Challenges and Opportunities for the Blue Perovskite Quantum Dot Light-Emitting Diodes
Abstract
:1. Introduction
2. Basic
2.1. Blue Light-Emitting Perovskite Quantum Dot Materials
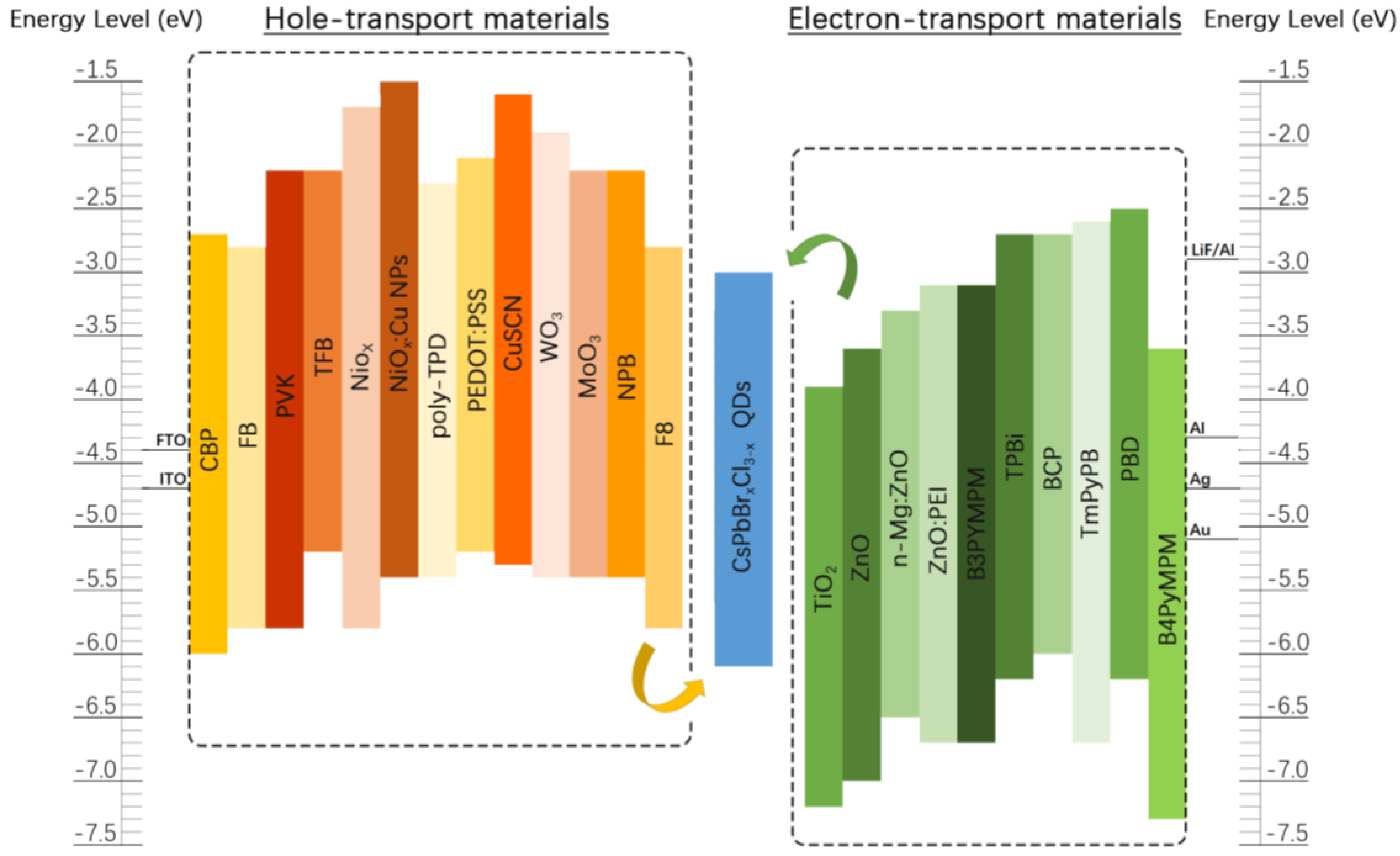
2.2. The Basic Stack of PQD-LEDs
| Perovskites | Device Structure | EL Peak [nm] | Vturn-on [V] | Lmax [cd·m−2] | EQEmax [%] | FWHM [nm] | LT50 | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Sky-blue PeLEDs | |||||||||
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/Ca/Ag | 496 | 3.2 | 603 | 2.6 | 18 | 2020 | [10] | |
| CsPb (Br1−xClx)3 | ITO/TiO2/perovskite/F8/MoO3/Au | 495 | 4 | 750 | 0.075 | 2016 | [11] | ||
| CsPbBr2.4Cl0.6 | ITO/PEDOT:PSS/PVK/perovskite/TPBi/LiF/Al | 495 | 7.8 | 2452 | 1.13 | 21 | 2016 | [12] | |
| CsPbBrxCl3−x | ITO/:ZnO/b-PEI/perovskite/PVK/V2O5/Al | 492 | 143.1 | 0.053 | 26 | 2021 | [13] | ||
| CsPbBrxCl3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/B3PYMPM/TPBi/LiF/Al | 490 | <3.4 | 2063 | 3.5 | 18–20 | 26 s@100 cd·m−2 | 2019 | [14] |
| RbxCs1−xPbBr3 | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 490 | 4 | 186 | 0.87 | 22 | 2019 | [15] | |
| CsPb (Br1−xClx)3 | ITO/PEDOT:PSS/perovskite/TPBi/LiF/Al | 490 | 3 | 35 | 1.9 | 19 | 2016 | [16] | |
| (Cs/FA)PbBrxCl3−x:Cu | ITO/PEDOT:PSS/PTAA/perovskite/TOPO/TPBi/LiF/Al | 490 | 2.6 | 130 | 5.02 | 19 | 2020 | [17] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/Ca/Ag | 489 | 3.4 | 182 | 1.8 | 18 | 2020 | [10] | |
| CsPbBrxCl4−x:La | ITO/PEDOT:PSS/PVK/perovskite/TPBi/LiF/Al | 489 | 4 | 192.6 | 3.25 | 21 | 2020 | [18] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/TFB/PFI/perovskite/TPBI/LiF/Al | 488 | 830 | 1.41 | 23 | 2018 | [19] | ||
| CsPbBrxCl3−x | ITO/ZnO/b-PEI/perovskite/PVK/V2O5/Al | 486 | 94.2 | 0.045 | 25 | 2021 | [13] | ||
| CsPbBrxCl3−x | ITO/ZnO/b-PEI/perovskite/PVK/V2O5/Al | 484 | 95.1 | 0.04 | 25 | 2021 | [13] | ||
| CsPbBrxCl3−x | ITO/PEDOT:PSS/TFB/PFI/perovskite/TPBI/LiF/Al | 481 | 212 | 0.44 | 23 | 2018 | [19] | ||
| CsPbBrxCl3−x:Nd | ITO/PEDOT:PSS/TFB/perovskite/TPBi/Liq/Al | 481 | 3 | 138 | 2.7 | 14 | 2020 | [20] | |
| CsPbBrxCl3−x:La | ITO/PEDOT:PSS/PVK/perovskite/TPBi/LiF/Al | 480 | 4 | 292.7 | 2.17 | 22 | 2020 | [18] | |
| CsPbBr3 (nanoplates) | ITO/PEDOT:PSS/Poly-TPD/perovskite/TPBi/LiF/Al | 480 | 25 | 0.1 | 2018 | [21] | |||
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/Ca/Ag | 479 | 3.2 | 119 | 1 | 18 | 2020 | [10] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 479 | 3.5 | 29.95 | 0.864 | 18 | 2019 | [22] | |
| CsPbBr3 | ITO/PEDOT:PSS/PTAA/perovskite/MoOx/Ag | 479 | 90 | 12.3 | 20 | 20 min@90 cd·m−2 | 2020 | [23] | |
| (K/Cs)PbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 477 | 3.2 | 86.95 | 1.96 | 19 | 2020 | [24] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/B3PYMPM/TPBi/LiF/Al | 476 | <3.4 | 678 | 2.25 | 18–20 | 26 s@100 cd·m−2 | 2019 | [14] |
| Pure-blue PeLEDs | |||||||||
| CsPbBrxCl3−x | ITO/ZnO/b-PEI/perovskite/PVK/V2O5/Al | 472 | 46.6 | 0.027 | 25 | 2021 | [13] | ||
| CsPb (BrxCl1−x)3 | ITO/TFB/PFI/MHP perovskite/3TPYMB/Liq/Al/Pt | 471 | 465 | 6.3 | 17 | 2020 | [25] | ||
| CsPbBrxCl3−x:Mn | ITO/TFB/PFI/perovskite/TPBi/LiF/Al | 470 | ~4.8 | 389 | 1.46 | 17 | 2018 | [26] | |
| Ni2+-CsPbClxBr3−x | ITO/PEDOT:PSS/TFB/PFI/perovskite/TPBi/LiF/Al | 470 | 3.2 | 612 | 2.4 | 2020 | [27] | ||
| CsPbClxBr3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/TmPyPB/TPBi/LiF/Al | 470 | 4.9 | 507 | 2.15 | 21 | 2020 | [28] | |
| CsPbClxBr3−x | ITO/NiOx/perovskite/TPBi/LiF/Al | 470 | 350 | 0.07 | 20 | 2017 | [29] | ||
| CsPbBr3 | ITO/PEDOT:PSS/PVK/perovskite/ZnO/Ag | 470 | 3850 | 4.7 | 27 | 12 h@102 cd·m−2 | 2021 | [30] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/Ca/Ag | 469 | 3.8 | 30 | 0.65 | 18 | 2020 | [10] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 469 | 4 | 11.95 | 0.44 | 18 | 2019 | [22] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/TFB:PFI/perovskite/TPBi/LiF/Al | 469 | 111 | 0.5 | 24 | 2018 | [19] | ||
| CsPbBrxCl3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/TmPyPB/TPBi/LiF/Al | 468 | 4.8 | 620 | 1.53 | 20 | 2020 | [28] | |
| (Rb/Cs)PbBrxCl3−x:Ni | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 467 | 3.5 | 10.4 | 2.14 | 16 | 2020 | [31] | |
| CsMnyPb1−yBrxCl3−x | ITO/TFB/PFI/perovskite/TPBi/LiF/Al | 466 | 245 | 2.12 | 18 | 2018 | [26] | ||
| Deep-blue PeLEDs | |||||||||
| CsPbBrxCl3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/TmPyPB/TPBi/LiF/Al | 465 | 4.6 | 518 | 0.92 | 19 | 2020 | [28] | |
| RbxCs1−xPbBr3 | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 464 | 4 | 63 | 0.11 | 18 | 2019 | [15] | |
| CsPb (Br/Cl)3 | ITO/PEDOT:PSS/Poly-TPD/CBP/perovskite/B3PYMPM/LiF/Al | 463 | 2.9 | 318 | 1.4 | 14 | 2019 | [32] | |
| CsPbBr3 | ITO/PEDOT:PSS/Poly-TPD/perovskite/TPBi/LiF/Al | 463 | 62 | 0.124 | 12 | 2018 | [33] | ||
| CsPbBrxCl3−x | ITO/ZnO/b-PEI/perovskite/PVK/V2O5/Al | 462 | 5 | 32.5 | 0.02 | 24 | 2021 | [13] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/B3PYMPM/TPBi/LiF/Al | 462 | <3.4 | 193 | 1 | 18–20 | 26 s@100 cd·m−2 | 2019 | [14] |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/polyTPD/PVK/perovskite/TmPyPB/TPBi/LiF/Al | 462 | 4.4 | 450 | 0.77 | 19 | 2020 | [28] | |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 461 | 4 | 763 | 0.8 | 16 | 2019 | [34] | |
| CsPbClxBr3−x | ITO/PEDOT:PSS/Poly-TPD/perovskite/TPBi/LiF/Al | 460 | 3.8 | 33 | 1.35 | 15 | 51.5 s@3.7 V | 2019 | [35] |
| CsPbBrxCl3−x | ITO/PEDOT:PSS/poly-TPD/perovskite/TPBi/LiF/Al | 458 | 4.5 | 3.865 | 0.101 | 17 | 2019 | [22] | |
| CsPb (Cl/Br)3 | ITO/PEDOT:PSS/TFB/perovskite/TPBi/Liq/Al | 456 | 5.4 | 43.2 | 1.1 | 16 | 5 s@10 cd·m−2 | 2020 | [36] |
| CsPb (Cl/Br)3 | ITO/PEDOT:PSS/PVK/perovskite/TPBi/LiF/Al | 455 | 5.1 | 742 | 0.07 | 20 | 2015 | [37] | |
| CsPbBr1.5Cl1.5 | ITO/PEDOT:PSS/PVK/perovskite/TPBi/LiF/Al | 445 | 7.8 | 2673 | 1.38 | <30 | 2016 | [12] | |
| CsPbBrxCl3−x | ITO/ZnO/b-PEI/perovskite/PVK/V2O5/Al | 435 | 5 | 12.3 | 0.01 | 25 | 2021 | [13] | |
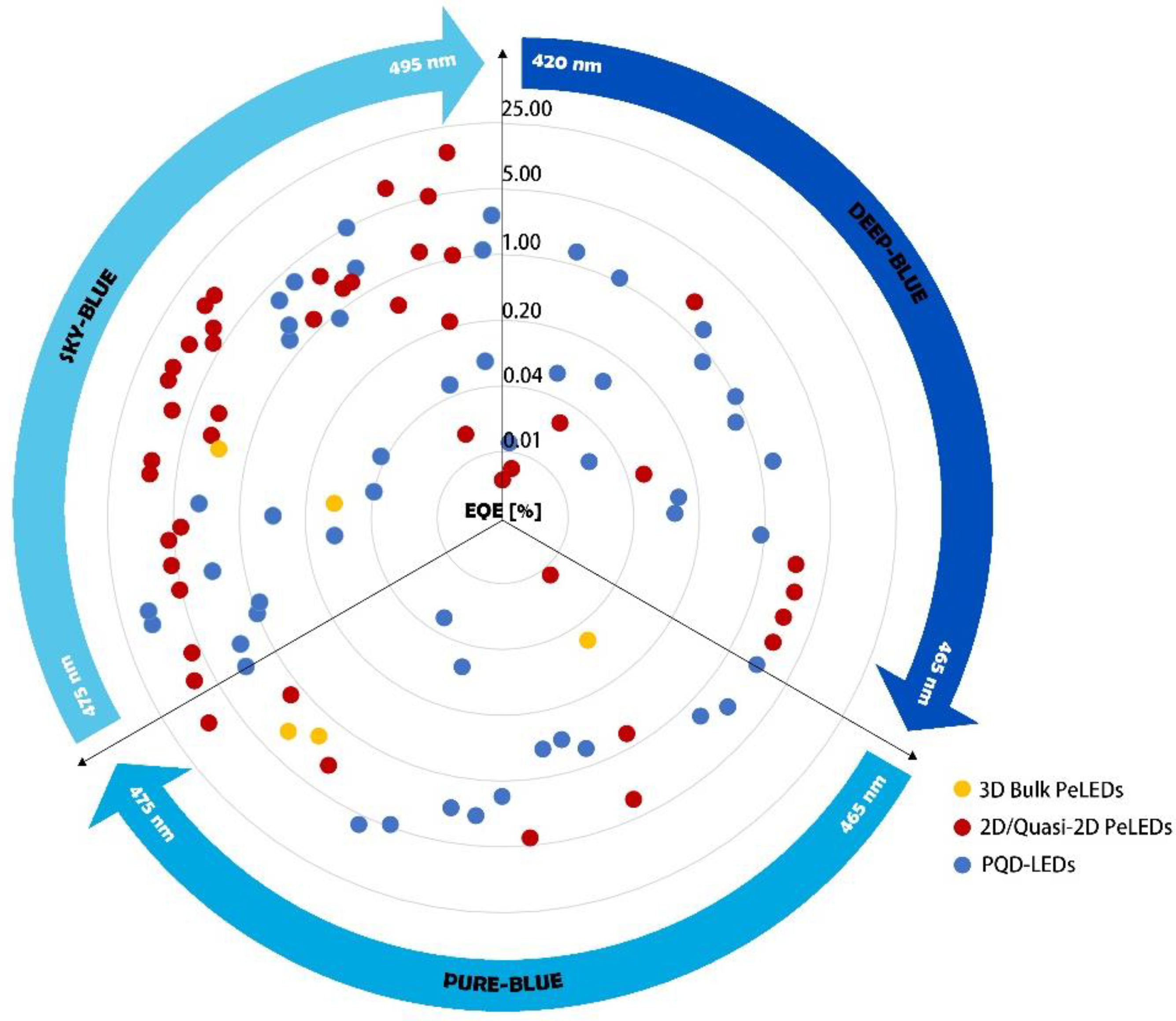
3. Uniqueness of Blue PQDs and PQD-LEDs
3.1. High-Quality Blue Light Emission
3.1.1. LIGHT Tuning Strategies
3.1.2. Deep-Blue Emission
3.2. Stability of PQD-LEDs
3.2.1. Material Stability
- Ambient stability
- Spectral stability
3.2.2. Device Stability
3.3. Defect Passivation
3.4. The Balance between Photoluminescence and Electroluminescence
3.5. Superior Light Purity
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, G.; Chen, Z.; Li, Z.; Yip, H.-L. Blue Perovskite Light-emitting Diodes: Opportunities and Challenges. Acta Phys. Chim. Sin. 2021, 37, 2009002. [Google Scholar] [CrossRef]
- He, H.; Mei, S.; Wen, Z.; Yang, D.; Yang, B.; Zhang, W.; Xie, F.; Xing, G.; Guo, R. Recent Advances in Blue Perovskite Quantum Dots for Light-Emitting Diodes. Small 2022, 18, 2103527. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef]
- Meng, L.; Yao, E.P.; Hong, Z.; Chen, H.; Sun, P.; Yang, Z.; Li, G.; Yang, Y. Pure Formamidinium-Based Perovskite Light-Emitting Diodes with High Efficiency and Low Driving Voltage. Adv. Mater. 2017, 29, 1603826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, F.; Sun, W.; Shi, H.; Hayat, T.; Alsaedi, A.; Fan, L.; Tan, Z.A. Multifunctional p-Type Carbon Quantum Dots: A Novel Hole Injection Layer for High-Performance Perovskite Light-Emitting Diodes with Significantly Enhanced Stability. Adv. Opt. Mater. 2019, 7, 1901299. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, T.; Wang, F.; Dai, S.; Tan, Z. Morphology Engineering for High-Performance and Multicolored Perovskite Light-Emitting Diodes with Simple Device Structures. Small 2016, 12, 4412–4420. [Google Scholar] [CrossRef]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Sun, W.; Zhang, J.; Hu, S.; Hayat, T.; Alsaedi, A.; Tan, Z. All-solution-processed perovskite light-emitting diodes with all metal oxide transport layers. Chem. Commun. 2018, 54, 13283–13286. [Google Scholar] [CrossRef]
- Shi, Z.; Li, S.; Li, Y.; Ji, H.; Li, X.; Wu, D.; Xu, T.; Chen, Y.; Tian, Y.; Zhang, Y.; et al. Strategy of Solution-Processed All-Inorganic Heterostructure for Humidity/Temperature-Stable Perovskite Quantum Dot Light-Emitting Diodes. ACS Nano 2018, 12, 1462–1472. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, H.; Wang, P.; Cai, J.; Wang, L.; Liu, D.; Wang, T. Spectral Tuning of Efficient CsPbBrxCl3−x Blue Light-Emitting Diodes via Halogen Exchange Triggered by Benzenesulfonates. Chem. Mater. 2020, 32, 3211–3218. [Google Scholar] [CrossRef]
- Yassitepe, E.; Yang, Z.; Voznyy, O.; Kim, Y.; Walters, G.; Castañeda, J.A.; Kanjanaboos, P.; Yuan, M.; Gong, X.; Fan, F.; et al. Amine-Free Synthesis of Cesium Lead Halide Perovskite Quantum Dots for Efficient Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 8757–8763. [Google Scholar] [CrossRef]
- Deng, W.; Xu, X.; Zhang, X.; Zhang, Y.; Jin, X.; Wang, L.; Lee, S.-T.; Jie, J. Organometal Halide Perovskite Quantum Dot Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 4797–4802. [Google Scholar] [CrossRef]
- Park, Y.R.; Kim, H.H.; Eom, S.; Choi, W.K.; Choi, H.; Lee, B.R.; Kang, Y. Luminance efficiency roll-off mechanism in CsPbBr3−xClx mixed-halide perovskite quantum dot blue light-emitting diodes. J. Mater. Chem. C 2021, 9, 3608–3619. [Google Scholar] [CrossRef]
- Shynkarenko, Y.; Bodnarchuk, M.I.; Bernasconi, C.; Berezovska, Y.; Verteletskyi, V.; Ochsenbein, S.T.; Kovalenko, M.V. Direct Synthesis of Quaternary Alkylammonium-Capped Perovskite Nanocrystals for Efficient Blue and Green Light-Emitting Diodes. ACS Energy Lett. 2019, 4, 2703–2711. [Google Scholar] [CrossRef] [Green Version]
- Todorović, P.; Ma, D.; Chen, B.; Quintero-Bermudez, R.; Saidaminov, M.I.; Dong, Y.; Lu, Z.H.; Sargent, E.H. Spectrally Tunable and Stable Electroluminescence Enabled by Rubidium Doping of CsPbBr3 Nanocrystals. Adv. Opt. Mater. 2019, 7, 1901440. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Chen, F.; Xu, L.; Li, Y.; Fang, T.; Wang, T.; Salerno, M.; Prato, M.; Song, J. Highly efficient sky-blue light-emitting diodes based on Cu-treated halide perovskite nanocrystals. J. Mater. Chem. C 2020, 8, 13445–13452. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Li, X.; Wang, S. Enhancing quantum yield of CsPb(BrxCl1−x)3 nanocrystals through lanthanum doping for efficient blue light-emitting diodes. Nano Energy 2020, 77, 105302. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Hou, S.; Quan, Q.; Congreve, D.N. Reducing Architecture Limitations for Efficient Blue Perovskite Light-Emitting Diodes. Adv. Mater. 2018, 30, 1706226. [Google Scholar] [CrossRef]
- Chiba, T.; Sato, J.; Ishikawa, S.; Takahashi, Y.; Ebe, H.; Sumikoshi, S.; Ohisa, S.; Kido, J. Neodymium Chloride-Doped Perovskite Nanocrystals for Efficient Blue Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 53891–53898. [Google Scholar] [CrossRef]
- Yang, D.; Zou, Y.; Li, P.; Liu, Q.; Wu, L.; Hu, H.; Xu, Y.; Sun, B.; Zhang, Q.; Lee, S.-T. Large-scale synthesis of ultrathin cesium lead bromide perovskite nanoplates with precisely tunable dimensions and their application in blue light-emitting diodes. Nano Energy 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Shin, Y.S.; Yoon, Y.J.; Lee, K.T.; Jeong, J.; Park, S.Y.; Kim, G.H.; Kim, J.Y. Vivid and Fully Saturated Blue Light-Emitting Diodes Based on Ligand-Modified Halide Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 23401–23409. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, H.; Zhang, R.; Liu, X.; Zhang, W.; Zhang, J.; Gao, F.; Wang, L. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Based on Potassium Passivated Nanocrystals. Adv. Funct. Mater. 2020, 30, 1908760. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, S.; Liu, J.; Yin, J.; Yuan, F.; Shen, W.-S.; Yao, K.; Wei, M.; Zhou, C.; Song, K.; et al. Chlorine Vacancy Passivation in Mixed Halide Perovskite Quantum Dots by Organic Pseudohalides Enables Efficient Rec. 2020 Blue Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 793–798. [Google Scholar] [CrossRef]
- Hou, S.; Gangishetty, M.K.; Quan, Q.; Congreve, D.N. Efficient Blue and White Perovskite Light-Emitting Diodes via Manganese Doping. Joule 2018, 2, 2421–2433. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Bai, X.; Xu, W.; Chen, X.; Zhai, Y.; Zhu, J.; Shao, H.; Ding, N.; Xu, L.; Dong, B.; et al. Bright Blue Light Emission of Ni2+ Ion-Doped CsPbClxBr3−x Perovskite Quantum Dots Enabling Efficient Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 14195–14202. [Google Scholar] [CrossRef]
- Shao, H.; Zhai, Y.; Wu, X.; Xu, W.; Xu, L.; Dong, B.; Bai, X.; Cui, H.; Song, H. High brightness blue light-emitting diodes based on CsPb(Cl/Br)3 perovskite QDs with phenethylammonium chloride passivation. Nanoscale 2020, 12, 11728–11734. [Google Scholar] [CrossRef]
- Yao, E.P.; Yang, Z.; Meng, L.; Sun, P.; Dong, S.; Yang, Y.; Yang, Y. High-Brightness Blue and White LEDs based on Inorganic Perovskite Nanocrystals and their Composites. Adv. Mater. 2017, 29, 1606859. [Google Scholar] [CrossRef]
- Bi, C.; Yao, Z.; Sun, X.; Wei, X.; Wang, J.; Tian, J. Perovskite Quantum Dots with Ultralow Trap Density by Acid Etching-Driven Ligand Exchange for High Luminance and Stable Pure-Blue Light-Emitting Diodes. Adv. Mater. 2021, 33, 2006722. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, Z.; Fang, F.; Wang, L.; Wang, G.; Liu, C.; Chen, J.; Xie, J.; Sun, J.; Wang, K.; et al. Multiple Cations Enhanced Defect Passivation of Blue Perovskite Quantum Dots Enabling Efficient Light-Emitting Diodes. Adv. Opt. Mater. 2020, 8, 2001494. [Google Scholar] [CrossRef]
- Ochsenbein, S.T.; Krieg, F.; Shynkarenko, Y.; Raino, G.; Kovalenko, M.V. Engineering Color-Stable Blue Light-Emitting Diodes with Lead Halide Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 21655–21660. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, C.; Li, X.; Li, Y.; Qiu, S.; Shen, W.; Cai, B.; Sun, Z.; Yang, D.; Deng, Z.; et al. In Situ Passivation of PbBr64− Octahedra toward Blue Luminescent CsPbBr3 Nanoplatelets with Near 100% Absolute Quantum Yield. ACS Energy Lett. 2018, 3, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Li, X.; Wu, Y.; Wei, C.; Qin, Z.; Zhang, C.; Sun, Z.; Li, Y.; Wang, Y.; Zeng, H. Surface Halogen Compensation for Robust Performance Enhancements of CsPbX3 Perovskite Quantum Dots. Adv. Opt. Mater. 2019, 7, 1900276. [Google Scholar] [CrossRef]
- Zhang, B.B.; Yuan, S.; Ma, J.P.; Zhou, Y.; Hou, J.; Chen, X.; Zheng, W.; Shen, H.; Wang, X.C.; Sun, B.; et al. General Mild Reaction Creates Highly Luminescent Organic-Ligand-Lacking Halide Perovskite Nanocrystals for Efficient Light-Emitting Diodes. J. Am. Chem. Soc. 2019, 141, 15423–15432. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Ishikawa, S.; Sato, J.; Takahashi, Y.; Ebe, H.; Ohisa, S.; Kido, J. Blue Perovskite Nanocrystal Light-Emitting Devices via the Ligand Exchange with Adamantane Diamine. Adv. Opt. Mater. 2020, 8, 2000289. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Zhang, B.; Xie, Z. Blue perovskite light-emitting diodes based on RbX-doped polycrystalline CsPbBr3 perovskite films. J. Mater. Chem. C 2019, 7, 5596–5603. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, Z.K.; Yuan, S.; Wang, Q.; Qin, C.; Wang, K.L.; Dong, C.; Li, M.; Liu, Y.; Liao, L.S. Synergistic Effect of Dual Ligands on Stable Blue Quasi-2D
Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2019, 30, 1908339. [Google Scholar] [CrossRef] - Dong, Y.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef]
- Parobek, D.; Dong, Y.; Qiao, T.; Son, D.H. Direct Hot-Injection Synthesis of Mn-Doped CsPbBr3 Nanocrystals. Chem. Mater. 2018, 30, 2939–2944. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Y.; Tu, Z.; Su, K.; Fan, X.; Liu, B.; Shi, Z.; Zhang, Y.; Zhao, C.; Zhang, B. Blue emitting CsPbBr3 perovskite quantum dot inks obtained from sustained release tablets. Nano Res. 2019, 12, 3129–3134. [Google Scholar] [CrossRef]
- Ge, W.; Shi, J.; Tian, Y.; Xu, M.; Wu, Y.; Li, Y. Core-shell CsPbBr3@Cs4PbBr6 nanocrystals dispersed in thermoplastic polyurethane as writeable heat-resistant fluorescent inks. J. Alloy. Compd. 2021, 865, 158768. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Wang, Z.; Li, X.; Chen, X.; Nalla, V.; Zeng, H.; Sun, H. Solution-Grown CsPbBr3/Cs4PbBr6 Perovskite Nanocomposites: Toward Temperature-Insensitive Optical Gain. Small 2017, 13, 1701587. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Quintero-Bermudez, R.; Voznyy, O.; Walters, G.; Jain, A.; Fan, J.Z.; Zheng, X.; Yang, Z.; Sargent, E.H. Highly Emissive Green Perovskite Nanocrystals in a Solid State Crystalline Matrix. Adv. Mater. 2017, 29, 1605945. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, Y.S.; Lim, J.; Lee, D.; Padilha, L.A.; McDaniel, H.; Robel, I.; Lee, C.; Pietryga, J.M.; Klimov, V.I. Controlling the influence of Auger recombination on the performance of quantum-dot light-emitting diodes. Nat. Commun. 2013, 4, 2661. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Lei, L.; Mendes, J.; So, F. Operational stability of perovskite light emitting diodes. J. Phys. Mater. 2020, 3, 012002. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Heo, J.M.; Pei, M.; Park, I.H.; Liu, Z.; Yun, H.J.; Park, M.H.; Jeong, S.H.; Kim, Y.H.; et al. Proton-transfer-induced 3D/2D hybrid perovskites suppress ion migration and reduce luminance overshoot. Nat. Commun. 2020, 11, 3378. [Google Scholar] [CrossRef]
- Chen, S.; Cao, W.; Liu, T.; Tsang, S.W.; Yang, Y.; Yan, X.; Qian, L. On the degradation mechanisms of quantum-dot light-emitting diodes. Nat. Commun. 2019, 10, 765. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Z.; Ma, Z.; Yang, D.; Zhang, F.; Ji, X.; Wang, M.; Chen, X.; Na, G.; Chen, S.; et al. Colloidal Synthesis of Ternary Copper Halide Nanocrystals for High-Efficiency Deep-Blue Light-Emitting Diodes with a Half-Lifetime above 100 h. Nano Lett. 2020, 20, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.E.; Park, J.H.; Jang, C.H.; Song, M.H.; Woo, H.Y. Multifunctional Charge Transporting Materials for Perovskite Light-Emitting Diodes. Adv. Mater. 2020, 32, 2002176. [Google Scholar] [CrossRef] [PubMed]
- Nenon, D.P.; Pressler, K.; Kang, J.; Koscher, B.A.; Olshansky, J.H.; Osowiecki, W.T.; Koc, M.A.; Wang, L.W.; Alivisatos, A.P. Design Principles for Trap-Free CsPbX3 Nanocrystals: Enumerating and Eliminating Surface Halide Vacancies with Softer Lewis Bases. J. Am. Chem. Soc. 2018, 140, 17760–17772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, N.K.; Abate, A.; Stranks, S.D.; Parrott, E.S.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Enhanced Photoluminescence and Solar Cell Performance via Lewis Base Passivation of OrganicInorganic Lead Halide Perovskites. ACS Nano 2014, 8, 9815–9821. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Dai, X.; Shu, Y.; Zhu, M.; Deng, Y.; Jin, Y.; Peng, X. Electrochemically-stable ligands bridge the photoluminescence-electroluminescence gap of quantum dots. Nat. Commun. 2020, 11, 937. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.-J.; Kim, J.S.; Lee, T.-W. Characterization of stability and challenges to improve lifetime in perovskite LEDs. Nat. Photonics 2021, 15, 630–634. [Google Scholar] [CrossRef]


| Performance | 2D/Quasi-2D Perovskites | PQDs | 3D Bulk Perovskites |
|---|---|---|---|
| Defects | Relatively passivated | More surface defects | Surface and bulk defects |
| FWHM | Relatively large | Small | Relatively large |
| PL performance | Good | Good | Poor |
| EL performance | Good | Poor | Poor |
| Stability | High | Moderate | Low |
| PeLEDs | OLEDs | |||||
|---|---|---|---|---|---|---|
| LT50 (hours) | Blue | Green | Red | Blue | Green | Red |
| 0.4 | 11 | 112 | 11,000 | 400,000 | 250,000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, S.; Yu, G.; Zhou, C.; Lin, F.; Han, Y.; Wang, H.; Huang, X.; Liu, X.; Hu, H.; Liu, W.; et al. Challenges and Opportunities for the Blue Perovskite Quantum Dot Light-Emitting Diodes. Crystals 2022, 12, 929. https://doi.org/10.3390/cryst12070929
Weng S, Yu G, Zhou C, Lin F, Han Y, Wang H, Huang X, Liu X, Hu H, Liu W, et al. Challenges and Opportunities for the Blue Perovskite Quantum Dot Light-Emitting Diodes. Crystals. 2022; 12(7):929. https://doi.org/10.3390/cryst12070929
Chicago/Turabian StyleWeng, Shuchen, Guicheng Yu, Chao Zhou, Fang Lin, Yonglei Han, Hao Wang, Xiaoxi Huang, Xiaoyuan Liu, Hanlin Hu, Wei Liu, and et al. 2022. "Challenges and Opportunities for the Blue Perovskite Quantum Dot Light-Emitting Diodes" Crystals 12, no. 7: 929. https://doi.org/10.3390/cryst12070929
APA StyleWeng, S., Yu, G., Zhou, C., Lin, F., Han, Y., Wang, H., Huang, X., Liu, X., Hu, H., Liu, W., Wang, Y., & Lin, H. (2022). Challenges and Opportunities for the Blue Perovskite Quantum Dot Light-Emitting Diodes. Crystals, 12(7), 929. https://doi.org/10.3390/cryst12070929








