2-Pyridinyl-Terminated Iminobenzoate: Type and Orientation of Mesogenic Core Effect, Geometrical DFT Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of 4-Formylbenzoic Acid
2.2.2. Synthesis of 4(((4-Alkylphenyl)imino)methyl)benzoic Acid (X)
2.2.3. Synthesis of Pyridin-2-yl 4(((4-alkylphenyl)imino)methyl)benzoate (A, B, and C)
3. Results and Discussion
3.1. Mesomorphic Behavior
3.2. DFT Calculations
3.2.1. Geometrical Structure
3.2.2. Molecular Electrostatic Potential (MEP)
3.2.3. Frontier Molecular Orbitals (FMOs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashikur Rahman Rabbi, J.A.F. Preparation, Characterization and Applications of Liquid Crystals: A Review. J. Appl. Chem. 2020, 13, 43–54. [Google Scholar]
- Khoo, I.-C. Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Andrienko, D. Introduction to liquid crystals. J. Mol. Liq. 2018, 267, 520–541. [Google Scholar] [CrossRef]
- Mitov, M. Liquid-Crystal Science from 1888 to 1922: Building a Revolution. ChemPhysChem 2014, 15, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Ashok, C.K.; Ramesh, D.R.; Ola, M.M.; Chaudhari, V.A. Liquid crystals: A review. Int. J. All Res. Writ. 2019, 1, 119–129. [Google Scholar]
- Priestly, E. Introduction to Liquid Crystals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Chester, A.N.; Martellucci, S. Phase Transitions in Liquid Crystals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 290. [Google Scholar]
- Jber, N.R.; Shukur, M.M.; Najaf, A.A. Schiff Base liquid Crystals with Terminal Alkoxy Group Synthesis and Thermotropic Properties. Al-Nahrain J. Sci. 2014, 17, 64–72. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Aouad, M. Mesomorphic and DFT diversity of Schiff base derivatives bearing protruded methoxy groups. Liq. Cryst. 2020, 47, 2222–2233. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, S.-T.; Yeap, G.-Y.; Boey, P.-L. Synthesis and liquid crystalline properties of new Schiff bases N-[4-(4-nalkanoyloxybenzoyloxy) benzylidene]-4-cyano-, 4-hydroxy-, 4-thio-and 4-nitroanilines. Aust. J. Basic Appl. Sci. 2009, 3, 3417–3422. [Google Scholar]
- Hagar, M.; Ahmed, H.; Saad, G. Mesophase stability of new Schiff base ester liquid crystals with different polar substituents. Liq. Cryst. 2018, 45, 1324–1332. [Google Scholar] [CrossRef]
- Ahmed, N.H.; Saad, G.R.; Ahmed, H.A.; Hagar, M. New wide-stability four-ring azo/ester/Schiff base liquid crystals: Synthesis, mesomorphic, photophysical, and DFT approaches. RSC Adv. 2020, 10, 9643–9656. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2020, 47, 114–124. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A.; Zaki, A.A. Optical and geometrical characterizations of non-linear supramolecular liquid crystal complexes. Crystals 2020, 10, 701. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; Alalawy, H.H.; Al Alwani, M.H. Synthesis, mesomorphic and computational characterizations of nematogenic schiff base derivatives in pure and mixed state. Molecules 2021, 26, 2038. [Google Scholar] [CrossRef] [PubMed]
- Nada, S.; Hagar, M.; Farahat, O.; Hasanein, A.A.; Emwas, A.-H.; Sharfalddin, A.A.; Jaremko, M.; Zakaria, M.A. Three Rings Schiff Base Ester Liquid Crystals: Experimental and Computational Approaches of Mesogenic Core Orientation Effect, Heterocycle Impact. Molecules 2022, 27, 2304. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2020, 47, 404–413. [Google Scholar] [CrossRef]
- Paterson, D.A.; Crawford, C.A.; Pociecha, D.; Walker, R.; Storey, J.M.; Gorecka, E.; Imrie, C.T. The role of a terminal chain in promoting the twist-bend nematic phase: The synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkyloxyanilinebenzylidene-4′-oxy) hexanes. Liq. Cryst. 2018, 45, 2341–2351. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision d. 01; Gaussian. Inc.: Wallingford, CT, USA, 2009; p. 201. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem, Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Adrienko, G.A. Chemcraft 1.8 (build445). Available online: https://www.chemcraftprog.com/download.html (accessed on 24 April 2022).
- Ribera, D.; Serra, A.; Mantecón, A. Dimeric liquid-crystalline epoxyimine monomers: Influence of dipolar moments on mesomorphic behavior and the formation of liquid-crystalline thermosets. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1465–1477. [Google Scholar] [CrossRef]
- Al-Hossainy, A.F.; Zoromba, M.S. Doped-poly (para-nitroaniline-co-aniline): Synthesis, semiconductor characteristics, density, functional theory and photoelectric properties. J. Alloys Compd. 2019, 789, 670–683. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S. Liquid crystals in photovoltaics: A new generation of organic photovoltaics. Polym. J. 2017, 49, 85–111. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.-C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702. [Google Scholar] [CrossRef] [PubMed]











| Compounds | TCr-SmA | TCr-N | TCr-I | TSmA-I | TN-I | ΔHCr-N | ΔSCr-N | ΔSCr-N/R | ΔHCr-SmA | ΔSCr-SmA | ΔSCr-SmA/R | ΔHCr-I | ΔSCr-I | ΔSCr-I/R | ΔHN-I | ΔSN-I | ΔSN-I/R | ΔHSmA-I | ΔSSmA-I | ΔSSmA-I/R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | - | 118.24 | - | - | 134.16 | 29.7 | 75.9 | 9.1 | - | - | - | - | - | - | 0.30 | 0.74 | 0.09 | - | - | - |
| B | - | 118.65 | - | - | 132.55 | 38.2 | 97.5 | 11.7 | - | - | - | - | - | - | 0.41 | 1.01 | 0.12 | - | - | - |
| C | - | 120.32 | - | - | 133.60 | 44.7 | 113.6 | 13.7 | - | - | - | - | - | - | 0.50 | 1.23 | 0.15 | - | - | - |
| D | - | - | 124.3 | - | - | - | - | - | - | - | - | 35.17 | 88.49 | 10.64 | - | - | - | - | - | - |
| E | 96.1 | - | - | 121.5 | - | - | - | - | 23.5 | 63.64 | 7.65 | - | - | - | - | - | - | 1.6 | 4.05 | 0.49 |
| F | - | 128.6 | - | - | 174.2 | 43.40 | 108.03 | 12.99 | - | - | - | - | - | - | 1.80 | 4.02 | 0.48 | - | - | - |
| G | 67.3 | - | - | 87.3 | - | - | - | - | 28.38 | 83.36 | 10.02 | - | - | - | - | - | - | 1.92 | 5.33 | 2.65 |
| H | - | 116.42 | - | - | 130.59 | 50.48 | 129.58 | 15.58 | - | - | - | - | - | - | 2.03 | 5.03 | 0.60 | - | - | - |
| I | - | 110.8 | - | - | 156.8 | 26.15 | 68.11 | 8.19 | - | - | - | - | - | - | 0.88 | 2.05 | 0.25 | - | - | - |
| Compounds | θI-II | θI-III | θII-III |
|---|---|---|---|
| A | 27.28 | 11.20 | 37.04 |
| B | 11.86 | 26.17 | 37.57 |
| C | 15.15 | 22.16 | 36.80 |
| D | 0.06 | 0.06 | 0.01 |
| E | 0.01 | 0.04 | 0.05 |
| F | 6.25 | 22.51 | 28.70 |
| G | 48.91 | 79.97 | 31.42 |
| H | 26.41 | 6.63 | 30.57 |
| I | 40.15 | 57.07 | 16.96 |
| Compounds | Cr | TSmA | TN | ΔTC | ΔTSmA | ΔTN | Dimensions | Aspect Ratio (L/D) | |
|---|---|---|---|---|---|---|---|---|---|
| Length (L) | Width (D) | ||||||||
| A | 118.24 | - | 134.16 | 15.92 | - | 15.92 | 29.62 | 7.55 | 3.92 |
| B | 118.65 | - | 132.55 | 13.9 | - | 13.9 | 34.58 | 8.42 | 4.11 |
| C | 120.32 | - | 133.60 | 13.28 | - | 13.28 | 37.16 | 8.45 | 4.40 |
| D | 124.3 | - | - | - | - | - | 31.32 | 7.93 | 3.95 |
| E | 96.1 | 121.5 | - | 25.4 | 25.4 | - | 41.70 | 7.89 | 5.29 |
| F | 128.6 | - | 174.2 | 45.6 | - | 45.6 | 31.15 | 8.06 | 3.86 |
| G | 67.3 | 87.3 | - | 20 | 20 | - | 28.78 | 7.27 | 3.96 |
| H | 116.42 | - | 130.59 | 14.17 | - | 14.17 | 31.35 | 7.48 | 4.19 |
| I | 110.8 | - | 156.8 | 46 | - | 46 | 29.05 | 7.47 | 3.89 |
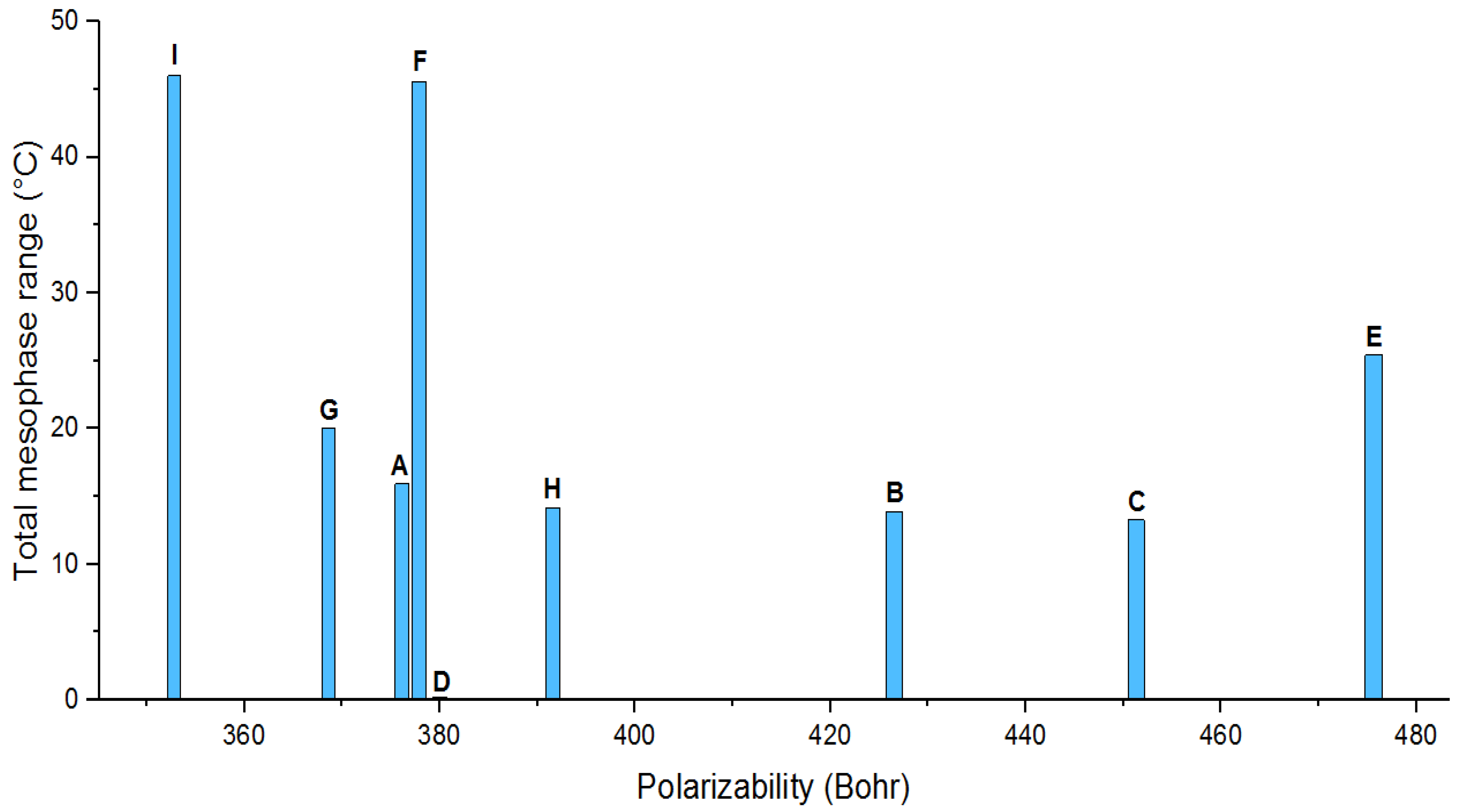
| Compounds | TSmA | TN | ΔTC | ΔTSmA | ΔTN | Polarizability | Dipole Moment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (x) | (y) | (z) | Total | |||||||
| A | - | 134.16 | 15.92 | - | 15.92 | 376.16 | 0.16 | −1.45 | −0.43 | 1.52 |
| B | - | 132.55 | 13.9 | - | 13.9 | 426.56 | 0.12 | −0.59 | −1.25 | 1.39 |
| C | - | 133.60 | 13.28 | - | 13.28 | 451.29 | −0.12 | 0.79 | −1.18 | 1.43 |
| D | - | - | - | - | - | 380.01 | −1.81 | 0.76 | 0.00 | 1.96 |
| E | 121.5 | - | 25.4 | 25.4 | - | 475.63 | −5.87 | −2.63 | 0.00 | 6.43 |
| F | - | 174.2 | 45.6 | - | 45.6 | 377.90 | 3.28 | 3.27 | 0.11 | 4.64 |
| G | 87.3 | - | 20 | 20 | - | 368.62 | −2.87 | −1.86 | 0.51 | 3.45 |
| H | - | 130.59 | 14.17 | - | 14.17 | 391.56 | −1.89 | −0.14 | −1.31 | 2.30 |
| I | - | 156.8 | 46 | - | 46 | 352.82 | 3.82 | 0.46 | 0.33 | 3.87 |
| Compounds | ELUMO | EHOMO | ΔE | S | η |
|---|---|---|---|---|---|
| A | −2.38 | −6.15 | 3.77 | 0.27 | 1.89 |
| B | −2.39 | −6.15 | 3.76 | 0.27 | 1.88 |
| C | −2.39 | −6.15 | 3.76 | 0.27 | 1.88 |
| D | −2.75 | −6.34 | 3.59 | 0.28 | 1.80 |
| E | −2.75 | −6.26 | 3.51 | 0.28 | 1.76 |
| F | −2.33 | −5.78 | 3.45 | 0.29 | 1.73 |
| G | −2.28 | −5.81 | 3.53 | 0.28 | 1.77 |
| H | −2.30 | −5.80 | 3.50 | 0.29 | 1.75 |
| I | −1.97 | −6.07 | 4.10 | 0.24 | 2.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Humaidi, J.Y.; Nada, S.; Gerges, M.; Ehab, M.; Jaremko, M.; Emwas, A.-H.; Hagar, M. 2-Pyridinyl-Terminated Iminobenzoate: Type and Orientation of Mesogenic Core Effect, Geometrical DFT Investigation. Crystals 2022, 12, 902. https://doi.org/10.3390/cryst12070902
Al-Humaidi JY, Nada S, Gerges M, Ehab M, Jaremko M, Emwas A-H, Hagar M. 2-Pyridinyl-Terminated Iminobenzoate: Type and Orientation of Mesogenic Core Effect, Geometrical DFT Investigation. Crystals. 2022; 12(7):902. https://doi.org/10.3390/cryst12070902
Chicago/Turabian StyleAl-Humaidi, Jehan Y., Shady Nada, Mariam Gerges, Marwa Ehab, Mariusz Jaremko, Abdul-Hamid Emwas, and Mohamed Hagar. 2022. "2-Pyridinyl-Terminated Iminobenzoate: Type and Orientation of Mesogenic Core Effect, Geometrical DFT Investigation" Crystals 12, no. 7: 902. https://doi.org/10.3390/cryst12070902





