Room Temperature Synthesis of Various Color Emission Rare-Earth Doped Strontium Tungstate Phosphors Applicable to Fingerprint Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SrWO4:RE3+ by Co-Precipitation at Room Temperature
2.2. Fabricated Fingerprint Identification Application
2.3. Characterization
3. Results & Discussion
3.1. Characteristics of SrWO4 and SrWO4:RE3+
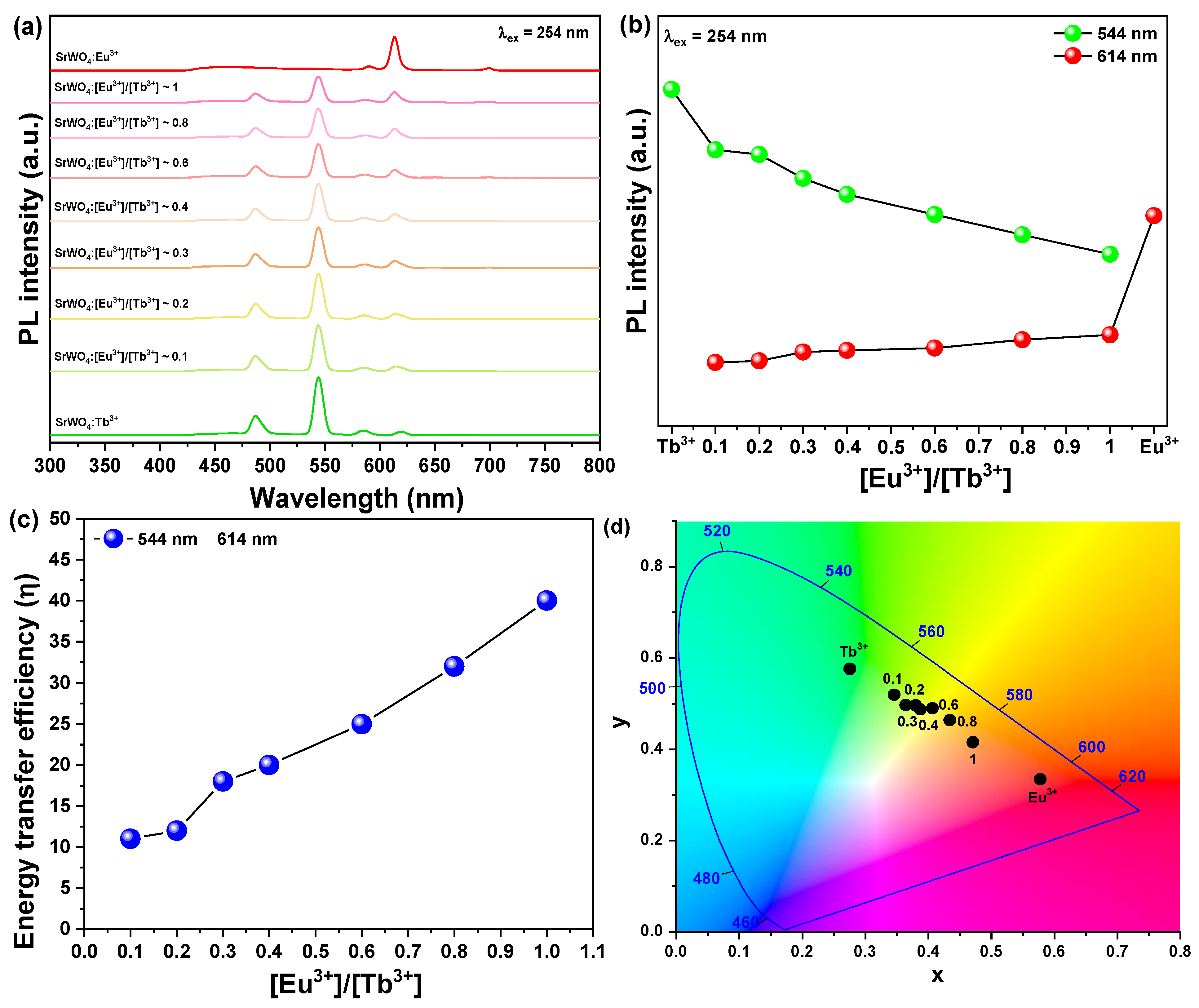
3.2. Characteristics of the SrWO4: [Eu3+]:[Tb3+] Phosphors
3.3. Applied for Fingerprint Identification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Do, Y.R.; Huh, Y.D. Optical Properties of Potassium Europium Tungstate Phosphors. J. Electrochem. Soc. 2000, 147, 4385–4388. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Zhang, Y.; Li, Y.; Yao, X. Winning wide-temperature-range and high-sensitive thermometry by a multichannel strategy of dual-lanthanides in the new tungstate phosphors. J. Alloys Compd. 2020, 834, 154998. [Google Scholar] [CrossRef]
- LEE, G.; KIM, T.; YOON, C.; KANG, S. Effect of local environment and Sm3+-codoping on the luminescence properties in the Eu3+-doped potassium tungstate phosphor for white LEDS. J. Lumin. 2008, 128, 1922–1926. [Google Scholar] [CrossRef]
- Hua, Y.; Yu, J.S. Double-excited states of charge transfer band and 4f-4f in single-phase K3Gd(VO4)2:Tb3+/Sm3+ phosphors with superior sensing sensitivity for potential luminescent thermometers. J. Mater. Sci. Technol. 2021, 91, 148–159. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.; Huang, W.; Bettinelli, M.; Liu, X. Lanthanide-Activated Phosphors Based on 4f-5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef]
- Li, L.; Yang, P.; Xia, W.; Wang, Y.; Ling, F.; Cao, Z.; Jiang, S.; Xiang, G.; Zhou, X.; Wang, Y. Luminescence and optical thermometry strategy based on emission and excitation spectra of Pr3+ doped SrMoO4 phosphors. Ceram. Int. 2021, 47, 769–775. [Google Scholar] [CrossRef]
- Wu, H.; Niu, P.; Pei, R.; Zheng, Y.; Jin, W.; Li, X.; Jiang, R. Tb3+ and Sm3+ co-doped CaWO4 white light phosphors for plant lamp synthesized via solid state method: Phase, photoluminescence and electronic structure. J. Lumin. 2021, 236, 118146. [Google Scholar] [CrossRef]
- Pollnau, M.; Romanyuk, Y.E.; Gardillou, F.; Borca, C.N.; Griebner, U.; Rivier, S.; Petrov, V. Double Tungstate Lasers: From Bulk Toward On-Chip Integrated Waveguide Devices. JSTQE 2007, 13, 661–671. [Google Scholar] [CrossRef]
- Semenov, P.A.; Meshchanin, A.P.; Davidenko, A.M.; Kormilitsin, V.A.; Batarin, V.A.; Goncharenko, Y.M.; Stone, S.; Kravtsov, V.I.; Matulenko, Y.A.; Semenov, V.K.; et al. Design and performance of LED calibration system prototype for the lead tungstate crystal calorimeter. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 556, 94–99. [Google Scholar] [CrossRef][Green Version]
- Jung, J.-Y. Luminescent Color-Adjustable Europium and Terbium Co-Doped Strontium Molybdate Phosphors Synthesized at Room Temperature Applied to Flexible Composite for LED Filter. Crystals 2022, 12, 552. [Google Scholar] [CrossRef]
- Feng, L.; Chen, X.; Mao, C. A facile synthesis of SrWO4 nanobelts by the sonochemical method. Mater. Lett. 2010, 64, 2420–2423. [Google Scholar] [CrossRef]
- Thongtem, T.; Phuruangrat, A.; Thongtem, S. Preparation and characterization of nanocrystalline SrWO4 using cyclic microwave radiation. Curr. Appl. Phys. 2008, 8, 189–197. [Google Scholar] [CrossRef]
- Gopal, R.; Kumar, A.; Manam, J. Enhanced photoluminescence and abnormal temperature dependent photoluminescence property of SrWO4:Dy3+ phosphor by the incorporation of Li+ ion. Mater. Chem. Phys. 2021, 272, 124960. [Google Scholar] [CrossRef]
- Long, Q.; Xia, Y.; Huang, Y.; Liao, S.; Gao, Y.; Huang, J.; Liang, J.; Cai, J. Na+ induced electric-dipole dominated transition (5D0→7F2) of Eu3+ emission in AMgPO4:Eu3+ (A = Li+, Na+, K+) phosphors. Mater. Lett. 2015, 145, 359–362. [Google Scholar] [CrossRef]
- Roh, H.; Lee, S.; Caliskan, S.; Yoon, C.; Lee, J. Luminescence and electric dipole in Eu3+ doped strontium phosphate: Effect of SiO4. J. Alloys Compd. 2019, 772, 573–578. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Luminescence of Phosphors Based on Host Lattices ABO4 (A is Sc, In; B is P, V, Nb). J. Chem. Phys. 1969, 50, 2974–2980. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Xu, X.; Liu, X.; Ge, X.; Meng, F. Crystal structure and magnetic-dipole emissions of Sr2CaWO6: RE3+ (RE = Dy, Sm and Eu) phosphors. J. Alloys Compd. 2018, 739, 660–668. [Google Scholar] [CrossRef]
- Yu, R.; Wang, C.; Chen, J.; Wu, Y.; Li, H.; Ma, H. Photoluminescence Characteristics of Eu3+-Doped Double-Perovskite Phosphors. ECS J. Solid State Sci. Technol. 2014, 3, R33–R37. [Google Scholar] [CrossRef]
- Tian, L.; Yu, B.; Pyun, C.; Park, H.L.; Mho, S. New red phosphors BaZr(BO3)2 and SrAl2B2O7 doped with Eu3+ for PDP applications. Solid State Commun. 2004, 129, 43–46. [Google Scholar] [CrossRef]
- Yu, P.; Su, L.; Xu, J. Synthesis and Luminescence Properties of Eu3+, Bi3+-Doped BaWO4 Phosphors. Opt. Rev. 2014, 21, 455–460. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.; Shim, Y.; Hwang, D.; Son, C.S. Structure and Photoluminescence Properties of Rare-Earth (Dy3+, Tb3+, Sm3+)-Doped BaWO4 Phosphors Synthesized via Co-Precipitation for Anti-Counterfeiting. Materials 2020, 13, 4165. [Google Scholar] [CrossRef]
- Shinde, K.N.; Dhoble, S.J.; Kumar, A. Combustion synthesis of Ce3+, Eu3+ and Dy3+ activated NaCaPO4 phosphors. J. Rare Earths 2011, 29, 527–535. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Thongtem, T.; Kungwankunakorn, S.; Kuntalue, B.; Phuruangrat, A.; Thongtem, S. Luminescence and absorbance of highly crystalline CaMoO4, SrMoO4, CaWO4 and SrWO4 nanoparticles synthesized by co-precipitation method at room temperature. J. Alloys Compd. 2010, 506, 475–481. [Google Scholar] [CrossRef]
- Shu, Y.; Travert, A.; Schiller, R.; Ziebarth, M.; Wormsbecher, R.; Cheng, W. Effect of Ionic Radius of Rare Earth on USY Zeolite in Fluid Catalytic Cracking: Fundamentals and Commercial Application. Top. Catal. 2015, 58, 334–342. [Google Scholar] [CrossRef]
- Krishna Bharat, L.; Lee, S.H.; Yu, J.S. Synthesis, structural and optical properties of BaMoO4:Eu3+ shuttle like phosphors. Mater. Res. Bull. 2014, 53, 49–53. [Google Scholar] [CrossRef]
- Liao, J.; Qiu, B.; Wen, H.; Chen, J.; You, W. Hydrothermal synthesis and photoluminescence of SrWO4:Tb3+ novel green phosphor. Mater. Res. Bull. 2009, 44, 1863–1866. [Google Scholar] [CrossRef]
- Barja, B.; Baggio, R.; Garland, M.T.; Aramendia, P.F.; Peña, O.; Perec, M. Crystal structures and luminescent properties of terbium(III) carboxylates. Inorg. Chim. Acta 2003, 346, 187–196. [Google Scholar] [CrossRef]
- Tseng, T.; Choi, J.; Davidson, M.; Holloway, P.H. Synthesis and luminescent characteristics of europium dopants in SiO2/Gd2O3 core/shell scintillating nanoparticles. J. Mater. Chem. 2010, 20, 6111–6115. [Google Scholar] [CrossRef]
- Dhara, S.; Imakita, K.; Mizuhata, M.; Fujii, M. Europium doping induced symmetry deviation and its impact on the second harmonic generation of doped ZnO nanowires. Nanotechnology 2014, 25, 225202. [Google Scholar] [CrossRef]
- Sun, S.; Guo, R.; Zhang, Q.; Lv, X.; Leng, P.; Wang, Y.; Huang, Z.; Wang, L. Efficient deep-blue thermally activated delayed fluorescence emitters based on diphenylsulfone-derivative acceptor. Dye. Pigment. 2020, 178, 108367. [Google Scholar] [CrossRef]
- Zhu, H.; Qian, B.; Zhou, X.; Song, Y.; Zheng, K.; Sheng, Y.; Zou, H. Tunable luminescence and energy transfer of Tb3+/Eu3+ co-doped cubic CaCO3 nanoparticles. J. Lumin. 2018, 203, 441–446. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, V.K.; Kumar, V.; Kumar, V.; Swart, H.C. Upconversion based temperature sensing ability of Er3+–Yb3+codoped SrWO4: An optical heating phosphor. Sens. Actuators B Chem. 2015, 209, 352–358. [Google Scholar] [CrossRef]
- Song, H.; Wang, C.; Han, Q.; Tang, X.; Yan, W.; Chen, Y.; Jiang, J.; Liu, T. Highly sensitive Tm3+/Yb3+ codoped SrWO4 for optical thermometry. Sens. Actuators A Phys. 2018, 271, 278–282. [Google Scholar] [CrossRef]
- Li, G.; Gao, S.; He, W. Preparation and photoluminescence properties of the Sm3+, Eu3+ co-doped CaWO4 phosphors. Optik 2015, 126, 3272–3275. [Google Scholar] [CrossRef]
- Kang, F.; Hu, Y.; Wu, H.; Mu, Z.; Ju, G.; Fu, C.; Li, N. Luminescence and red long afterglow investigation of Eu3+–Sm3+ CO-doped CaWO4 phosphor. J. Lumin. 2012, 132, 887–894. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Yang, R. A series of color tunable yellow–orange–red-emitting SrWO4:RE (Sm3+, Eu3+–Sm3+) phosphor for near ultraviolet and blue light-based warm white light emitting diodes. Superlattices Microstruct. 2016, 91, 138–147. [Google Scholar] [CrossRef]








| No. | Host | Rare Earth | Type | Wavalength (nm) |
|---|---|---|---|---|
| 1 [33] | SrWO4 | Er3+/Yb3+ | Up conversion | 489, 525 |
| 2 [34] | SrWO4 | Tm3+/Yb3+ | Up conversion | 684, 814 |
| 3 [35] | CaWO4 | Sm3+/Eu3+ | Down conversion | 592, 615 |
| 4 [36] | CaWO4 | Eu3+/Sm3+ | Down conversion | 622, 630 |
| 5 [37] | SrWO4 | Eu3+/Sm3+ | Down conversion | 590, 613 |
| This work | SrWO4 | Eu3+/Tb3+ | Down conversion | 544, 614 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.-S.; Jung, J.-Y. Room Temperature Synthesis of Various Color Emission Rare-Earth Doped Strontium Tungstate Phosphors Applicable to Fingerprint Identification. Crystals 2022, 12, 915. https://doi.org/10.3390/cryst12070915
Yi S-S, Jung J-Y. Room Temperature Synthesis of Various Color Emission Rare-Earth Doped Strontium Tungstate Phosphors Applicable to Fingerprint Identification. Crystals. 2022; 12(7):915. https://doi.org/10.3390/cryst12070915
Chicago/Turabian StyleYi, Soung-Soo, and Jae-Yong Jung. 2022. "Room Temperature Synthesis of Various Color Emission Rare-Earth Doped Strontium Tungstate Phosphors Applicable to Fingerprint Identification" Crystals 12, no. 7: 915. https://doi.org/10.3390/cryst12070915
APA StyleYi, S.-S., & Jung, J.-Y. (2022). Room Temperature Synthesis of Various Color Emission Rare-Earth Doped Strontium Tungstate Phosphors Applicable to Fingerprint Identification. Crystals, 12(7), 915. https://doi.org/10.3390/cryst12070915






