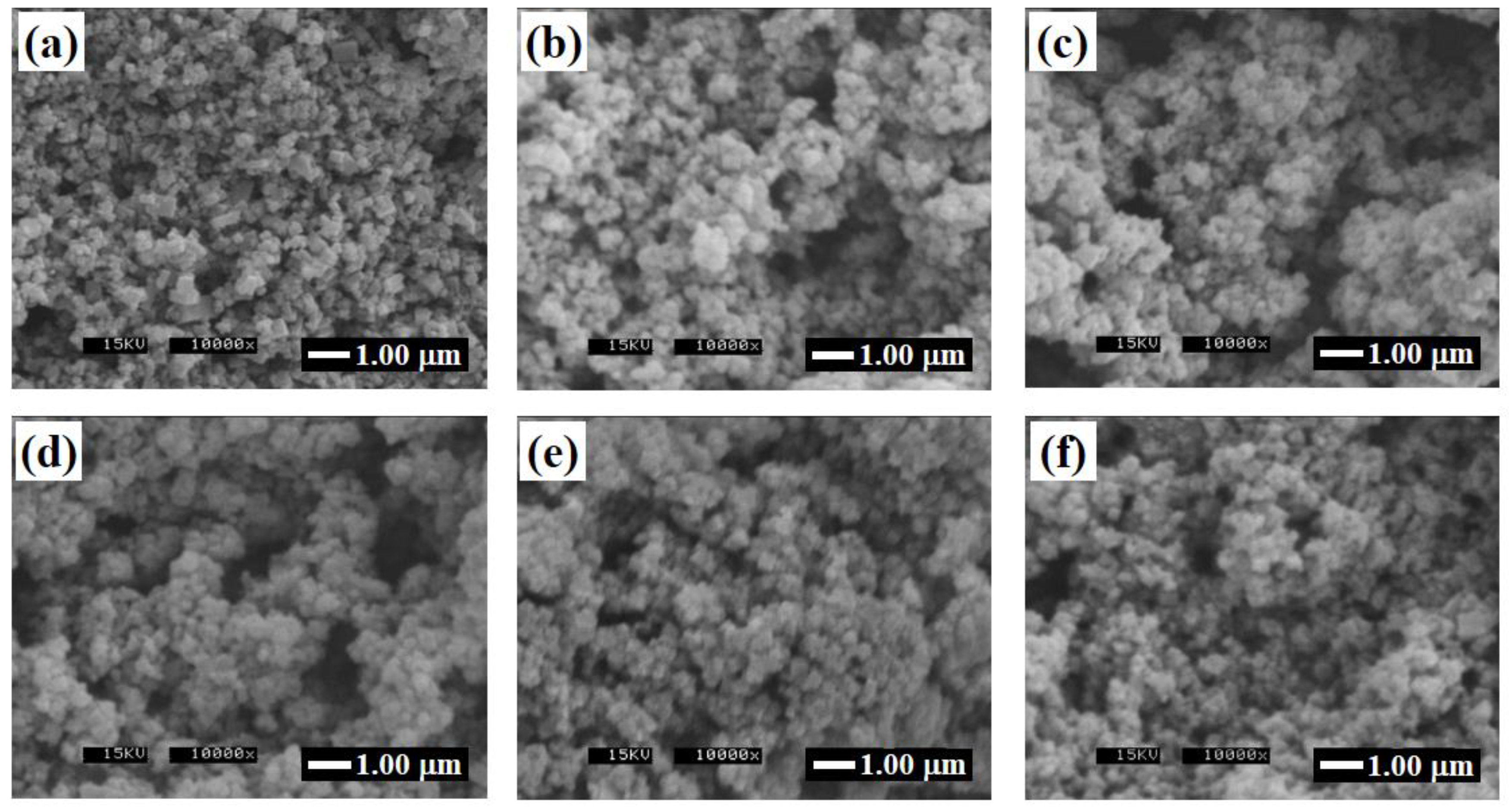
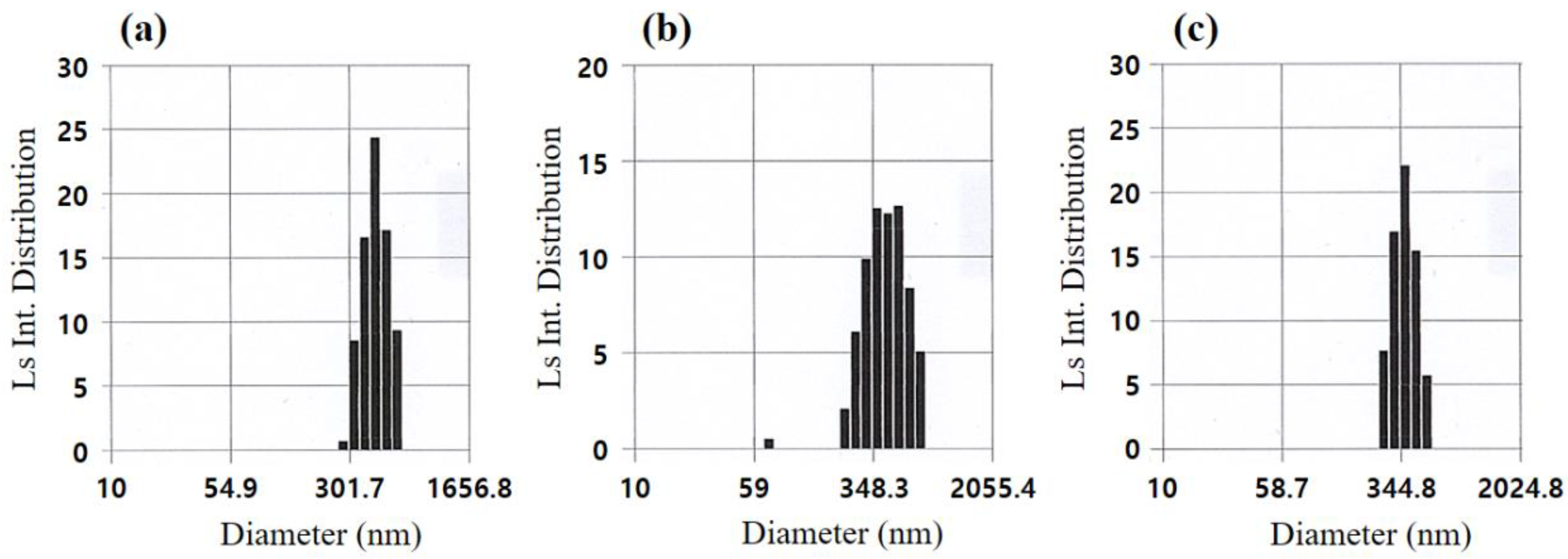
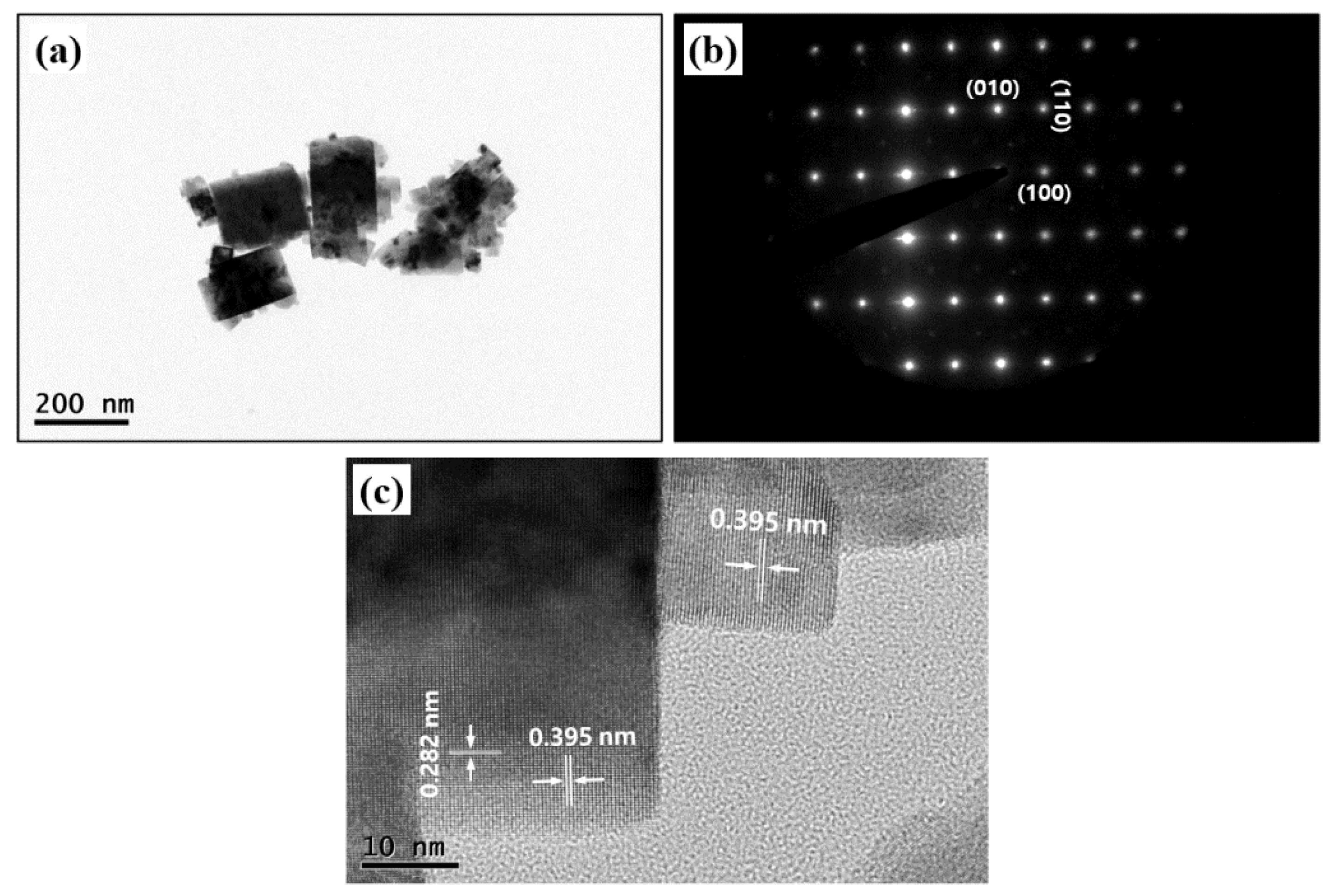
3.1. Glycothermal Synthesis of KNN Particles
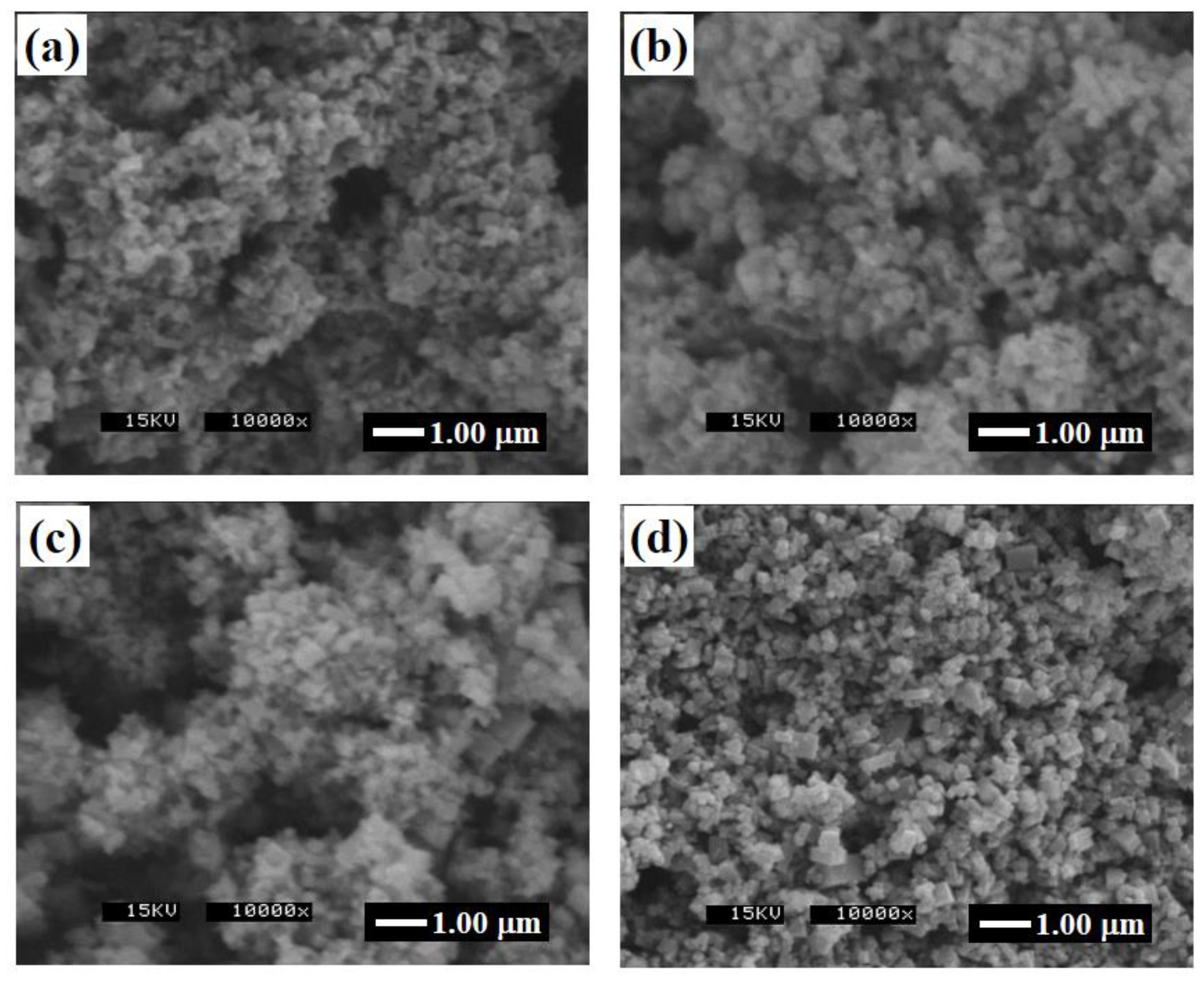
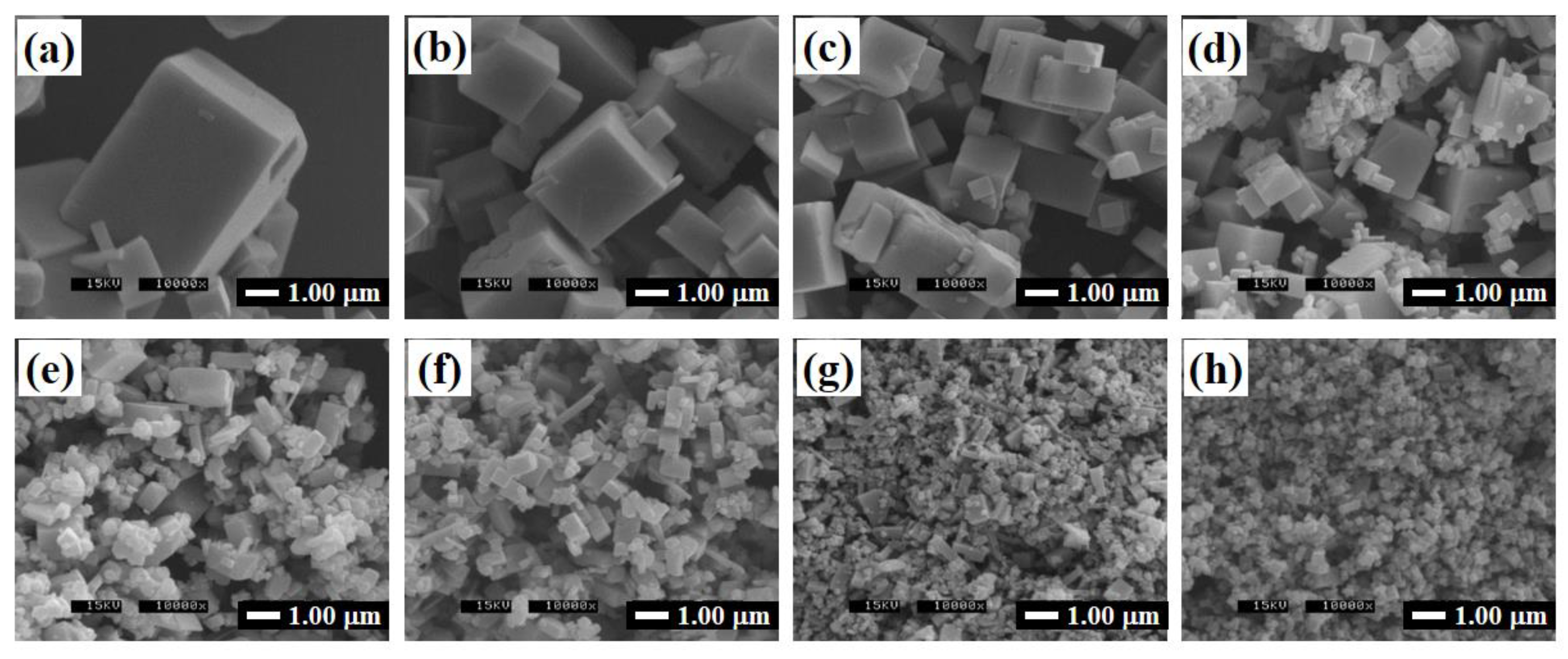
3.2. Glycothermal Synthesis of K0.5Na0.5NbO3 Particles at the MPB Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics; Academic Press: New York, NY, USA, 1971; pp. 271–280. [Google Scholar]
- Lu, Y.; Jeong, D.Y.; Cheng, Z.Y.; Zhang, Q.M.; Luo, H.S.; Yin, Z.W.; Viehland, D. Phase Transitional Behavior and Piezoelectric Properties of the Orthorhombic Phase of Pb(Mg1/3Nb2/3)O3–PbTiO3 Single Crystals. Appl. Phys. Lett. 2001, 78, 3109–3111. [Google Scholar] [CrossRef]
- Ren, W.; Liu, S.F.; Mukherjee, B.K. Piezoelectric Properties and Phase Transitions of <001>-Oriented Pb(Zn1/3Nb2/3)O3–PbTiO3 Single Crystals. Appl. Phys. Lett. 2002, 80, 3174–3176. [Google Scholar] [CrossRef]
- Park, S.E.; Shrout, T.R. Ultrahigh Strain and Piezoelectric Behavior in Relaxor Based Ferroelectric Single Crystals. J. Appl. Phys. 1997, 82, 1804–1811. [Google Scholar] [CrossRef]
- Guo, R.; Cross, L.E.; Park, S.E.; Noheda, B.; Cox, D.E.; Shirane, G. Origin of the High Piezoelectric Response in PbZr1−xTixO3. Phys. Rev. Lett. 2000, 84, 5423–5426. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, J.; Xiao, D.; Zhu, J. Recent Development in Lead-Free Perovskite Piezoelectric Bulk Materials. Progress Mater. Sci. 2018, 98, 552–624. [Google Scholar] [CrossRef]
- Panda, P.K. Review: Environmental friendly lead-free piezoelectric materials. J. Mater. Sci. 2009, 44, 5049–5062. [Google Scholar] [CrossRef]
- Kosec, M.; Malič, B.; Benčan, A.; Rojac, T. KNN-Based Piezoelectric Ceramics. In Piezoelectric and Acoustic Materials for Transducer Applications; Safari, A., Akdoğan, E.K., Eds.; Springer: New York, NY, USA, 2008; pp. 81–102. [Google Scholar]
- Zuo, R.; Rödel, J.; Chen, R.; Li, L. Sintering and Electrical Properties of Lead-Free Na0.5K0.5NbO3 Piezoelectric Ceramics. J. Am. Ceram. Soc. 2006, 89, 2010–2015. [Google Scholar] [CrossRef]
- Lv, J.H.; Zhang, M.; Guo, M.; Li, W.C.; Wang, X.D. Hydrothermal Synthesis and Characterization of KxNa(1−x)NbO3 Powders. Int. J. Appl. Ceram. Technol. 2007, 4, 571–577. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Zhu, J. Potassium–Sodium Niobate Lead-Free Piezoelectric Materials: Past, Present, and Future of Phase Boundaries. Chem. Rev. 2015, 115, 2559–2595. [Google Scholar] [CrossRef]
- Lin, D.; Kwok, K.W.; Chan, H.L.W. Phase transition and electrical properties of (K0.5Na0.5)(Nb1−xTax)O3 lead-free piezoelectric ceramics. Appl. Phys. A-Mater. Sci. Process. 2008, 91, 167–171. [Google Scholar] [CrossRef]
- Yang, S.L.; Tsai, C.C.; Hong, C.S.; Chu, S.Y. Effects of sintering aid CuTa2O6 on piezoelectric and dielectric properties of sodium potassium niobate ceramics. Mater. Res. Bull. 2012, 47, 998–1003. [Google Scholar] [CrossRef]
- Kupec, A.; Malic, B.; Tellier, J.; Tchernychova, E.; Glinsek, S.; Kosec, M. Lead-Free Ferroelectric Potassium Sodium Niobate Thin Films from Solution: Composition and Structure. J. Am. Ceram. Soc. 2012, 95, 515–523. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, K.; Zhu, F.Y.; Cheng, L.Q.; Yao, F.Z. (K, Na)NbO3-Based Lead-Free Piezoceramics: Fundamental Aspects, Processing Technologies, and Remaining Challenges. J. Am. Ceram. Soc. 2013, 96, 3677–3696. [Google Scholar] [CrossRef]
- López-Juárez, R.; González, F.; Villafuerte-Castrejón, M.E. Lead-free ferroelectric ceramics with perovskite structure. Ferroelectr. Mater Aspects. 2011, 5, 305–330. [Google Scholar]
- Gu, H.; Zhu, K.; Pang, X.; Shao, B.; Qiu, J.; Ji, H. Synthesis of (K, Na) (Nb, Ta)O3 lead-free piezoelectric ceramic powders by high temperature mixing method under hydrothermal conditions. Ceram. Int. 2012, 38, 1807–1813. [Google Scholar] [CrossRef]
- Park, S.H.; Ahn, C.W.; Nahm, S.; Song, J.S. Microstructure and Piezoelectric Properties of ZnO-added (Na0.5K0.5)NbO3 Ceramics. Jpn. J. Appl. Phys. 2004, 43, L1072–L1074. [Google Scholar] [CrossRef]
- Lim, J.B.; Zhang, S.; Jeon, J.-H.; Shrout, T.R. (K,Na)NbO3-based ceramics for piezoelectric hard lead-free material. J. Am. Ceram. Soc. 2010, 93, 1218–1220. [Google Scholar] [CrossRef]
- Zeng, J.T.; Kwok, K.W.; Chan, H.L.W. KxNa1−xNbO3 powder synthesized by molten-salt process. Mater. Lett. 2007, 61, 409–411. [Google Scholar] [CrossRef]
- López-Juárez, R.; Castañeda-Guzmán, R.; Rubio-Marcos, F.; Villafuerte-Castrejón, M.E.; Barrera-Calvad, E.; González, F. Insights into the dielectric and luminescent properties of Na0.5Pr0.003Bi0.497-xLaxTiO3 synthesized by the Pechini method. Dalton Trans. 2013, 42, 6879–6885. [Google Scholar] [CrossRef]
- Hussain, F.; Khesro, A.; Muhammad, R.; Wang, D. Effect of Ta-doping on functional properties of K0.51Na0.49NbO3. Mater. Res. Express 2019, 6, 106309. [Google Scholar] [CrossRef]
- Malic, B.; Bernard, J.; Holc, J.; Jenko, D.; Kosec, M. Alkaline-earth doping in (K,Na)NbO3 based piezoceramics. J. Eur. Ceram. Soc. 2005, 25, 2707–2711. [Google Scholar] [CrossRef]
- Ribeiroa, C.A.; Eiras, J.A.; Lente, M.H. Sintering kinects of (K0.48Na0.52)NbO3 lead-free piezoelectric ceramics densified by spark plasma sintering: Construction of master sintering curve. Ferroelectrics 2019, 545, 80–88. [Google Scholar] [CrossRef]
- Li, X.; Fan, B.; Lu, H.; Wang, H.; Shao, G.; Zhang, R. Investigation on preparation of Na0.5K0.5NbO3 nanoparticles by microwave heating method. Mater. Res. Express 2018, 5, 115018. [Google Scholar] [CrossRef]
- Khorrami, G.H.; Kompany, A.; Zak, A.K. Structural and optical properties of (K,Na)NbO3 nanoparticles synthesized by a modified sol–gel method using starch media. Adv. Powder Technol. 2015, 26, 113–118. [Google Scholar] [CrossRef]
- Wang, H.; Zuo, R.; Fu, J.; Liu, Y. Sol–gel derived (Li, Ta, Sb) modified sodium potassium niobate ceramics: Processing and piezoelectric properties. J. Alloy. Compd. 2011, 509, 936–941. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Qin, Z.C.; Yang, X.Y.; Zhu, H.B.; Cao, M.S. Study on synthesis and evolution of sodium potassium niobate ceramic powders by an oxalic acid-based sol–gel method. J. Sol-Gel Sci. Technol. 2011, 57, 31–35. [Google Scholar] [CrossRef]
- Sun, C.; Xing, X.; Chen, J.; Deng, J.; Li, L.; Yu, R.; Qiao, L.; Liu, G. Hydrothermal Synthesis of Single Crystalline (K,Na)NbO3 Powders. Eur. J. Inorg. Chem. 2007, 13, 1884–1888. [Google Scholar] [CrossRef]
- Piskin, C.; Karacasulu, L.; Bortolotti, M.; Vakifahmetoglu, C. Synthesis of potassium–sodium niobate (KNN) from NbO2. Open Ceram. 2021, 7, 100159. [Google Scholar] [CrossRef]
- Skjærvø, S.L.; Wells, K.H.; Beek, W.; Grande, T.; Einarsrud, M.A. Kinetics of the hydrothermal synthesis of nanosized KxNa1−xNbO3. CrystEngComm 2018, 20, 6795–6802. [Google Scholar] [CrossRef]
- Shi, G.; Wang, J.; Wang, H.; Wu, Z.; Wu, H. Hydrothermal synthesis of morphology-controlled KNbO3, NaNbO3, and (K,Na)NbO3 powders. Ceram. Int. 2017, 43, 7222–7230. [Google Scholar]
- Zhang, F.; Han, L.; Bai, S.; Sun, T.; Karaki, T.; Adachi, M. Hydrothermal synthesis of (K,Na)NbO3 particles. Jpn. J. Appl. Phys. 2008, 47, 7685–7688. [Google Scholar] [CrossRef]
- Ramajo, L.A.; Rubio-Marcos, F.; Campo, A.D.; Fernández, J.F.; Castro, M.S.; Parra, R. New insights into the properties of KxNa(1−x)NbO3 ceramics obtained by hydrothermal synthesis. Ceram. Int. 2014, 40, 14701–14712. [Google Scholar] [CrossRef]
- Kim, B.K.; Lim, D.Y.; Riman, R.E.; Nho, J.S.; Cho, S.B. A New Glycothermal Process for Barium Titanate Nanoparticle Synthesis. J. Am. Ceram. Soc. 2003, 86, 1793–1796. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lim, D.Y.; Nho, J.S.; Cho, S.B.; Riman, R.E.; Lee, B.W. Glycothermal synthesis and characterization of tetragonal barium titanate. J. Cryst. Growth 2005, 274, 638–652. [Google Scholar] [CrossRef]
- Cho, S.B.; Noh, J.S.; Park, S.J.; Lim, D.Y.; Choi, S.H. Morphological control of Fe3O4 particles via glycothermal process. J. Mater. Sci. 2007, 42, 4877–4886. [Google Scholar]
- Ryu, J.H.; Kil, H.S.; Song, J.H.; Lim, D.Y.; Cho, S.B. Glycothermal synthesis of 3 mol% yttria stabilized tetragonal ZrO2 nano powders at low temperature without mineralizers. Powder Technol. 2012, 221, 228–235. [Google Scholar] [CrossRef]
- Kil, H.S.; Jung, Y.J.; Moon, J.I.; Song, J.H.; Lim, D.Y.; Cho, S.B. Glycothermal Synthesis and Photocatalytic Properties of Highly Crystallized Anatase TiO2 Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 6193–6200. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kongsy, P.; Lim, D.Y.; Cho, S.B.; Song, J.H. Facile glycothermal synthesis of ZnO nanopowder at low temperature. Ceram. Int. 2016, 42, 17565–17570. [Google Scholar] [CrossRef]
- Park, S.J.; Song, J.H. Glycothermally Synthesized Self-aggregated ZnS Spherical Particles for Methyl Orange Photodecomposition. Korean J. Met. Mater. 2021, 59, 732–740. [Google Scholar] [CrossRef]
- Deblonde, G.J.-P.; Chagnes, A.; Bélair, S.; Cote, G. Solubility of niobium(V) and tantalum(V) under mild alkaline conditions. Hydrometallurgy 2015, 156, 99–106. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystal growth in pure and impure systems. In Industrial Crystallization 78; de Jong, E.J., Jancic, S.J., Eds.; North Holland: Amsterdam, The Netherland, 1979; pp. 93–103. [Google Scholar]
- Randolph, A.D.; Larson, M.A. Theory of Particulate Processes: Analysis and Techniques of Continuous Crystallization, 2nd ed.; Academic Press, Inc.: San Diego, CA, USA, 1988; pp. 109–134. [Google Scholar]
- Baker, D.W.; Thomas, P.A.; Zhang, N.; Glazer, A.M. Structural study of KxNa1−xNbO3 (KNN) for compositions in the range x = 0.24–0.36. Acta Cryst. B 2009, 65, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Peddigari, M.; Kim, J.H.; Kim, E.; Hwang, G.-T.; Kim, J.-W.; Ahn, C.-W.; Choi, J.-J.; Hahn, B.-D.; Choi, J.-H.; et al. Selective phase control of dopant-free potassium sodium niobate perovskites in solution. Inorg. Chem. 2020, 59, 3042–3052. [Google Scholar] [CrossRef]
- Lin, T.-H.; Yund, R. Potassium and sodium self-diffusion in alkali feldspar. Contrib. Mineral. Petrol. 1972, 34, 177–184. [Google Scholar] [CrossRef]
- Malic, B.; Jenko, D.; Holc, J.; Hrovat, M.; Kosec, M. Synthesis of sodium potassium niobate: A diffusion couples study. J. Am. Ceram. Soc. 2008, 91, 1916–1922. [Google Scholar] [CrossRef]
- Zhang, C.; LV, H.; Guo, M.; Zhang, M.; Wang, X. Thermodynamic evaluation and hydrothermal preparation of KxNa1−xNbO3. Rare Metals 2008, 27, 371–377. [Google Scholar] [CrossRef]


| Solvent Volume Ratio (B/W) | Lattice Parameters | ||
|---|---|---|---|
| a (nm) | b (nm) | c (nm) | |
| 0/70 | 0.5551 | 0.5588 | 1.5589 |
| 10/60 | 0.5546 | 0.5588 | 1.5589 |
| 20/50 | 0.5543 | 0.5586 | 1.5582 |
| 30/40 | 0.5545 | 0.5584 | 1.5582 |
| 40/30 | 0.5545 | 0.5584 | 1.5582 |
| 50/20 | 0.5543 | 0.5581 | 1.5589 |
| 60/10 | 0.5532 | 0.5582 | 1.5595 |
| 70/0 | 0.5539 | 0.5576 | 1.5595 |
| Molar Ratio (K+/Na+) | Lattice Parameters | Structure | ||
|---|---|---|---|---|
| a (nm) | b (nm) | c (nm) | ||
| 1.0/1.0 | 0.5539 | 0.5576 | 1.5595 | Orthorhombic |
| 1.4/0.6 | 0.5557 | 0.5567 | 1.5609 | Orthorhombic |
| 1.5/0.5 | 0.5573 | 0.5576 | 1.5704 | Orthorhombic |
| 1.6/0.4 | 0.5567 | 0.3931 | 0.5618 | Orthorhombic and Monoclinic [45] |
| 1.7/0.3 | 0.5646 | 0.3933 | 0.5679 | Orthorhombic |
| 1.8/0.2 | 0.5713 | 0.3996 | 0.5740 | Orthorhombic |
| Samples (KOH/NaOH /Nb2O5) | 1.0/1.0 /0.1 | 1.4/0.6 /0.1 | 1.5/0.5 /0.1 | 1.6/0.4 /0.1 | 1.7/0.3 /0.1 | 1.8/0.2 /0.1 |
| Mole Content of K+ (%) * | 1 | 6 | 15 | 26 | 50 | 72 |
| Phase | Na-rich KNN | MPB | K-rich KNN | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-C.; Lim, D.-Y.; Song, J.-H. Facile Glycothermal Synthesis of KxNa(1−x)NbO3 Particles. Crystals 2022, 12, 827. https://doi.org/10.3390/cryst12060827
Cho H-C, Lim D-Y, Song J-H. Facile Glycothermal Synthesis of KxNa(1−x)NbO3 Particles. Crystals. 2022; 12(6):827. https://doi.org/10.3390/cryst12060827
Chicago/Turabian StyleCho, Hong-Chan, Dae-Young Lim, and Jeong-Hwan Song. 2022. "Facile Glycothermal Synthesis of KxNa(1−x)NbO3 Particles" Crystals 12, no. 6: 827. https://doi.org/10.3390/cryst12060827
APA StyleCho, H.-C., Lim, D.-Y., & Song, J.-H. (2022). Facile Glycothermal Synthesis of KxNa(1−x)NbO3 Particles. Crystals, 12(6), 827. https://doi.org/10.3390/cryst12060827






